Abstract
Hydrophobins are a class of small proteins that fulfill a wide spectrum of functions in fungal growth and development. They do so by self-assembling into an amphipathic membrane at hydrophilic-hydrophobic interfaces. The SC3 hydrophobin of Schizophyllum commune is the best-studied hydrophobin. It assembles at the air-water interface into a membrane consisting of functional amyloid fibrils that are called rodlets. Here we examine the dynamics of SC3 assembly at an oil-water and air-water interface and the permeability characteristics of the assembled layer. Hydrophobin assembled at an oil-water interface is a dynamic system capable of emulsifying oil. It accepts soluble-state SC3 oligomers from water in a unidirectional process and sloughs off SC3 vesicles back into the water phase enclosing a portion of the oil phase in their hydrophobic interior. The assembled layer is impermeable to solutes >200 Da from either the water phase or the oil phase; however, due to the emulsification process, oil and the hydrophobic marker molecules in the oil phase can be transferred into the water phase, thus giving the impression that the assembled layer is permeable to the marker molecules. By contrast, the layer assembled at an air-water interface is permeable to water vapor from either the hydrophobic or hydrophilic side.
INTRODUCTION
Hydrophobins are a class of small proteins that play an important role in fungal growth and development (Wösten, 2001). For instance, they allow fungi to escape an aqueous environment, confer hydrophobicity to fungal surfaces in contact with air, and mediate attachment of fungi to hydrophobic surfaces. Hydrophobins have diverse amino acid sequences but share eight conserved cysteine residues (Wessels, 1997). Class I and class II hydrophobins are distinguished on the basis of differences in hydropathy patterns and biophysical properties (Wessels, 1994). SC3 of Schizophyllum commune is the best characterized class I hydrophobin. It self-assembles at interfaces between water and air, water and oil, and water and a hydrophobic solid (Wösten et al., 1993, 1994a, 1995) Mature SC3 consists of 112 amino acids (de Vocht et al., 1998). Its eight cysteine residues form four disulfide bridges (de Vries et al., 1993) that prevent spontaneous self-assembly in solution and thus account for the controlled assembly at hydrophilic-hydrophobic interfaces (de Vocht et al., 2000). SC3 contains 17–22 mannose residues that are attached to threonine residues in the N-terminal part of the protein (de Vocht et al., 1998). These sugar residues are exposed at the hydrophilic side after self-assembly and thus determine the surface properties of this side (Wösten et al., 1994b; de Vocht et al., 1998; Scholtmeijer et al., 2002).
SC3 undergoes several conformational changes during self-assembly. Hydrophobin exists in water as oligomers that are rich in β-sheet (de Vocht et al., 1998; Wang et al., 2002). Oligomerization of SC3 takes place above a critical concentration of ∼4 μg/ml. (Wang et al., 2002, 2004a). Upon assembly at the air-water interface, SC3 proceeds through an intermediate form with increased α-helical structure (α-helical state) to a film which has a β-sheet signature but no clear ultrastructure (β-sheet I state) and, finally, after prolonged incubation, to the β-sheet II state consisting of amyloid-like fibrils 10 nm in diameter that are called rodlets (Wösten et al., 1993; Wösten and de Vocht, 2000; de Vocht et al., 1998, 2002). The rodlets formed by SC3 are composed of two tracks of 2–3 protofilaments that are 2.5 nm wide (Wösten and de Vocht, 2000). Like other amyloid fibrils (LeVine, 1993), they increase the fluorescence of thioflavin T and bind Congo red (Wösten and de Vocht, 2000; Mackay et al., 2001; Butko et al., 2001). Assembly of SC3 on a Teflon surface is similar to that at the air-water interface except that it does not spontaneously adopt its stable end form at this interface. Instead it stops at the intermediate α-helical state and only proceeds to the β-sheet state after treating the SC3 coated Teflon surface with detergent at elevated temperatures (de Vocht et al., 1998, 2002). A predicted amphipathic helix between the third and fourth cysteine has been identified as responsible for the binding to a Teflon surface and inducing significant structural change to the α-helical-state structure (Wang et al., 2004b).
Here we show that functional amyloid fibrils spontaneously form not only at an air-water interface but also at an oil-water interface. The SC3 membrane consisting of amyloid fibrils is permeable to water vapor but the diffusion of molecules >200 Da is blocked. However, the molecules >200 Da can pass through the membrane via soluble-state SC3-assisted emulsification which is driven by one-way insertion of SC3 into the membrane and the resulting increase of surface area.
MATERIALS AND METHODS
Purification and labeling of hydrophobins
A monokaryotic strain of S. commune with a deleted SC15 gene (Lugones et al., 2004) was used for production of the SC3 hydrophobin. The fungus was grown in production medium (Scholtmeijer et al., 2002) in 1 L shaken cultures (225 rpm) for 5–7 days at 24°C. SC3 was purified from the culture medium as described (Wösten et al., 1993; Wessels, 1997). SC3 was labeled with dansyl or dabcyl according to Wang et al. (2002).
Self-assembly of SC3 at the air-water and oil-water interface
Freeze-dried labeled or unlabeled SC3 was treated with trifluoroacetic acid to dissociate assemblages into soluble-state SC3 (de Vries et al., 1993; Wösten et al., 1993). After removing the solvent by a stream of air, the protein was taken up in 50 mM sodium phosphate, pH 7.0 (1 mg/ml). In the case of FRET experiments, stock solutions of dabcyl and dansyl labeled SC3 were mixed 1:1 and incubated for 30 min before use, or stock solution of dansyl-SC3 was used for coating first, followed by the addition of a dabcyl-SC3 stock solution to a ratio of 1:1.
Assembly of SC3 at the air-water interface was done as follows. A soluble-state SC3 stock solution (1 mg/ml) was diluted 10-fold in a cuvette containing 50 mM sodium phosphate, pH 7.0, and the solution was allowed to stand at room temperature for a stated time before measurements were carried out. In the case of assembly at an oil-water interface, paraffin oil was layered on top of the buffer solution immediately after soluble-state SC3 was added to the buffer. In the case of assembly at an oil-water interface in an emulsion, emulsions of organic solvents (butanol, hexane, and hexadecane) and oils (olive oil, paraffin oil) in 50 mM sodium phosphate, pH 7.0 (2.5% v/v) were prepared. These were obtained by bath sonication for 10 min just before adding SC3 to a final concentration of 100 μg/ml.
