Abstract
The membrane-disruptive antimicrobial peptide PGLa is found to change its orientation in a dimyristoyl-phosphatidylcholine bilayer when its concentration is increased to biologically active levels. The alignment of the α-helix was determined by highly sensitive solid-state NMR measurements of 19F dipolar couplings on CF3-labeled side chains, and supported by a nonperturbing 15N label. At a low peptide/lipid ratio of 1:200 the amphiphilic peptide resides on the membrane surface in the so-called S-state, as expected. However, at high peptide concentration (≥1:50 molar ratio) the helix axis changes its tilt angle from ∼90° to ∼120°, with the C-terminus pointing toward the bilayer interior. This tilted “T-state” represents a novel feature of antimicrobial peptides, which is distinct from a membrane-inserted I-state. At intermediate concentration, PGLa is in exchange between the S- and T-state in the timescale of the NMR experiment. In both states the peptide molecules undergo fast rotation around the membrane normal in liquid crystalline bilayers; hence, large peptide aggregates do not form. Very likely the obliquely tilted T-state represents an antiparallel dimer of PGLa that is formed in the membrane at increasing concentration.
INTRODUCTION
PGLa (GMASKAGAIAGKIAKVALKAL-nh2) is an antibiotic peptide found in frog skin (Hoffmann et al., 1983; Richter et al., 1985; Soravia et al., 1988; Latal et al., 1997). As a member of the magainin family it assumes an amphiphilic α-helical structure upon membrane binding (Bechinger et al., 1998). The cationic peptide has a high affinity for bacterial membranes and is able to permeabilize them. A specific receptor is not involved in this antimicrobial action (Wade et al., 1990); hence, it is unlikely that bacteria develop resistance against such peptides. Several different models of antimicrobial action have been proposed (McElhaney and Prenner, 1999; Shai, 1999; Matsuzaki, 1999; Huang, 2000; Zasloff, 2002; Strandberg and Ulrich, 2004). Initially, amphiphilic peptides are supposed to bind flat to the membrane surface in the so-called “S-state”. Above a threshold concentration the membrane may then get disrupted nonspecifically by the high surface density, as in the carpet mechanism. Alternatively, a realignment of the peptide molecules into a membrane-inserted “I-state” has been demonstrated, leading to the formation of oligomeric pores. Here, it is appropriate to differentiate between the “barrel-stave” model in which the pore is exclusively made up of peptide molecules, and the “toroidal wormhole” model in which anionic lipids contribute to the pore lining. A better understanding of the structural basis for these different functional mechanisms may help to improve the activity and specificity of antimicrobial peptides.
We have recently developed a highly sensitive solid-state 19F-NMR strategy for characterizing the orientation and dynamics of membrane-bound peptides, based on collecting a number of orientational constraints (Salgado et al., 2001; Afonin et al., 2003, 2004; Glaser and Ulrich, 2003; Glaser et al., 2004; Ulrich, 2005; Ulrich et al., 2005). The most appropriate label for obtaining these structural data is l-4-trifluoromethyl-phenylglycine (CF3-Phg), when substituted for a single nonpolar amino acid in the peptide sequence. The anisotropic 19F-19F dipolar coupling within the CF3 group is resolved by 19F-NMR measurements using macroscopically aligned membrane samples (Glaser et al., 2004). The dipolar splittings from several individual labels serve as experimental constraints to determine the orientation of the peptide within the lipid bilayer. For PGLa reconstituted in DMPC (dimyristoyl-phosphatidylcholine) at a peptide/lipid molar ratio (P/L) of 1:200 we have previously demonstrated that the helix is aligned flat in the plane of the membrane. The tilt angle of ∼90° between the helix axis and the membrane normal corresponds to the surface-bound S-state, as expected from the amphiphilic character of the peptide. We have now studied PGLa at higher concentration (P/L ≥ 1:50), since antimicrobial peptides tend to become biologically active only above such threshold value (Blazyk et al., 2001). Here, we observed an unexpected realignment of the peptide, whereby the helix axis assumes an oblique tilt angle. This new orientation is clearly distinct from a transmembrane alignment, and it does not correspond to an I-state, which would have been expected for such peptides when a pore is formed (Huang, 2000). Instead, it appears that PGLa is able to self-assemble as a dimer on the bilayer surface at high peptide concentration, which would explain how its tilted orientation in the membrane is stabilized as a distinct state.
