Abstract
To understand the structure and the function of the Golgi apparatus, it is essential to establish how resident Golgi enzymes are localized in only a few Golgi cisternae. In particular it is crucial to establish whether Golgi enzymes are retained specifically in cisternae, or if they are continuously transported from cisterna to cisterna. Here we report that a resident Golgi enzyme is largely excluded from peri-Golgi transport vesicles in normal rat kidney cells, a cell type in which conflicting results have been reported. Analysis of the lateral distribution of two markers within Golgi cisternae led to the same conclusion: a protein incorporated in vesicles (KDEL receptor) is concentrated at the rims of cisternae where vesicles form, while mannosidase II is not. These results suggest that localization of resident Golgi enzymes is achieved primarily by selective retention within cisternae and exclusion from transport vesicles. These observations cannot easily be reconciled with the vision of rapidly maturing Golgi cisternae as the principal means of intra-Golgi transport.
Keywords: Golgi apparatus‖cisternal maturation‖coatomer‖glycosyltransferase‖secretion
After translocation in the endoplasmic reticulum (ER), secreted proteins are transported to the Golgi apparatus, then to the cell surface. Despite this continuous flow, each of these compartments maintains its specific composition. In the case of the ER, it has been shown that although many resident ER proteins can escape to the Golgi apparatus, they are then efficiently retrieved back to the ER via coatomer (COP1)-coated vesicles (1). A number of enzymes are also specifically localized within the Golgi stack, each one of them concentrated in the cis, the medial, or the trans cisternae.
Two conflicting views of the Golgi apparatus have emerged in recent years (for review see ref. 2; the specific issue of Golgi enzyme localization is discussed at length in refs. 3 and 4). Briefly, according to the classical model, each cisterna of the Golgi is a relatively stable compartment. The specific composition of each cisterna is ensured by selective retention of its components, while secreted proteins are successively transported from one cisterna to the next. On the contrary, the cisternal maturation model proposes that each cisterna matures successively from cis to medial to trans because of a constant flow of Golgi enzymes from cisterna to cisterna. Accordingly, Golgi enzymes would be expected to be enriched in transport vesicles surrounding the Golgi apparatus. Note that although both models predict very different results for Golgi enzymes, they do not predict precisely how cargo proteins should be localized. Vesicular transport of the cargo could be accomplished either with or without concentration in transport vesicles, whereas cisternal maturation could also allow for some retrograde transport of cargo proteins. Finally it should be emphasized that the two views are not necessarily mutually exclusive. As proposed earlier (2) cisternal maturation and vesicular transport might operate simultaneously, though the relative importance of these two transport mechanisms could differ between cell types and between organisms. In any case, an elucidation of the mechanisms of sorting of resident Golgi enzymes is essential to clarify our understanding of the general transport mechanisms through the Golgi apparatus.
Unfortunately, different laboratories have reported conflicting results: Orci et al. (4) did not detect significant amounts of Golgi resident enzymes in peri-Golgi vesicles in fibroblasts and insulin cells. In a more recent study in normal rat kidney (NRK) cells Martinez-Menarguez et al. (3) observed higher amounts of Golgi resident enzymes in peri-Golgi vesicles and in lateral cisternal rims from which they emanate. The discrepancy was attributed to the use of distinct cell types: peri-Golgi vesicles in fibroblasts and insulin cells might actually include vesicles of other origins, from which Golgi enzymes are absent, a possibility formally excluded in NRK cells. Here we report that in NRK cells mannosidase II (Man II) is largely excluded from peri-Golgi transport vesicles.
Materials and Methods
Unless otherwise specified, cells were fixed for 15 min in the culture medium containing 2% paraformaldehyde and 0.2% glutaraldehyde. The medium was then aspirated and replaced with Phosphate buffer (100 mM NaPO4, pH 7.4) containing the same fixative and incubated further for 1 h. The cells were then detached and pelleted, the fixative was rinsed out three times with Phosphate buffer and the cells processed for cryosectioning essentially as described (5). Briefly, the cell pellet was infiltrated with sucrose and frozen in liquid nitrogen. Frozen sections (45-nm thickness) were cut with a Leica FCS cryotome, transferred to grids, and incubated with the indicated antibodies. Grids were examined in a Philips CM10 transmission electron microscope.
Polyclonal antiserum to Man II was supplied by K. W. Moremen (Univ. of Georgia, Athens) (6, 7). This is the same antiserum that was used in previous studies (3, 4). Unless otherwise specified, it was used 1:50 and the secondary reagent was protein A coupled to 10-nm gold particles. Polyclonal antiserum to KDEL receptor (8) was obtained from H. D. Söling (Georg-August-Universität, Göttingen, Germany), used 1:500 followed by a goat anti-rabbit antiserum coupled to 15-nm gold particles.