Incorporation of SC3 into an SC3 membrane already assembled at an oil-water interface
SC3 was added to an oil-water emulsion to a final concentration of 100 μg/ml. After overnight incubation, the oil droplets were either washed or not washed. All procedures were carried out at room temperature. Washing was done in the following way. An aliquot of 2 ml was centrifuged at 1000 rpm in an Eppendorf table centrifuge for 2 min. The buffer was carefully removed with a glass pipette, and the oil was resuspended in 2 ml of buffer. This was repeated 3 times with 30-min incubation periods between the washes. A dansyl-SC3 solution was then added to all the samples including a control sample with SC3 alone (no oil droplets). At given times, a 1-ml aliquot was filtered through a 0.2-μm low-protein-binding membrane (Millex-LG, Millipore, Billerica, MA), and the dansyl fluorescence in the filtrate was measured to determine the loss of dansyl-SC3 from the solution, i.e., the incorporation of dansyl-SC3 into the membrane.
The converse experiment was carried out with dansyl-SC3 being added to the oil emulsion first, followed by the addition of unlabeled-SC3 after the washes. The gain of dansyl fluorescence in the filtered solution was measured at the end to determine the release of dansyl-SC3 from the membrane.
Thickness of an SC3 membrane formed at an air-water interface
A 100 μl volume of SC3 solution (100 μg/ml) in 50 mM phosphate, pH 7.0, was dried down on a 1 × 1-cm mica surface, and the thickness of the membrane formed was determined to be ∼47 nm by AFM. The refractive index of assembled SC3 in the membrane was determined with a Nanofilm EP3 ellipsometer (LayTec, Berlin, Germany), and the resulting value, 1.535, was used to determine the thickness of the membrane formed at an air-water interface using the same equipment. For this measurement, a plastic cuvette was filled with freshly prepared soluble-state SC3 in 50 mM sodium phosphate, pH 7.0, at a concentration of 100 μg/ml or 10 μg/ml. The position and the height of the cuvette were adjusted until the laser from the ellipsometer was maximally reflected by the water surface. The measurement was started ∼10 min after the preparation of SC3 solution. The sample was left at the same position without disturbance and the data were collected at given times. The data were then analyzed using the software, AnalysR.
Thioflavin T fluorescence of SC3 at a hydrophobic-hydrophilic interface
A thioflavin T (ThT) stock solution (300 μM) was diluted 100-fold at fixed time intervals in mixtures of oil and water to which hydrophobin was added. ThT fluorescence was measured using a SPF-500C spectrofluorometer (SLM Aminco, Foster City, CA). The excitation wavelength was 450 ± 4 nm and emission was measured at 500 ± 4 nm. Data for each sample were corrected for the signal before the addition of ThT.
Permeability of the SC3 membrane formed at an air-water interface
For the measurement of water permeability from the hydrophilic side to the hydrophobic side of the membrane, a cuvette was filled with 2 ml buffer (50 mM phosphate, pH 7.0) or 2 ml of freshly prepared soluble-state SC3 (10 or 100 μg/ml) in the same buffer. The samples were incubated at 37°C and were weighed at various time points. The water evaporated from the sample was then calculated by subtracting the actual weight from the original weight.
For the measurement of permeability from the hydrophobic side to the hydrophilic side, the same SC3 and control (no SC3) samples as mentioned above were placed in an open dessicater. After overnight incubation, the bottom of the dessicater was filled with deuterium oxide (D2O) and the dessicater was closed immediately. One SC3 and one control sample were taken out at each time point from the dessicater and the content of D2O in each sample was determined by NMR using a Varian Unity INOVA 600 NMR spectrometer.
Permeability of the SC3 membrane formed at a paraffin oil/buffer interface
Pyrene (molecular mass 202.26 Da), neutral Texas Red dextran (molecular mass 3,000 Da), and neutral rhodamine B dextran (molecular mass 10,000 Da) were used to study the permeability of the SC3 membrane. Pyrene was purchased from Fluka (St. Louis, MO), the other compounds from Molecular Probes (Eugene, OR). Pyrene was dissolved in paraffin oil or buffer (50 mM sodium phosphate, pH 7.0) to a saturated concentration. All of the dextrans were dissolved in paraffin oil to saturated concentrations or dissolved in buffer to a final concentration of ∼100 μg/ml. Sample preparation and measurements were done in a 1 × 1-cm (4-ml) quartz cuvette with a stirring bar at the bottom. The excitation and emission wavelength of pyrene were 347 and 395 nm, respectively. Those of Texas Red-labeled dextran and rhodamine B-labeled dextran were 582 and 610 nm and 570 and 603 nm, respectively. The change of fluorescence intensity in the buffer phase was followed for 2.5 h with a SPF-500C spectrofluorometer (SLM Aminco). For this, the cuvette was placed in the spectrofluorometer in such a way that the light only passed through the buffer phase. The slit width for excitation and emission was 4 nm. Experiments were carried out at room temperature, except for those with dextran 10,000, which were done at 50°C to accelerate transfer between the phases.
Transfer from buffer to paraffin oil
A cuvette was filled with 2 ml of a solution of soluble-state SC3 supplemented with one of the fluorescent markers. For pyrene, a saturated concentration was used, and for the dextrans, the final concentration was ∼100 μg/ml. Paraffin oil (1 ml) saturated with the same fluorescent marker was then carefully layered on top of the SC3 solution. The hydrophobin was allowed to assemble at the oil-water interface at room temperature overnight. The cuvette was then placed in the spectrofluorometer in such a way that light only passed through the aqueous phase. The transfer experiment was started by replacing 0.5 ml of marker-saturated paraffin oil top layer with 1.5 ml of the oil lacking the marker. Measurements were immediately started under conditions of slow stirring. The control experiment was done in the same way but in the absence of SC3.
Transfer from paraffin oil to buffer
A cuvette was filled with 2 ml of a solution of soluble-state SC3 on top of which 1 ml of paraffin oil was layered. The cuvette was left at room temperature overnight to allow SC3 to fully assemble at the oil-water interface. The cuvette was then placed in the spectrofluorometer. The transfer experiment was started by carefully adding 1 ml of paraffin oil saturated with a fluorescent marker on top of the original paraffin oil layer. The stirring-bar was then immediately set to slow rotation. The control experiment was done in the same way but in the absence of SC3.