MATERIALS AND METHODS
19F-NMR analysis
A series of CF3-Phg labeled PGLa analogs were synthesized, purified, and characterized to be functionally fully intact, as previously reported (Afonin et al., 2003; Glaser et al., 2004). A single label was introduced either at position Ile-9, Ala-10, Ile-13, or Ala-14. Macroscopically oriented samples with a mixture of 0.2–0.5 mg peptide plus lipid in different molar ratios were prepared as previously described, and one-pulse 19F-NMR spectra were acquired on a 500-MHz wide-bore Unity Inova spectrometer (Varian, Palo Alto, CA) with a 19F/1H double-tuned flat-coil probe (Doty Scientific, Columbia, SC) that can be manually rotated and allows 1H decoupling with 10–15 kHz (Glaser et al., 2004).
15N-NMR analysis
15N-labeled PGLa was synthesized by standard Fmoc solid phase synthesis with 15N-glycine at Gly-11. For the sample with P/L = 1:200, ∼1 mg of peptide plus 70 mg DMPC were oriented on 25 glass plates, and 2.7 mg PGLa plus 47 mg lipid were used for the P/L 1:50 sample. 15N-NMR experiments were performed at 50.68 MHz on a Bruker Avance 500 MHz NMR spectrometer with a fixed flat-coil triple-resonance probe (Bruker, Karlsruhe, Germany). We used a ramped cross-polarization sequence with a power of 40 kHz, 2.5 s relaxation delay time, 100 kHz spectral width, 2048 data points, and tppm20 proton decoupling. The contact time was 1 ms in hydrated samples, and 2 ms for the lyophilized peptide. Between 30,000 and 200,000 scans were collected. Spectra were referenced to 15NH4NO3 by setting the signal of solid 15NH4Cl to 18.0 ppm. We determined the principal axis values of the 15N chemical shift anisotropy (CSA) tensor as σ11 = 14, σ22 = 33, and σ33 = 197 ppm by fitting the lineshape to the experimental spectrum of the lyophilized peptide powder. We used an orientation of the 15N tensor of the labeled glycine with σ33 tilted 17° from N-H toward the N-C′ bond, and σ11 normal to the plane of the peptide bond (Sternberg et al., 2004).
RESULTS AND DISCUSSION
Concentration-dependent realignment of PGLa in DMPC
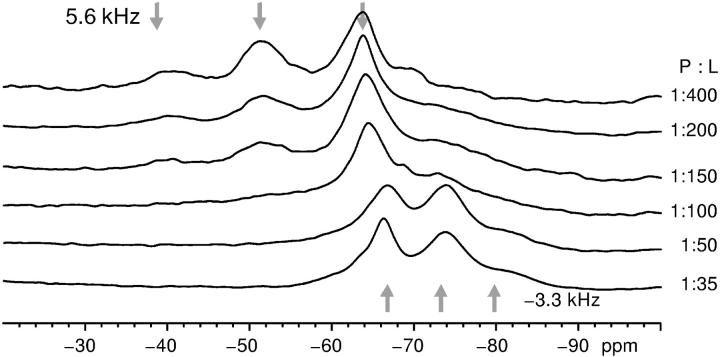
The one-pulse 19F-NMR spectra of the four different labeled PGLa analogs were measured at different peptide/lipid ratios in DMPC membranes at 35°C (Fig. 1). At low peptide concentration, i.e., between 1:1000 and 1:200, the dipolar analysis yielded a surface alignment as previously described (Glaser et al., 2004). Upon increasing the P/L to 1:50 and beyond, the chemical shift and dipolar couplings of the labels changed (Table 1). In the following, we will analyze and interpret the structural meaning of these spectral changes, providing new insight into the peptide-lipid interactions of antimicrobial peptides. A similar observation of a concentration-dependent change in NMR parameters had been reported for a 4F-Phg labeled PGLa (Glaser and Ulrich, 2003) as well as a related model peptide K3 (Toke et al., 2004). At that time, no structural interpretation had been possible in these systems.
FIGURE 1.
Solid-state 19F-NMR spectra of PGLa-Ile13-CF3Phg at 35°C in oriented DMPC membranes with different P/L ratios. The membrane normal is oriented parallel to the B0-field. The dipolar coupling within the CF3-group is read from the splitting of the triplet, and its sign is taken from the chemical shift. The asymmetric shape of the triplet results from differential relaxation properties (Glaser et al., 2004).
TABLE 1.