Quantitative evaluation followed the procedures described previously (9). Note that the average density of gold particles over a whole stack can be slightly different from the average of the densities over each cisterna (C1–C5), because the size of the different cisternae (e.g., C1 vs. C2) can be slightly different. The average diameter of peri-Golgi vesicles was 58.3 ± 7.1 nm (mean ± SD; n = 300).
Results and Discussion
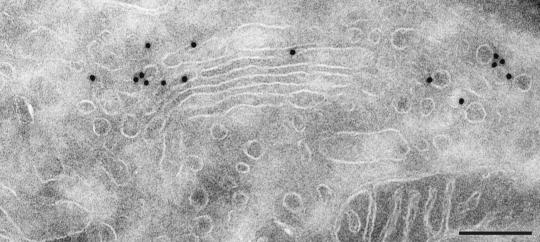
In this study, we examined the distribution of the Golgi resident enzyme Man II and of the KDEL receptor in NRK cells. The KDEL receptor binds escaped ER proteins bearing a KDEL C-terminal peptide in the Golgi apparatus and ensures their transport back to the ER. Consistent with this function and with previous reports, the KDEL receptor was concentrated in the most cis cisternae of the Golgi apparatus (C1 and C2), and abundantly represented in peri-Golgi transport vesicles, presumably destined to the ER (Fig. 1; Table 1). Indeed, the labeling of KDEL receptor in peri-Golgi vesicles was found to be increased in peri-Golgi vesicles compared with the labeling within the Golgi stack (Table 1).
Figure 1.
The KDEL receptor is found in cis-Golgi cisternae and in peri-Golgi vesicles. Immunogold labeling of KDEL receptor in NRK cells reveals an abundant localization in cis-Golgi cisternae and in peri-Golgi vesicles. (Bar = 200 nm.)
Table 1.
Localization of Man II and KDEL receptor in Golgi cisternae and peri-Golgi vesicles
| Data set | Man II experiment 1 (60 stacks) | Man II experiment 2 (60 stacks) | Man II experiment 3 (64 stacks) | KDEL receptor experiment 4 (60 stacks) |
|---|---|---|---|---|
| No. of vesicles per Golgi stack | 5.8 | 6.9 | 7.5 | 7.3 |
| % of total labeling in peri-Golgi vesicles | 4.4 | 5.8 | 5.6 | 41 |
| Labeling densities, gold per μm | ||||
| Cisternae | ||||
| C1 | 0.13 | 0.30 | 0.17 | 1.47 |
| C2 | 0.97 | 1.20 | 1.07 | 0.82 |
| C3 | 0.98 | 1.01 | 0.68 | 0.41 |
| C4 | 0.19 | 0.38 | 0.38 | 0.28 |
| C5 | 0.04 | 0.17 | 0.38 | 0.11 |
| Whole stack | 0.53 | 0.61 | 0.54 | 0.62 |
| Peri-Golgi vesicles | 0.16 | 0.19 | 0.11 | 1.43 |
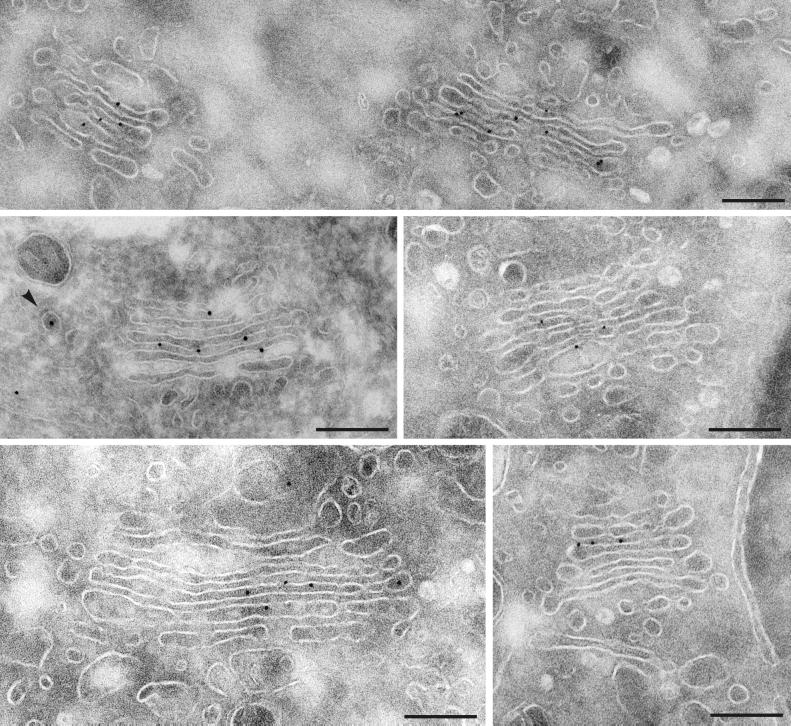
As reported previously, Man II was found concentrated in medial Golgi cisternae (C2–C3) and less abundant in C1 and C5 (Fig. 2; Table 1). We observed only a small fraction of this enzyme (≈5% of the total) in peri-Golgi vesicles. This corresponded to a labeling density within the vesicles lower than in the Golgi cisternae (approximately seven times less than in the most concentrated cisterna, four times less than in the whole stack). Because this result is in contradiction with results published by Martinez-Menarguez et al. (3), we tested many variations in the conditions of fixation, concentration of antibody, and length of incubations. The results obtained invariably indicated a fraction of labeling in peri-Golgi vesicles representing 2–5% of the total labeling, and at least four times lower labeling density in the peri-Golgi vesicles as in the cisternae (Table 2).
