Abstract
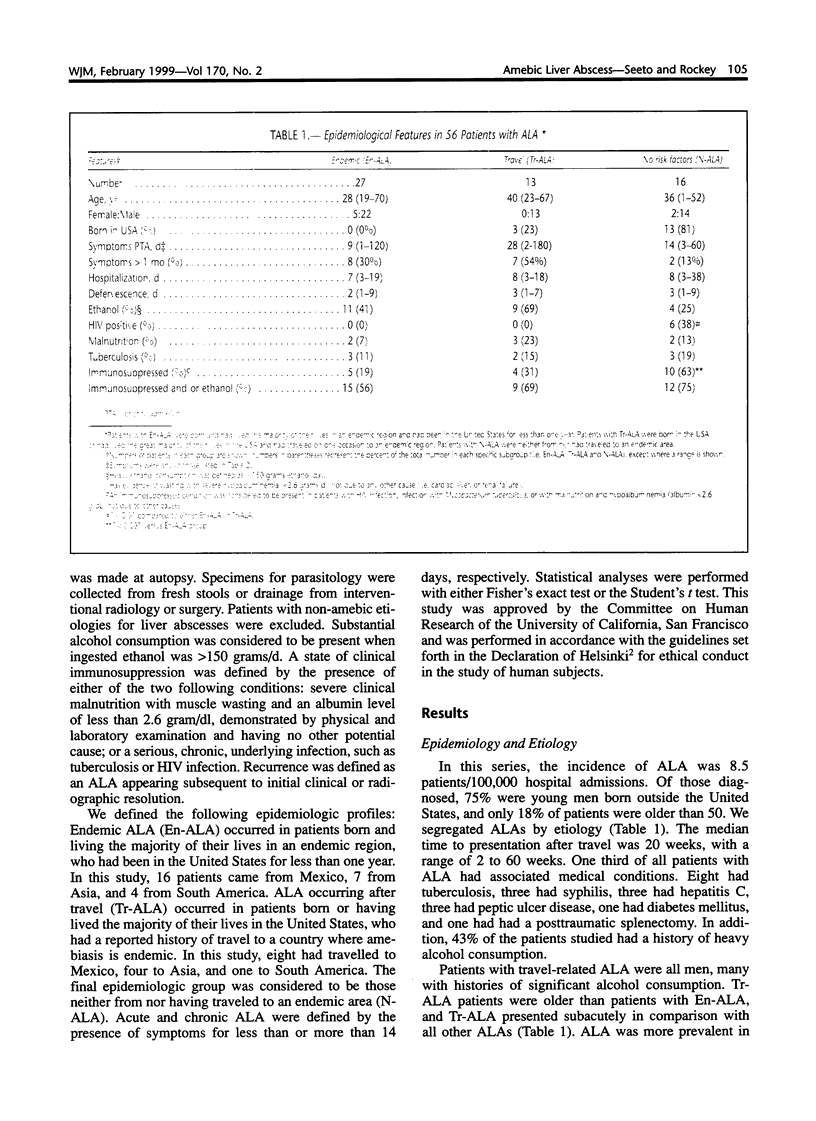
Amebic liver abscess (ALA) is a serious, but readily treatable form of hepatic infection. In order to understand the clinical features of this condition in the United States, we reviewed the medical histories of 56 patients with ALA at two large San Francisco Hospitals from 1979 to 1994. Patients were divided into the following groups based on the presumed manner in which they had acquired ALA: those born or raised in the United States, with a history of travel to an endemic area (Tr-ALA); those from an endemic area, but living in the United States for less than one year (En-ALA); and those neither from nor having traveled to an endemic area (N-ALA). We found distinct clinical patterns in patients from different epidemiological groups. Patients with Tr-ALA were a decade older than those from endemic regions, were more likely to be male, and tended to have an insidious onset. Furthermore, compared to patients with En-ALA, those with Tr-ALA were more likely to have hepatomegaly (P < 0.0001) and large abscesses (ALA > 10 cm; P < 0.01). One third of the patients studied had no associated travel history or endemic origin as risk factors. Of these, 63% had a condition consistent with severe immunosuppression, such as infection with the human immunodeficiency virus (HIV), malnourishment with severe hypoalbuminemia, or chronic infection. In patients with N-ALA, the presence of a presumed immunosuppressed state increased significantly, as compared to patients with endemic or travel risk factors for ALA. During the last five years of the study, one third of all patients diagnosed with ALA were HIV positive (including 2 with a new diagnosis of AIDS), many of whom were discovered to be HIV-infected only after presentation with ALA. We conclude that travel to and origin in an endemic area are important risk factors for the development of ALA, and patients in these different epidemiological groups appear to have distinct clinical features. Further, in the absence of recognized risk factors, the development of ALA may suggest an immunocompromised host.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ghadirian E., Meerovitch E. Effect of immunosuppression on the size and metastasis of amoebic liver abscesses in hamsters. Parasite Immunol. 1981 Winter;3(4):329–338. doi: 10.1111/j.1365-3024.1981.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E. Effect of splenectomy on the size of amoebic liver abscesses and metastatic foci in hamsters. Infect Immun. 