Fluorescence and confocal microscopy
For fluorescence microscopy of SC3-coated oil droplets, a 20-μl sample was put on a glass slide and covered with a coverslip. The sample was examined using an Olympus Provis AX70 microscope. Pictures were taken with a 3.3 MegaPixel-camera Color View II and analyzed with AnalySIS docu software by Soft Imaging System (Münster, Germany).
For confocal laser scanning microscopy experiments, pyrene-saturated paraffin oil and 50 mM sodium phosphate, pH 7.0 (2% v/v) were sonicated for 2 min on ice. Triton X-100 (10% v/v) or an SC3 solution (1 mg/ml) was added to the emulsion to a final concentration of 0.01% and 100 μg/ml, respectively, immediately after sonication. The emulsions were incubated overnight at room temperature in the dark and subsequently washed or not washed. Washing was done in the same way as mentioned above. Samples of 20 μl were mounted on glass slides and analyzed by confocal microscopy (TCS Leica SP2 confocal laser scanning microscope, Welzlar, Germany). Two to four images were taken for each sample, and the fluorescence intensity of 10–20 droplets (middle plane of the vesicles) of each image was quantified by averaging the signals using Leica Confocal Software, version 2.5 (Leica Microsystems, Heidelberg, Germany).
RESULTS
Spontaneous SC3 membrane formation at the paraffin oil/buffer interface
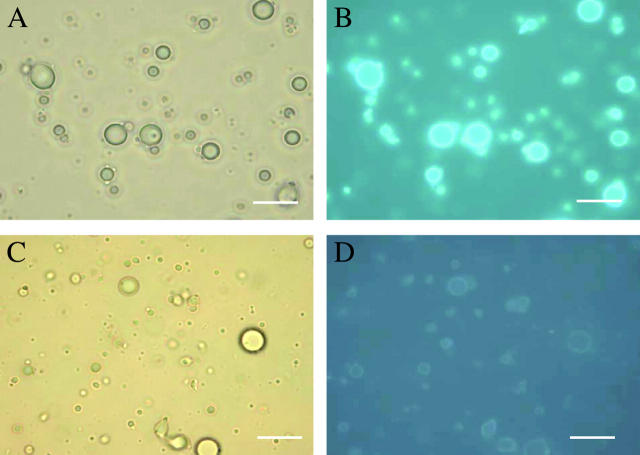
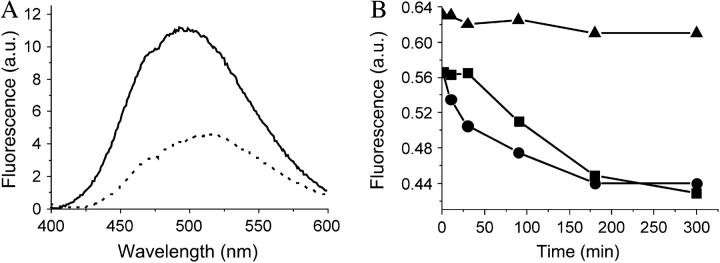
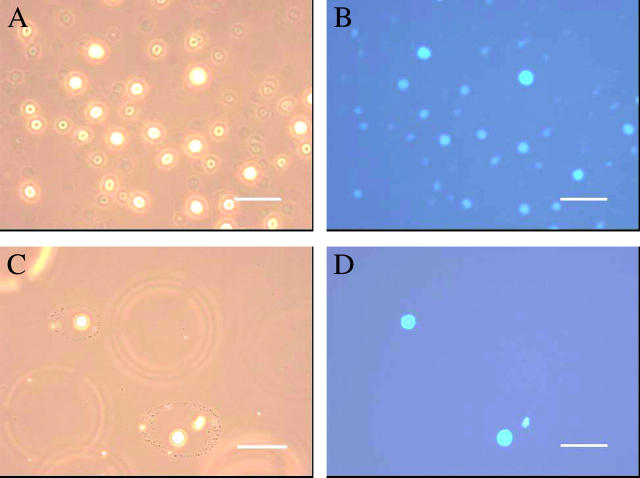
Emulsions of butanol, hexane, hexadecane, olive oil, or paraffin oil were mixed with soluble-state SC3 to a final concentration of 100 μg/ml. The paraffin oil emulsion showed the highest stability as deduced from the turbidity of the aqueous phase and, consequently, was used for the remainder of the study. Dansyl-SC3 (fluorescence resonance energy transfer (FRET) donor) also stabilized the paraffin oil emulsion. When a sample was taken 30 min after adding the labeled SC3 to the paraffin oil emulsion, membranes coating the paraffin oil droplets showed a green fluorescence that was somewhat enhanced after overnight incubation (Fig. 1, A and B). When a mixture of dansyl-SC3 and dabcyl-SC3 (FRET acceptor) were used together to stabilize the paraffin oil emulsion, the fluorescence of dansyl-SC3 was quenched at the oil-water interface (Fig. 1, C and D), indicating that the membrane is composed of spatially proximate SC3 molecules. A similar quenching was observed if oil droplets were first coated overnight with dansyl-SC3, then washed to remove excess labeled SC3 and exposed to dabcyl-SC3. Quenching to 50% of the original fluorescence occurred within 10 min (Fig. 2 A). Washing of the quenched sample or addition of an excess amount of unlabeled SC3 did not lead to recovery of dansyl fluorescence at all (data not shown), indicating that once incorporated, SC3 remains strongly associated in the membrane. In another experiment (Fig. 2 B), the droplets were coated overnight with native SC3 and the resulting emulsions were washed (dots) or not washed (squares). Dansyl-SC3 was then added to the samples and its incorporation into the coating around the oil droplet was followed as a loss of dansyl-SC3 from the supernatant after removing the oil droplets by filtration (see Materials and Methods). The decrease in fluorescence (Fig. 2 B) suggests that SC3 continues to incorporate into the assembled membrane over a period of hours. Conversely, when dansyl-SC3 was used to coat the droplets first, followed by the addition of SC3, no dansyl-SC3 could be competed out of the membrane into the solution, indicating that the incorporation of SC3 into the assembled membrane is a one-way process (data not shown). If oil droplets were not present (triangles) the fluorescence was constant, confirming that the fluorescence decrease was due to incorporation of labeled protein into the oil droplet membrane rather than removal of aggregated and precipitated protein by filtration.
FIGURE 1.