Figure 2.
Immunogold labeling of Man II in NRK cells. The immunogold particles are predominantly associated with the cisternal profiles of the Golgi complex. Labeling of peri-Golgi vesicles (arrowhead) was very infrequent. For quantitation see Table 1. (Bars = 200 nm.)
Table 2.
Distribution of Man II in Golgi cisternae and vesicles in different experimental conditions
| Experimental conditions (fixation/dilution of antibody and length of incubation) | No. of stacks | % of total labeling in peri-Golgi vesicles | Labeling density, gold per μm
|
||
|---|---|---|---|---|---|
| Cisternae | Vesicles | Ratio cisternae/vesicles | |||
| PAF 2% + Glut. 0.2%/1:20 1 h | 40 | 2.0 | 2.31 | 0.13 | 17.8 |
| PAF 2% + Glut. 0.2%/1:50 1 h* | 184 | 5.3 | 0.56 | 0.15 | 3.7 |
| PAF 2% + Glut. 0.2%/1:100 2 h | 48 | 6.4 | 0.91 | 0.25 | 3.6 |
| PAF 1% + Glut. 0.5%/1:50 1 h | 65 | 1.4 | 1.22 | 0.13 | 9.4 |
| PAF 1% + Glut. 0.5%/1:100 1 h | 21 | 1.8 | 0.90 | 0.06 | 15 |
| PAF 1% + Glut. 0.5%/1:100 ON | 40 | 2.5 | 2.07 | 0.17 | 12.2 |
PAF, paraformaldehyde; Glut., glutaraldehyde; ON, overnight.
For comparison the experiments described in Table 1 are summarized and indicated in bold characters.
An alternative to visualize active transport of a subset of proteins out of a compartment is to measure its concentration at the sites of formation of exit vesicles. For example, in the case of endocytosis, accumulation of membrane proteins in clathrin-coated pits precedes their specific internalization in clathrin-coated vesicles (10). Because coatomer (COP1)-coated vesicles form by budding at the rim of the Golgi cisternae, we compared the concentration of proteins at the rim of cisternae with their concentration at the center of the cisternae. For each Golgi stack, gold particles were counted in the rim of the cisternae (50 nm on each side) and in the most central area of the cisternae (100 nm). As could be expected from its abundance in peri-Golgi vesicles, the KDEL receptor was more concentrated in the lateral rims of the Golgi apparatus (Table 3). On the contrary, for Man II, labeling was not increased in lateral rims compared with the center of the cisternae, but rather slightly depleted (Table 3). Thus, the lateral distribution of KDEL receptor and Man II within the Golgi stack reflects their differential localization in peri-Golgi transport vesicles: the KDEL receptor is concentrated in the lateral rims of the cisternae and in transport vesicles, whereas Man II is not.
Table 3.
Lateral distribution of Man II and KDEL receptor in Golgi cisternae
| Data set | No. of stacks | Localization of gold particles
|
||
|---|---|---|---|---|
| Rim (2 × 50 nm) | Center (100 nm) | Rim/center ratio | ||
| KDEL receptor | ||||
| Exp. 4 | 60 | 62 | 16 | 3.88 |
| Exp. 5 | 53 | 49 | 22 | 2.23 |
| Exp. 6 | 18 | 8 | 4 | 2.0 |
| Total | 131 | 119 | 42 | 2.83 |
| Man II | ||||
| Exp. 1 | 60 | 31 | 33 | 0.93 |
| Exp. 2 | 60 | 13 | 20 | 0.65 |
| Exp. 3 | 64 | 20 | 23 | 0.87 |
| Exp. 7 | 67 | 18 | 28 | 0.64 |
| Total | 251 | 82 | 104 | 0.79 |
Overall, these observations are in good agreement with previous observations indicating that Golgi resident enzymes are excluded from peri-Golgi vesicles (4), and extend these results to NRK cells. Furthermore, they indicate that Man II is not concentrated in the rim of the Golgi cisternae where peri-Golgi vesicles form. These results are in apparent contradiction with the results of Martinez-Menarguez et al. (3), who reported an enrichment of Man II in peri-Golgi vesicles, whereas we find that Man II is present in peri-Golgi vesicles at a concentration at least four times lower than in the cisternae. This is all of the more surprising as the localization of the KDEL receptor in peri-Golgi vesicles was very similar in the two studies. To the best of our knowledge, unless otherwise specified, the conditions used in both studies (cell culture, fixation, antibody, concentration, and length of incubation) are identical. Because the number of vesicles observed by Martinez-Menarguez et al. (3) was not presented, we cannot directly compare the number of vesicles observed in both studies, but the same definition was adopted for peri-Golgi vesicles: “vesicular profiles within 200 nm lateral from a Golgi cisterna” (3). How can such a discrepancy be explained?