19F dipolar couplings (in kHz) of CF3-Phg-labeled PGLa
| Labeled position | Ile-9 | Ala-10 | Ile-13 | Ala-14 |
|---|---|---|---|---|
| P/L ratio 1:200 | 0 | 0 | 5.6 | −5.4 |
| P/L ratio 1:50 | −4.7 | −3.1 | −3.3 | −5.3 |
Using 31P-NMR, we confirmed that the phospholipids remain in a lamellar bilayer phase at all concentrations, irrespective of the peptide orientation (Sachse, 2003). We observed a slight smooth change of the anisotropic 31P chemical shift of the lipids, roughly proportional to peptide concentration. Such a qualitative reduction of lipid headgroup order was also observed with many other molecules dissolved in membranes (Zidovetzki et al., 1988). There was no indication of phase separation between bound and free lipids, nor between peptide-rich and peptide-depleted regions. The uncharged lipid DMPC allowed a highly reproducible preparation of samples with well-oriented bilayers. As the oriented samples contain no excess bulk water, anionic lipids are not necessary to attract the peptides to the membranes.
Confirmation of realignment in DMPC/DMPG (dimyristoyl-phosphatidylglycerol)
To determine the alignment of PGLa in the membrane from the local orientational constraints of its four labels, the peptide secondary structure has to be verified. Circular dichroism was used to confirm that the helix content of all four analogs is comparable to that of the wild-type peptide in the presence of small unilamellar vesicles at a P/L of 1:50 and 1:200 (see Supplementary Material). Unlike the solid-state 19F-NMR experiments above, circular dichroism (CD) measurements are performed on vesicles in dilute suspension. Hence, negatively charged lipids are required to attract the cationic PGLa to the membrane. Under those conditions we have previously shown by solution-state 19F-NMR that the fraction of membrane-bound PGLa increases when negatively charged dimyristoyl-phosphatidylglycerol (DMPG) is mixed into the vesicles of DMPC (Afonin et al., 2003). Once the overall negative charge of the lipids exceeded the positive charge of PGLa (+5 at neutral pH), virtually all PGLa was associated to the membrane and the concentration in bulk solution became immeasurably small.
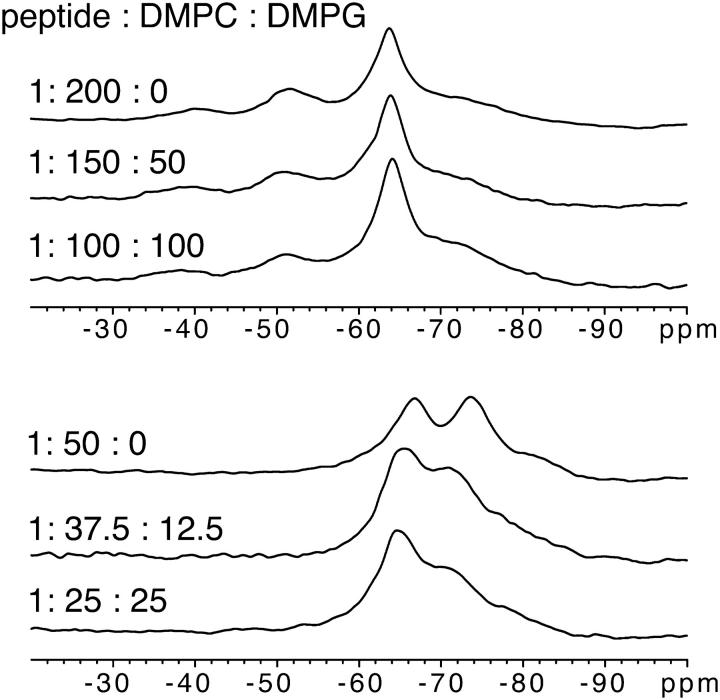
To test the influence of negative lipid charge on peptide orientation and to justify the CD-derived secondary structure information, we acquired further solid-state 19F-NMR spectra of a labeled PGLa analog in mixtures of DMPC and DMPG. Measurements in oriented membranes were performed at 1:200 and 1:50 molar peptide/lipid ratio (Fig. 2). The linewidth on these spectra was higher than in samples containing only DMPC. The reason for the line broadening is not clear at this point, but differences in hydration properties and 1H decoupling are likely. Nevertheless, for 3:1 and 1:1 DMPC/DMPG we found the same qualitative changes in the spectra as in DMPC alone, which is clearly indicative of a reorientation from the S-state at 1:200 to a new state at 1:50. Given that the conformation of PGLa remains α-helical in all cases, the observed changes in the 19F-NMR spectra must therefore be attributed to a concentration-induced realignment of PGLa in the membrane.
FIGURE 2.
19F-NMR spectra of PGLa (as in Fig. 1) in oriented membranes with different mixtures of DMPC and DMPG.