Fluorescence of SC3-coated paraffin oil droplets in buffer. Dansyl-SC3-coated oil droplets shown by light (A) and fluorescence microscopy (B) and dansyl-SC3 (donor)/dabcyl-SC3 (acceptor)-coated oil droplets shown by light (C) and fluorescence microscopy (D). Bar indicates 10 μm.
FIGURE 2.
Incorporation of SC3 into the membrane assembled at an oil-water interface. (A) Overnight-incubated dansyl-SC3-coated oil droplets were washed (solid line) and then supplemented with dabcyl-SC3 to the same concentration as for the dansyl-SC3 that was originally present. Dansyl fluorescence was recorded after 10 min (dotted line). (B) Overnight-incubated unlabeled-SC3-coated oil droplets were washed (circles) and not washed (squares). Unlabeled-soluble-state SC3 alone served as a control (triangles). At T = 0, dansyl-SC3 was added to the same concentration as the original unlabeled SC3. Aliquots of 1 ml were taken at each time point from each sample, filtered through a 0.2-μm membrane, and the dansyl fluorescence in the filtrate was measured.
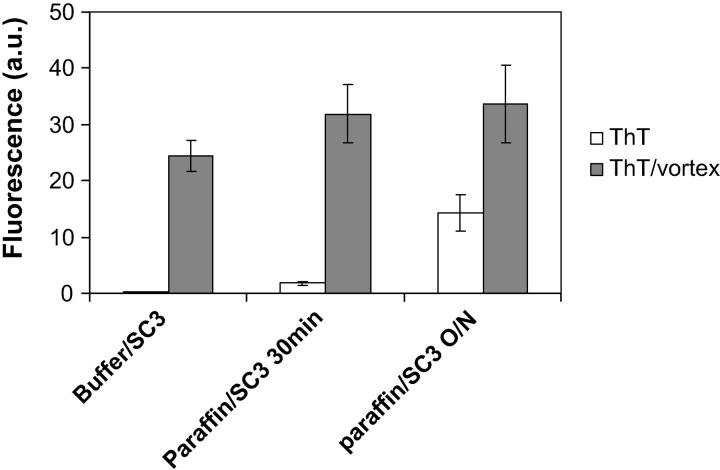
The amyloid-specific fluorescent dye ThT has been shown to interact with self-assembled SC3 in the β-sheet II state (Wösten and de Vocht, 2000; Butko et al., 2001). Fig. 3 shows that soluble-state SC3 in buffer hardly interacts with ThT. Vortexing, however, results in conversion to the β-sheet assembled state and results in increased ThT fluorescence, as has already been reported by Wösten and de Vocht (2000). In the case of SC3 and a paraffin oil emulsion, the ThT fluorescence increased twofold 30 min after adding soluble-state SC3 and from 10–15-fold after overnight incubation. This is ∼40% of the maximum ThT fluorescence obtained by vortexing the sample. When ThT was added to the overnight-incubated sample that had been washed with buffer to remove free SC3, the fluorescence reached the same level as the open columns but, after vortexing, increased only slightly (data not shown). Therefore, the additional increase in ThT fluorescence in the paraffin oil emulsions in Fig. 3 upon vortexing can be attributed to SC3 association on air bubbles just as in the case of the buffer/SC3 sample. These data show that SC3 spontaneously self-assembles at the oil-water interface into the stacked β-sheet state.
FIGURE 3.
ThT fluorescence study of interfacial assembly of SC3. Amyloid-like β-sheet-state formation of SC3 at the air-water and oil-water interfaces as determined by the increase in ThT fluorescence. Open and shaded columns, respectively, represent samples before and after vortexing for 5 min. Error bars indicate the standard deviation of the results of three independent experiments.
Permeability of the SC3 membrane at the paraffin oil-buffer interface
Transfer from the hydrophilic to hydrophobic side
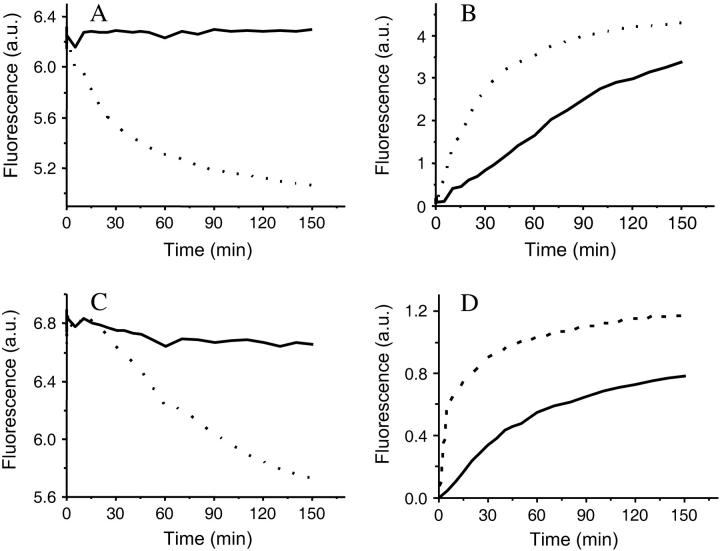
To measure the permeability of the SC3 membrane from the hydrophilic side, hydrophobin was allowed to self-assemble at a paraffin oil-water interface in a cuvette as stated in Materials and Methods. A downwardly curved interface was formed immediately after adding the paraffin oil on top of the SC3 solution, whereas without SC3 it formed a flat surface. The tight adhesion of the edge of the SC3 membrane to the cuvette wall might have contributed to such an unusual phenomenon. The transfer of pyrene (202 mol wt) from water to oil was monitored by following the fluorescence decrease in the buffer phase (see Materials and Methods for details). But first, to exclude that SC3 changes the fluorescence properties of pyrene in the buffer phase, the fluorescence emission spectrum was determined in the absence and presence of the hydrophobin. Pyrene excited at 345 nm showed an emission spectrum composed of two bands, a monomer band with peaks near 390 nm and an excimer band with a broad peak near 460 nm. The excimer/monomer ratio, which can be calculated from the fluorescence intensities at 460 and 390 nm, has been used by others to report the environmental hydrophobicity once pyrene accesses the interior of micelles, membranes, or proteins (Sen and Chakrabarti, 1990; Bhattacharyya et al., 2002). If pyrene bound to a hydrophobic environment in SC3 assemblies or formed SC3-coated micelles, a significant change in fluorescence intensities at 390 and 460 nm would have been expected. As shown in Fig. 4 A, the pyrene excimer/monomer ratio increased only slightly in time in the presence of SC3, indicating that a large amount of pyrene monomers (at least 90%) still remained in solution even after overnight incubation. Therefore, changes in pyrene fluorescence intensity, when they occur in the experiments described here, must be due to transfer across the hydrophobin membrane and not to binding of the marker to the protein.