One difference between the two studies is that labeling was counted by Martinez-Menarguez et al. (3) in both coated buds and vesicles, though the relative amounts found in buds vs. vesicles was not specified. Labeling at the rim can represent a significant fraction of the total Golgi labeling. Assuming a random distribution of Man II in Golgi cisternae, for a 500-nm-long stack (the average size of Golgi stacks in our experiments), 20% of the total labeling would be present in the rims (50 nm on each side). By counting a portion of this as buds, a variable amount of labeling could be counted together with the vesicles.
In our experience, buds can be identified best as rounded protruding structures connected to the cisterna by a restricted neck. With this definition, we observed an average of 0.7 buds per Golgi profile (184 Golgi, experiments 1, 2, and 3), but only 0.7% of the total mannosidase labeling was present in these structures. Although coatomer (COP1) coats can be clearly visualized in sections of Epon-embedded samples (9), they are more difficult to identify unambiguously in cryosections. Only a fraction of our presumptive buds (17%) exhibited a visible coat. Because the designation of bona fide buds is somewhat arbitrary, we counted the labeling associated with presumptive buds as cisternal labeling. Our observation that there is no accumulation of mannosidase at the rims of the Golgi cisternae, and even a slight depletion, further confirms that by doing so we did not overlook a high amount of labeling concentrated in forming vesicles.
Statistical variation may be another source of discrepancy. Observations reported by Martinez-Menarguez et al. (3) were the result of analysis of 50 Golgi stacks in table 2 and 25 Golgi stacks in table 3 of ref. 3. We have analyzed more than 400 Golgi stacks. Note also that key experiments reported here were conducted independently by two entirely different groups of researchers (L.O. and P.C.), and virtually identical results were obtained.
Finally, it is possible that minor experimental differences (e.g., in cell culture conditions) could account for the differences observed. Because secretion through the Golgi apparatus is a constitutive process, a minimal conclusion of this study would be that in the conditions used here, transport through the Golgi apparatus proceeds with no evidence of massive vesicular transport of resident Golgi enzymes.
In summary no evidence was found suggesting a massive transport of Golgi resident enzymes in peri-Golgi vesicles. The absence of resident Golgi enzymes in peri-Golgi vesicles suggests that their specific localization in a few cisternae is achieved primarily by selective retention within these cisternae and exclusion from transport vesicles. These observations cannot easily be reconciled with the vision of rapidly maturing Golgi cisternae as the principal means of intra-Golgi transport.
Acknowledgments
We thank Dr. K.W. Moremem for kindly providing the Man II antibody. This work was supported by grants from the Swiss National Science Foundation (to P.C. and L.O.) and from the National Institutes of Health (to J.E.R.) and by the Fondation Gabriella Giorgi-Cavaglieri (to P.C.).
Abbreviations
- ER
endoplasmic reticulum
- Man II
mannosidase II
- NRK
normal rat kidney
References
- 1.Letourneur F, Gaynor E C, Hennecke S, Demolliere C, Duden R, Emr S D, Riezman H, Cosson P. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Pelham H R, Rothman J E. Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Menarguez J A, Prekeris R, Oorschot V M, Scheller R, Slot J W, Geuze H J, Klumperman J. J Cell Biol. 2001;155:1213–1224. doi: 10.1083/jcb.200108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orci L, Amherdt M, Ravazzola M, Perrelet A, Rothman J E. J Cell Biol. 2000;150:1263–1270. doi: 10.1083/jcb.150.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou W, Geuze H J, Slot J W. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- 6.Moremen K W, Robbins P W. J Cell Biol. 1991;115:1521–1534. doi: 10.1083/jcb.115.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moremen K W, Touster O, Robbins P W. J Biol Chem. 1991;266:16876–16885. [PubMed] [Google Scholar]
- 8.Griffiths G, Ericsson M, Krijnse-Locker J, Nilsson T, Goud B, Soling H D, Tang B L, Wong S H, Hong W. J Cell Biol. 1994;127:1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner T H, Rothman J E. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 10.Anderson R G W, Brown M S, Goldstein J L. Cell. 1977;10:351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]