Calculation of helix tilt angle
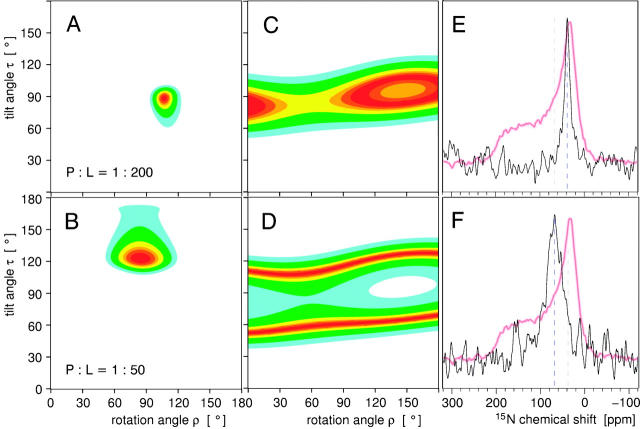
The orientation of PGLa in the bilayer can be calculated from the signed dipolar splittings of the individual CF3-Phg labels (see Supplementary Material). The peptide orientation is fully described by two angles: the tilt angle τ between the helix axis and the membrane normal, and the azimuthal rotation angle ρ around the helix axis. With our definition of Cα of Lys-12 and the helix axis as the base of the molecular coordinate system, τ ≈ 0° corresponds to an inserted orientation (I-state), and τ ≈ 90°, ρ ≈ 80 … 120° corresponds to a surface orientation according to the amphiphilic side-chain distribution of the peptide. Additionally, the degree of motional averaging is described by a molecular order parameter Smol. These three parameters, τ, ρ, and Smol are derived from the four dipolar couplings by means of a least-squares fit (Glaser et al., 2004; Afonin et al., 2004). For PGLa at P/L = 1:200 the minimum in the resulting ρ/τ map corresponds to the S-state, with τ ≈ 89°, ρ ≈ 106°, and Smol ≈ 0.6 (Fig. 3 A), as described before (Glaser et al., 2004). At P/L = 1:50, on the other hand, we find that the allowed region in the ρ/τ map has moved to τ ≈ 123°, ρ ≈ 85°, with Smol ≈ 0.6 (Fig. 3 B). Now the helix axis is tilted at an oblique angle, with the C-terminus of PGLa more deeply inserted into the lipid bilayer than the N-terminus. The azimuthal angle has also changed to some extent, though the amphiphilicity profile of PGLa is still compatible with the charged side chains reaching up toward the aqueous layer. We shall name this novel tilted state the “T-state”.
FIGURE 3.
Orientation of the PGLa helix in a DMPC bilayer at a 1:200 (upper panels) and 1:50 (lower panels) molar peptide/lipid ratio. The contour plots A–D show the sum of squared deviations between the experimental data and calculated values as a function of the helix tilt angle τ and the azimuthal rotation ρ, with best-fitting orientations in dark red. (A and B) Summary of the 19F-NMR analysis based on four CF3-Phg labels, and (C and D) a single 15N label. The corresponding 15N spectra are shown in E and F, with the powder lineshape of the lyophilized peptide superimposed.
In a concentration-dependent series of experiments from 1:400 to 1:35, at low peptide concentration (1:400–1:150) the splitting in Fig. 1 is +5.6 kHz, whereas at high concentration (1:50, 1:35) the splitting is −3.3 kHz. There is not a gradual change of the splitting, which indicates that there is not a gradual change of peptide orientation. At intermediate concentration the spectra are broadened to such an extent that the two outer peaks of the triplet are not detectable, i.e., the peptide changes between the S- and T-states in the timescale of the NMR experiment. The same concentration-dependent change between two distinct states has also been observed for PGLa labeled with 4F-phenylglycine, when an extensive concentration range was studied from 1:3000 to 1:8. Here, the chemical shift underwent a distinct change at a limiting threshold concentration of around 1:100 (Glaser and Ulrich, 2003).
Peptide mobility in the membrane
The orientational analysis above yielded an order parameter of Smol ≈ 0.6 for PGLa in both the S- and the T-states in liquid crystalline DMPC. On a scale of 1.0 (no averaging) to 0.0 (isotropic tumbling) this value accounts for both the global orientational mobility of the peptide with respect to the membrane normal and the local wobbling motion of the label.