FIGURE 4.
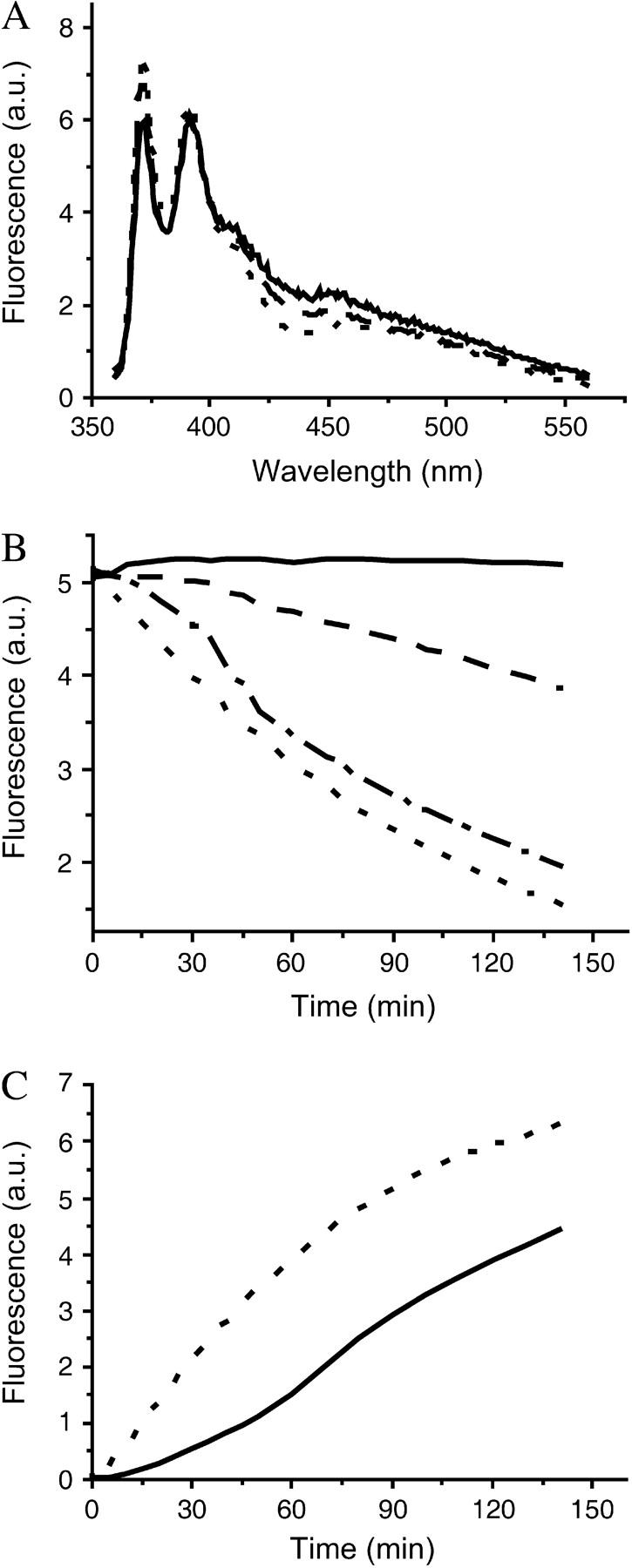
Pyrene transfers through the SC3 membrane formed at the buffer-paraffin oil interface. (A) Pyrene fluorescence is only slightly changed in the presence of SC3. Soluble-state SC3 was mixed with a saturated pyrene solution in 50 mM sodium phosphate, pH 7.0. The final concentration of SC3 was 100 μg/ml. The fluorescence spectrum of pyrene was taken after a 30-min (dashed line) and a 16-h (solid line) incubation. The same pyrene solution in the absence of SC3 served as a control (dotted line). (B) Pyrene transfer from buffer to paraffin oil after overnight assembly of SC3 at the oil-water interface starting from a concentration of the hydrophobin in the buffer phase of 3 μg/ml (dash-dotted line), 10 μg/ml (dashed line), and 100 μg/ml (solid line). A solution without SC3 served as a control (dotted line). (C) Pyrene transfer from paraffin oil to buffer in the absence (dotted line) or presence (solid line) of an SC3 membrane formed during overnight incubation from a solution containing 100 μg/ml of protein.
Pyrene rapidly transferred from buffer to paraffin oil in the absence of SC3 or after overnight assembly of SC3 from a 3-μg/ml solution at the oil-water interface (Fig. 4 B). However, the rate of transfer was reduced when SC3 had assembled overnight from a 10-μg/ml SC3 solution, and it was blocked completely by overnight assembly from a 100-μg/ml SC3 solution (Fig. 4 B). By contrast, the transfer of pyrene was reduced by only 20% when SC3 was replaced by bovine serum albumin or lysozyme (100 μg/ml) (data not shown). Similar results were obtained with dextran 3000 and dextran 10,000. Dextran 3000 equilibrated easily between buffer and paraffin oil in the absence of an SC3 membrane. Dextran 10,000 diffused more slowly, and therefore, a higher temperature (50°C) was used to accelerate transfer of this marker. After overnight assembly of SC3 (100 μg/ml), transfer of dextran 3,000 and 10,000 from buffer to oil was completely blocked (Fig. 5, A and C). From these data we can conclude that an SC3 membrane completely blocks the movement of molecules >200 Da from the hydrophilic to the hydrophobic phase.
FIGURE 5.
Transfer of dextran 3000 (A and B) and dextran 10,000 (C and D) in the absence (dotted lines) or presence (solid lines) of an SC3 membrane formed overnight at the paraffin oil/buffer interface from a protein solution of 100 μg/ml. Transfer was measured from buffer to paraffin oil (A and C) and from paraffin oil to buffer (B and D).
Transfer from the hydrophobic to the hydrophilic side
In contrast to transfer from buffer to the oil, pyrene apparently still transferred at room temperature from paraffin oil to buffer after overnight assembly of 100 μg/ml SC3 (Fig. 4 C), although the transfer rate was reduced compared to that observed across an interface without hydrophobin. Like pyrene, dextran 3000 and dextran 10,000 passed through the SC3 membrane (Fig. 5, B and D). Cytochrome c, a globular protein with a molecular weight of ∼12,000 Da, behaved similarly to dextran 10,000 (data not shown).