1981 Feb;31(2):571–573. doi: 10.1128/iai.31.2.571-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E. Macrophage requirement for host defence against experimental hepatic amoebiasis in the hamster. Parasite Immunol. 1982 Jul;4(4):219–225. doi: 10.1111/j.1365-3024.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Gupta R. K. Amebic liver abscess: a report of 100 cases. Int Surg. 1984 Jul-Sep;69(3):261–264. [PubMed] [Google Scholar]
- Kanani S. R., Knight R. Relapsing amoebic colitis of 12 year's standing exacerbated by corticosteroids. Br Med J. 1969 Jun 7;2(5657):613–614. doi: 10.1136/bmj.2.5657.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein D., Rickerson V., Braude A. New concepts of amebic liver abscess derived from hepatic imaging, serodiagnosis, and hepatic enzymes in 67 consecutive cases in San Diego. Medicine (Baltimore) 1982 Jul;61(4):237–246. doi: 10.1097/00005792-198207000-00003. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Chayen A., Aust-Kettis A., Diamond L. S. Entamoeba histolytica: effect of growth conditions and bacterial associates on isoenzyme patterns and virulence. Exp Parasitol. 1986 Aug;62(1):142–148. doi: 10.1016/0014-4894(86)90017-2. [DOI] [PubMed] [Google Scholar]
- Nordestgaard A. G., Stapleford L., Worthen N., Bongard F. S., Klein S. R. Contemporary management of amebic liver abscess. Am Surg. 1992 May;58(5):315–320. [PubMed] [Google Scholar]
- Pearce R. B. Intestinal protozoal infections in AIDS. Lancet. 1983 Jul 2;2(8340):51–51. doi: 10.1016/s0140-6736(83)90038-7. [DOI] [PubMed] [Google Scholar]
- Schmerin M. J., Gelston A., Jones T. C. Amebiasis. An increasing problem among homosexuals in New York City. JAMA. 1977 Sep 26;238(13):1386–1387. doi: 10.1001/jama.238.13.1386. [DOI] [PubMed] [Google Scholar]
- Stuiver P. C., Goud T. J. Corticosteroids and liver amoebiasis. Br Med J. 1978 Aug 5;2(6134):394–395. doi: 10.1136/bmj.2.6134.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Jr, Freischlag J., Thomas D. S. Amebic liver abscess in a homosexual man. Sex Transm Dis. 1983 Jul-Sep;10(3):153–155. doi: 10.1097/00007435-198307000-00013. [DOI] [PubMed] [Google Scholar]
- Weinke T., Friedrich-Jänicke B., Hopp P., Janitschke K. Prevalence and clinical importance of Entamoeba histolytica in two high-risk groups: travelers returning from the tropics and male homosexuals. J Infect Dis. 1990 May;161(5):1029–1031. doi: 10.1093/infdis/161.5.1029. [DOI] [PubMed] [Google Scholar]