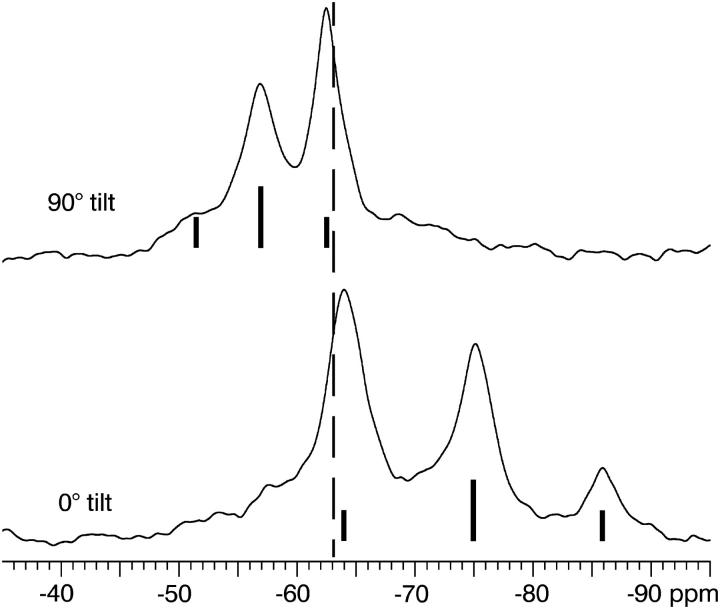
Rotational diffusion of PGLa around the membrane normal is not visible in the spectra acquired with the membrane normal aligned parallel to the static magnetic field direction, but it is revealed when the sample is positioned at a 90° angle, i.e., with the membrane normal perpendicular to the field (Salgado et al., 2001; Ulrich, 2004; Glaser et al., 2004). All spectra at P/L ratios of 1:200 and 1:50 were found to scale around the isotropic chemical shift by a factor of −1/2 compared to the respective spectrum acquired at 0° angle, and no powder pattern or signal broadening was observed (Fig. 4). This indicates that all NMR interactions are averaged around the sample axis on the timescale of the experiment (10 kHz spectral width). Thus, the PGLa molecules rotate freely around the membrane normal, in both the S- and T-states.
FIGURE 4.
19F-NMR spectra of PGLa (as in Fig. 1) at a P/L of 1:50 in oriented DMPC membranes acquired with two different sample orientations. Upper spectrum with the membrane normal aligned perpendicular to the magnetic field (90° tilt), and lower spectrum with the membrane normal parallel to the magnetic field (0° tilt). In addition, the idealized triplet structure of the spectrum along with the isotropic chemical shift of CF3-Phg (dotted line) are indicated.
We can furthermore exclude that PGLa rotates around its helical axis, since this would lead to identical dipolar splittings for all labeled positions. Especially the dipolar couplings of the epimeric D-CF3-Phg labels (see Supplementary Material) are incompatible with such motion, which besides would be energetically very unfavorable in view of the amphiphilic character of PGLa.
Verification of the peptide tilt angle by 15N-NMR
To decide whether the observed spectral changes at high peptide concentration are genuinely due to an oblique tilt of PGLa, we studied a peptide labeled with 15N at Gly-11. 15N-NMR experiments have a much lower sensitivity compared to 19F-NMR experiments (Afonin et al., 2003), but we can be sure that this label does not perturb the local peptide conformation nor its interactions with other molecules. At low peptide concentration (P/L = 1:200) the 15N signal of an oriented sample is at 40 ppm, and at high concentration (1:50) it moves to 68 ppm (Fig. 3, E and F). To find out which alignments of the peptide helix are compatible with these data, the principal axis values of the 15N CSA tensor need to be known. As Gly differs slightly from other amino acids (Sternberg et al., 2004), the values were determined from the lyophilized peptide powder, which is shown superimposed over the oriented spectra.
Orientational analysis of the 15N data in terms of τ yielded a tilt angle of 90° for a P/L of 1:200, confirming the S-state, as expected (Fig. 3 C). At a high peptide concentration of 1:50, we determined a helix tilt of about τ ≈ 65° or 115°, which is also in full agreement with the 19F analysis (Fig. 3 D). This orientational analysis was performed with Smol = 0.7. For the 15N label in the backbone, a slightly higher order parameter than 0.6 was chosen to compensate for any extra wobble of the CF3-Phg side chain and any motion in the lyophilized peptide. The signal lies close to (but not at) the isotropic position of 15N, but free tumbling or micellarization of the peptide can be excluded in view of the 19F- and 31P-NMR data on equivalent samples. For any reasonable choice of 0.5 < Smol < 1 only an oblique helix tilt can explain the 15N data at P/L = 1:50. The evaluation of an oblique helix tilt angle thus unequivocally confirms the self-consistent picture derived above by 19F-NMR.