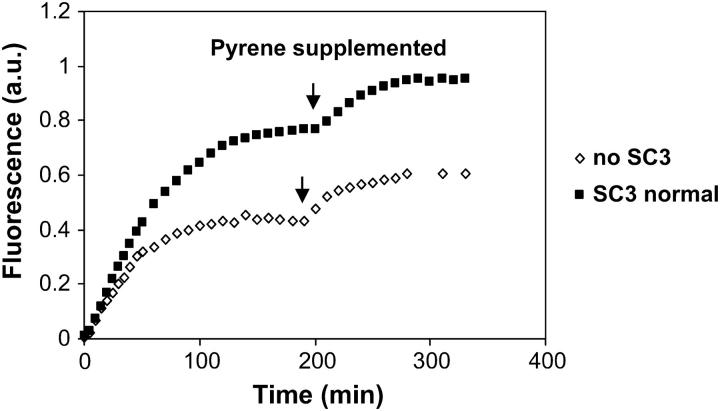
Unexpectedly, when the experiment shown in Fig. 4 C was repeated using a low concentration of pyrene in oil (e.g., one-tenth of the saturated concentration), the concentration of pyrene that accumulated in the aqueous phase reached a higher level than that in the control sample in the absence of SC3. An even further accumulation of pyrene in the buffer phase was observed if, after the system reached equilibrium, extra pyrene/oil was added to the sample (Fig. 6). This result suggests that another process apart from diffusion must be involved in transferring pyrene across the membrane. A closer examination of the physical state of the fluorescent markers in the aqueous phase provided the answer.
FIGURE 6.
Lowering the pyrene concentration used in oil resulted in more partition of pyrene in the aqueous phase in a buffer/SC3/oil system (▪) than in a buffer/oil control system (⋄). Pyrene concentration in oil was ∼10 times as low as that in a saturated solution, and only 100 μl of such pyrene/oil solution was added on the top of the oil phase. After the pyrene transfer reached equilibrium, 50 μl of extra pyrene/oil was added at the time indicated by the arrow.
When soluble-state SC3 was present in the aqueous phase, the fluorescent markers, pyrene and the dextrans that had transferred from the paraffin oil phase, were found in small oil droplets. No such oil droplets were observed in the sample without hydrophobin or in samples containing 0.01% (v/v) Triton X-100 instead of SC3 (Fig. 7). Moreover, these oil droplets were absent when the transfer experiment was done in the absence of stirring. This correlated with the absence of pyrene in the aqueous phase. Apparently, under stirring conditions, SC3 in the aqueous phase emulsifies oil droplets from the paraffin oil phase. Pyrene transfer from oil to water was also monitored in an emulsion of paraffin oil and buffer stabilized either by Triton X-100 (control) or SC3. The change of pyrene fluorescence in the oil droplets was monitored in time by confocal laser scanning microscopy. In both cases, pyrene fluorescence slightly decreased in the first 2 days and then remained constant for the next 7 days (Fig. 8). However, as Fig. 9 shows, Triton X-100 coated droplets rapidly destabilized when excess surfactant was washed away. Both the number and the size of the droplets decreased in time and the pyrene fluorescence in the remaining oil droplets decreased significantly, suggesting that pyrene transferred out of the oil droplets in this case. In contrast, when excess of SC3 was washed away from the buffer phase, the oil droplets remained stable and no pyrene fluorescence was lost. Clearly, SC3 can stabilize the oil droplets by a mechanism different from the detergent molecules. Further supplementing the solution containing washed SC3-coated oil droplets with freshly prepared soluble-state SC3 (to 100 μg/ml) seemed to restore the pyrene transfer to some extent. If the washed SC3-coated oil droplets were supplemented with Triton X-100 (to 0.01% v/v), an even larger amount of pyrene was released from oil after long incubation, whereas the size and the number of the droplets did not change significantly (Fig. 9).
FIGURE 7.
Large oil droplets were observed in the aqueous phase in the cuvette containing buffer/pyrene/SC3 (100 μg/ml)/paraffin oil (A and B). After the membrane was formed overnight at the interface, the solution was slowly stirred for 2.5 h and an aliquot of the aqueous solution was taken for microscopic measurement. No droplets were observed in a control sample using 0.01% (v/v) Triton X-100 instead of SC3 (C and D). Samples were visualized by light microscopy (A and C) and fluorescence microscopy (B and D). Scale bar, 10 μm.
FIGURE 8.
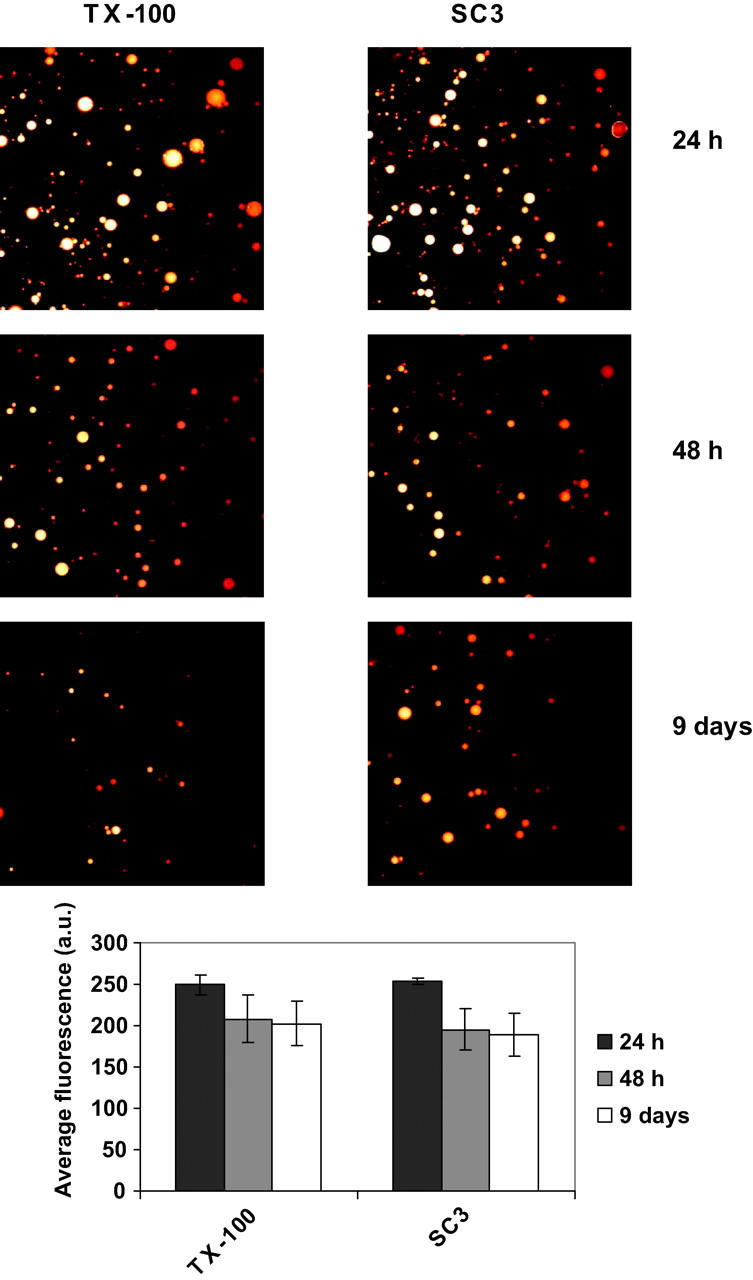
Time-dependent change of pyrene fluorescence in oil droplets stabilized with 0.01% (v/v) Triton X-100 and 100 μg/ml SC3, as detected by confocal laser scanning microscopy. The emulsions were left at room temperature for 1 day (black columns), 2 days (gray columns), and 9 days (white columns). Aliquots of the samples were taken and subjected to the microscopic detection. Error bars indicate the standard deviation of the fluorescence values for various oil droplets in each sample.