We note that the 15N-NMR signal at 40 ppm of the 1:50 sample is significantly broader than the signal of the 1:200 sample at 68 ppm. This is an inevitable consequence of the differential effect of the mosaic spread on the 15N chemical shift in the different CSA tensor orientations. Namely, the same mosaic spread produces an apparent linewidth that is ∼3 times smaller near the high-field and low-field edge of the CSA range (S-state) than in the middle of the CSA range (T-state).
Further evidence for a realignment of PGLa from an S-state to a T-state stems from analyzing the epimeric byproducts of our peptide synthesis, namely those PGLa analogs carrying a d-CF3-Phg label (see Supplementary Material). The bulky and stiff d-amino acid is expected to cause slight changes in the structure and in the hydrophobic surface of the peptide; therefore, the dipolar couplings only provide qualitative information about the orientation of the peptide (Glaser et al., 2004). Nevertheless, these measurements also confirmed the obliquely tilted orientation of PGLa at high concentration in the membrane, and they clearly excluded the possibility of a transmembrane orientation of the PGLa helix.
Interpretation in terms of peptide dimerization
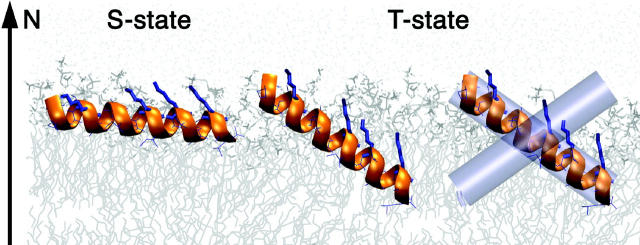
Notably, the concentration-dependent series of experiments never showed a gradual shift of the signals (e.g., Fig. 1) (as also observed in Glaser and Ulrich, 2003). At peptide/lipid ratios around 1:100, the NMR signal deteriorates because of exchange between the two populations in the intermediate timescale. This is taken as evidence for the presence of two distinct populations of peptides in different orientational states, separated by an energy barrier. A tilted state with τ = 120° would not seem to be a particularly favorable alignment for a monomer of PGL, which is already perfectly stable in a flat alignment (S-state). Hence, the change from the S- to the T-state must involve more than just a change in orientation with respect to the membrane. Significant conformational changes were excluded by CD experiments, and are very unlikely since our NMR data fits well with a helical conformation in both states. The formation of large peptide aggregates can also be excluded in view of the observed fast rotation around the membrane normal and the same Smol at low concentration. Therefore, although we have no direct experimental evidence, the most probable explanation for the occurrence of the T-state is the formation of peptide dimers upon increasing the PGLa concentration in the membrane. Indeed, PGLa is known to form dimers in a lipid monolayer at the air-water interface (Konovalov et al., 2002). Dimerization in the membrane has even been described for related antimicrobial peptides: REDOR distance measurements on the model peptide K3 showed parallel dimers (Toke et al., 2004), transferred NOE of a magainin analog indicated antiparallel dimers (Wakamatsu et al., 2002), and PGLa was found to associate with magainin as an antiparallel heterodimer (Hara et al., 2001). The tilt angle of such peptide dimers in the membrane has not yet been determined, as these experiments provided distance constraints but no orientational information. In our case of PGLa, the homodimers in DMPC must be rotationally symmetric with respect to the membrane normal, since we observe only a single set of NMR signals. The tilt of the monomer is then a consequence of the dimer orientation according to its overall amphiphilic shape. Self-assembly of PGLa into antiparallel dimers may thus account for the observation of the distinctly tilted T-state, as schematically illustrated in Fig. 5.
FIGURE 5.
Illustration of the observed realignment of PGLa in a DMPC membrane. At low peptide concentration, the amphiphilic helix lies flat on the surface in the S-state. With increasing concentration, it assumes a tilted T-state, which appears to involve the formation of antiparallel peptide dimers.
Assessment of error margins
An experimental inaccuracy of ±0.5 kHz in the dipolar splitting translates into an intrinsic error in τ and ρ of <±10° (Glaser et al., 2004). Nevertheless, this margin includes the ambiguity of choosing one or another helical conformation for the data analysis, as demonstrated by our systematic analysis of different conformational models (see Supplementary Material). Since the l-CF3-Phg labels used to replace Ile or Ala may induce slight distortions in the peptide backbone, the error margins should be considered more generously, say at ±20°. Especially in the T-state, which appears to involve specific peptide-peptide interactions, the stiff CF3-Phg labels might be more problematic than in the monomeric S-state. The information from a single nonperturbing 15N-label is not very accurate either (about ±20° in τ, and ρ is generally not accessible by 15N CSA analysis of single labels). Nonetheless, the distinct changes in the 19F dipolar couplings and in the 15N chemical shift unequivocally show that PGLa undergoes a concentration-dependent realignment.
With a small number of orientational constraints we can only test whether a certain helix conformation and orientation is compatible with the NMR data. In fact, the four constraints from the l-CF3-Phg labels alone would also be compatible with a distorted helix that is slightly tilted away from a transmembrane orientation (see Supplementary Material). However, the independent additional constraints (1× 15N-Gly, 4× d-CF3-Phg) supporting the oblique tilt of the central helical segment of PGLa make any alternative interpretation of the data very unlikely.
CONCLUSIONS
The tilted T-state of PGLa exhibits quite unexpected properties that do not fit well into the currently discussed structural models of antimicrobial action (Shai, 1999; Matsuzaki, 1999; Huang, 2000; Zasloff, 2002; Strandberg and Ulrich, 2004). It is clearly not compatible with a barrel-stave pore, and the rotational diffusion of the peptide around the membrane normal does not support the carpet model either. The oblique tilt with an order parameter nearly identical to that of the S-state is also not compatible with a toroidal wormhole. Although the high peptide concentration of the T-state may appear to correlate with the threshold for pore formation (Wieprecht et al., 2000), there is no evidence from our experiments that this state should be directly responsible for the destruction of the membrane barrier. Dimerization of PGLa at the membrane surface is a plausible hypothesis to explain our data in light of observations from similar amphiphilic helical membrane peptides from the same family. The T-state may thus be an intermediate in the formation of pores on the way from the monomeric S-state toward a cooperative I-state.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
CD experiments, 19F-NMR experiments with d-CF3-Phg labeled peptides, and effect of structural variation on peptide orientation are available as supporting material in PDF format; the orientational calculation of τ and ρ is available as MATHEMATICA notebook.
This work was supported by Sonderforschungsbereich 604 (TP B6) and by the Center for Functional Nanostructures of the Deutsche Forschungsgemeinschaft.
References
- Afonin, S., R. W. Glaser, M. Berditchevskaia, P. Wadhwani, K. H. Gührs, U. Möllmann, A. Perner, and A. S. Ulrich. 2003. 4-Fluorophenylglycine as a label for 19F-NMR structure analysis of membrane-associated peptides. ChemBioChem. 4:1151–1163. [DOI] [PubMed] [Google Scholar]
- Afonin, S., U. H. N. Dürr, R. W. Glaser, and A. S. Ulrich. 2004. “Boomerang”-like insertion of a fusogenic peptide in a lipid membrane revealed by solid state 19F-NMR. Magn. Reson. Chem. 42:195–203. [DOI] [PubMed] [Google Scholar]
- Bechinger, B., M. Zasloff, and S. J. Opella. 1998. Structure and dynamics of the antibiotic peptide PGLa in membranes by solution and solid-state nuclear resonance spectroscopy. Biophys. J. 74:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazyk, J., R. Wiegand, J. Klein, J. Hammer, R. M. Epand, R. F. Epand, W. L. Maloy, and U. P. Kari. 2001. A novel linear amphipathic beta-sheet cationic antimicrobial peptide with enhanced selectivity for bacterial lipids. J. Biol. Chem. 276:27899–27906. [DOI] [PubMed] [Google Scholar]
- Glaser, R. W., C. Sachse, U. H. N. Dürr, P. Wadhwani, and A. S. Ulrich. 2004. Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F-NMR dipolar couplings of 4-CF3-phenylglycine labels. J. Magn. Reson. 168:153–163. [DOI] [PubMed] [Google Scholar]
- Glaser, R. W., and A. S. Ulrich. 2003. Susceptibility corrections in solid state NMR experiments with oriented membrane samples. Part I: Applications. J. Magn. Reson. 164:104–114. [DOI] [PubMed] [Google Scholar]
- Hara, T., Y. Mitani, K. Tanaka, N. Uematsu, A. Takakura, T. Tachi, H. Kodama, M. Kondo, H. Mori, A. Otaka, F. Nobutaka, and K. Matsuzaki. 2001. Heterodimer formation between the antimicrobial peptides magainin 2 and PGLa in lipid bilayers: a cross-linking study. Biochemistry. 40:12395–12399. [DOI] [PubMed] [Google Scholar]
- Hoffmann, W., K. Richter, and G. Kreil. 1983. A novel peptide designated PYLa and its precursor as predicted from cloned mRNA of Xenopus laevis skin. EMBO J. 2:711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. W. 2000. Action of antimicrobial peptides: two-state model. Biochemistry. 39:8347–8352. [DOI] [PubMed] [Google Scholar]