FIGURE 9.
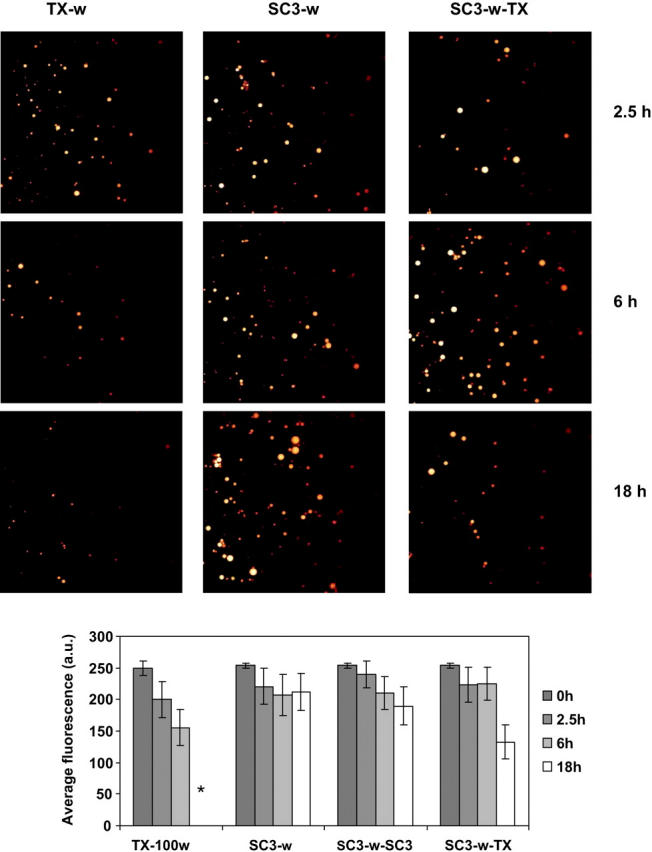
Time-dependent change of pyrene fluorescence in oil droplets as detected by confocal laser scanning microscopy. Oil droplets stabilized with 0.01% (v/v) Triton X-100 or 100 μg/ml SC3 were washed with buffer (TX-w and SC3-w, respectively), followed by supplementation with freshly prepared soluble-state SC3 to 100 μg/ml (SC3-w-SC3) and Triton X-100 to 0.01% (v/v) (SC3-w-TX). Samples were incubated at room temperature for 2.5 h (black columns), 6 h (gray columns), and 18 h (white columns). The asterisk indicates that the fluorescence in the sample TX-100w after 18 h incubation was not determined because the oil droplets were too small to be measured. Error bars indicate the standard deviation of the fluorescence values for various oil droplets in each sample.
Permeability of SC3 membrane to water at an air-water interface
Ellipsometry measurements showed that SC3 forms a monolayer membrane at an air-water interface after several hours of incubation. When protein concentration was high (100 μg/ml), the membrane thickness after 10 min was determined to be 2.3 nm, and it slowly increased to the maximum of ∼3 nm after 5 h incubation. This thickness agrees well with the expected diameter of an SC3 monomer, assuming that the molecule is spherical in shape. When a lower protein concentration (10 μg/ml) was used, the membrane thickness reached ∼1.7 nm in 5 h, and it only grew to 2.1 nm after overnight incubation. This suggests that the membrane formed at low protein concentration is less compact; either a larger space exists between molecules or the orientation of the protein in the membrane is different.
The water permeability of the membrane formed at the air-water interface by overnight incubation of a 100-μg/ml SC3 solution was monitored in both directions. Water transfer over the membrane from the hydrophilic to hydrophobic side was determined by simply following the evaporation of water from a cuvette containing SC3 which had been allowed to form a membrane at the air-water interface. Water transfer from the hydrophobic side was monitored as the exchange of deuterium from D2O outside a cuvette to H2O covered by an SC3 membrane in a cuvette. (see Materials and Methods for details). The time used to monitor the process was much longer than that needed for an SC3 membrane to mature (5 h). For both determinations, over a period of more than 80 h, the sample containing SC3 at 100 μg/ml or 10 μg/ml did not show significant differences from the control sample in the absence of SC3 (Tables 1 and 2), indicating that the SC3 membrane is permeable for water molecules from both the hydrophilic and the hydrophobic side.
TABLE 1.
Water permeability of the hydrophilic side of an SC3 membrane formed at an air-water interface, as detected by the evaporation of water
| Time (h) | 10 μg/ml SC3 (g) | 100 μg/ml SC3 (g) | No SC3 (g) |
|---|---|---|---|
| 3 | 0.025 | 0.024 | 0.026 |
| 8 | 0.066 | 0.064 | 0.068 |
| 22 | 0.191 | 0.186 | 0.194 |
| 96 | 0.789 | 0.772 | 0.804 |
TABLE 2.