- Konovalov, O., I. Myagkov, B. Struth, and K. Lohner. 2002. Lipid discrimination in phospholipid monolayers by the antimicrobial frog skin peptide PGLa. A synchrotron X-ray grazing incidence and reflectivity study. Eur. Biophys. J. 31:428–437. [DOI] [PubMed] [Google Scholar]
- Latal, A., G. Degovics, R. F. Epand, R. M. Epand, and K. Lohner. 1997. Structural aspects of the interaction of peptidyl-glycylleucine-carboxyamide, a highly potent antimicrobial peptide from frog skin, with lipids. Eur. J. Biochem. 248:938–946. [DOI] [PubMed] [Google Scholar]
- Matsuzaki, K. 1999. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta. 1462:1–10. [DOI] [PubMed] [Google Scholar]
- McElhaney, R. N., and E. J. Prenner, editors. 1999. Special issue. The interaction of antimicrobial peptides with model lipid bilayers and biological membranes. Biochim. Biophys. Acta. 1462:1–234. [DOI] [PubMed] [Google Scholar]
- Richter, K., H. Aschauer, and G. Kreil. 1985. Biosynthesis of peptides in the skin of Xenopus laevis: isolation of novel peptides predicted from the sequence of cloned cDNAs. Peptides. 6(Suppl 3):17–21. [DOI] [PubMed] [Google Scholar]
- Sachse, C. 2003. 19F-Festkörperuntersuchungen zur Orientierung und Dynamik des antimikrobiellen Peptides PGLa in Lipidmembranen. Diploma thesis, Friedrich-Schiller-University Jena. http://www.db-thueringen.de/servlets/DocumentServlet?id=1898
- Salgado, J., S. L. Grage, L. H. Kondejewski, R. S. Hodges, R. N. McElhaney, and A. S. Ulrich. 2001. Membrane-bound structure and alignment of the antimicrobial beta-sheet peptide gramicidin S derived from angular and distance constraints by solid state 19F-NMR. J. Biomol. NMR. 21:191–208. [DOI] [PubMed] [Google Scholar]
- Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phopholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1462:55–70. [DOI] [PubMed] [Google Scholar]
- Soravia, E., G. Martini, and M. Zasloff. 1988. Antimicrobial properties of peptides from Xenopus granular gland secretions. FEBS Lett. 228:337–340. [DOI] [PubMed] [Google Scholar]
- Sternberg, U., R. Witter, and A. S. Ulrich. 2004. 3D structure elucidation using NMR chemical shifts. Annu. Rep. NMR Spectrosc. 52:53–104. [Google Scholar]
- Strandberg, E., and A. S. Ulrich. 2004. NMR methods for studying membrane-active antimicrobial peptides. Concepts Magn. Reson. 23A:89–120. [Google Scholar]
- Ulrich, A. S. 2005. Solid state 19F-NMR methods for studying biomembranes. Prog. Nucl. Magn. Reson. Spectrosc. In press.
- Ulrich, A. S., P. Wadhwani, U. H. N. Dürr, S. Afonin, R. W. Glaser, E. Strandberg, P. Tremouilhac, C. Sachse, M. Berditchevskaia, and S. Grage. 2005. Solid-state 19F-NMR analysis of membrane-active peptides. In NMR Spectroscopy of Biological Solids. H. Ramamoorthy, editor. Marcel Dekker, New York. In press.
- Toke, O., R. D. O'Connor, T. K. Weldeghiorghis, W. L. Maloy, R. W. Glaser, A. S. Ulrich, and J. Schaefer. 2004. Structure of (KIAGKIA)3 aggregates in phospholipid bilayers by solid-state NMR. Biophys. J. 87:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, D., A. Boman, B. Wåhlin, C. M. Drain, D. Andreu, H. G. Boman, and R. B. Merrifield. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA. 87:4761–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu, K., A. Takeda, T. Tachi, and K. Matsuzaki. 2002. Dimer structure of magainin 2 bound to phospholipid vesicles. Biopolymers. 64:314–327. [DOI] [PubMed] [Google Scholar]
- Wieprecht, T., O. Apostolov, M. Beyermann, and J. Seelig. 2000. Membrane binding and pore formation of the antibacterial peptide PGLa: thermodynamic and mechanistic aspects. Biochemistry. 39:442–452. [DOI] [PubMed] [Google Scholar]
- Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. [DOI] [PubMed] [Google Scholar]
- Zidovetzki, R., U. Banerjee, D. W. Harrington, and S. I. Chan. 1988. NMR study of the interactions of polymyxin B, gramicidin S, and valinomycin with dimyristoyllecithin bilayers. Biochemistry. 27:5686–5692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.