Water permeability of the hydrophobic side of SC3 membrane formed at an air-water interface as detected by the exchange of deuterium oxide
| Time (h) | SC3 (S/N*) | No SC3 (S/N) |
|---|---|---|
| 3.5 | 83 | 82 |
| 8 | 200 | 206 |
| 24 | 700 | 740 |
| 72 | 2200 | 2153 |
S/N, signal/noise ratio in NMR.
DISCUSSION
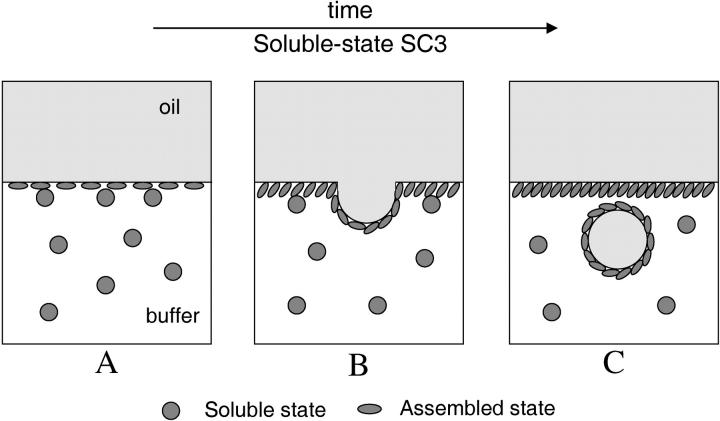
This study shows that SC3 spontaneously assembles into the β-sheet II state at the oil-water interface. When a mixture of dansyl-SC3 (donor) and dabcyl-SC3 (acceptor) was used to coat the oil droplets, the fluorescence of dansyl-labeled SC3 was efficiently quenched, confirming that donor and acceptor were in close proximity, as is typical for β-sheet-state hydrophobin (Wang et al., 2002). If one starts with a membrane composed only of dansyl-SC3, subsequent addition of dabcyl-SC3 quickly quenches the fluorescence of dansyl-SC3, which has assembled at the oil-water interface, indicating that SC3 can incorporate into an already formed membrane. The incorporation is best characterized as a one-way process because no evidence can be found for soluble-state SC3 reappearing in solution once it has been in the membrane. The insertion process has a fast and a slow kinetic regime. The fast regime only appears if the oil droplets are washed and centrifuged several times before adding fresh soluble-state SC3, suggesting that the handling procedure itself is creating easily accessible insertion sites (Fig. 10 A). The slow regime is observed under all circumstances. It reflects the process in which, for further insertion to occur, room must be created by budding off of SC3-coated oil droplets (Fig. 10, B and C). The slow regime is also reported by ThT fluorescence, which increased significantly in time when SC3 was added to a paraffin oil emulsion. Apparently, as long as there is soluble-state SC3 in solution, the incorporation process will continue, and the β-sheet II structure formation in the assembled membrane and vesicles will continue. The increased fluorescence of ThT is in good agreement with a previous study (Wösten et al., 1994a) showing rodlets of SC3 at the oil-water interface after freeze-fracture. However, at that time it could not be excluded that these rodlets were first formed at the air-water interface (due to sonication) and then associated with the oil droplets.
FIGURE 10.
A schematic model of SC3 membrane formed at an oil-water interface. SC3 undergoes a dynamic process of incorporation and association (assembly) into the membrane. As a result, certain parts of the membrane are pressed and protrude, forming vesicles that contain oil or oil solutes. Hydrophobic molecules >200 Da in the oil phase can therefore pass through the membrane and enter the aqueous phase via SC3-assisted emulsification. The whole process will stop when soluble-state SC3 is not present in the aqueous phase. Water molecules, however, can freely permeate the membrane from both sides.
The increased transfer of pyrene from the paraffin oil to the buffer phase beyond the equilibrium value is confirmation of a dynamic SC3-coated oil droplet formation process. Microscopy revealed that the fluorescent molecules were contained in oil droplets that were stabilized by a hydrophobin membrane. Apparently, SC3 in the aqueous phase in combination with slow stirring needed to accelerate the transfer of fluorescent molecules emulsifies oil from the layer on top of the SC3 membrane. This is consistent with a continuous incorporation of SC3 into the membrane, which increases the surface area and, therefore, causes parts of the membrane to protrude into the aqueous phase and further form SC3-coated oil droplets. Confocal microscopy strongly supports this conclusion. When soluble-state SC3 was washed away from the aqueous phase, pyrene transfer from the oil to the aqueous solution was eliminated. The system remained stable for many days, until freshly prepared soluble-state SC3 was added. The presence of detergent in the aqueous phase in this case even facilitated the transfer of pyrene, while the membrane remained intact.
The data presented here indicate that SC3 in the β-sheet II state forms a membrane permeable to water vapor but excluding molecules with a molecular weight >200 Da. Formation of this membrane is protein concentration-dependent. It is completely formed at 100 μg/ml, but only partially formed at 10 μg/ml and not at all at 3 μg/ml. At the lowest concentration, diffusion of marker molecules is not hampered at all, indicating the absence of a membrane. This may be due to the fact that assembly of SC3 depends on a critical concentration of 3.7 μg/ml (de Vocht, 2001). A concentration of SC3 in the aqueous phase of 10 μg/ml should be sufficient for self-assembly. However, at this concentration rodlets could not be observed at the air-water interface by electron microscopy even though a discrete SC3 membrane was formed (de Vocht et al., 2002). This suggests that at such a low concentration SC3 is arrested in the β-sheet I state. The secondary structure of this state cannot be discriminated from that of the β-sheet II state but the ultrastructure is clearly different, i.e., a featureless film versus a rodlet-decorated film (de Vocht et al., 2002). This difference in organization apparently affects the permeability of the protein film. Dextran 3000 seemed to transfer faster from paraffin to buffer than dextran 10,000 in the presence of SC3 membrane (Fig. 5, B and D), but this should not occur if the dextran transfer is independent of membrane permeability, i.e., because of the size exclusion limit. The experiment was repeated several times, and it was found that this trend is not reproducible. In some cases, the two dextrans transferred at a similar rate (data not shown). A likely reason for this discrepancy is that the stirring speed used was somehow different from measurement to measurement, leading to a difference in the formation of SC3-coated oil droplets.
The model of SC3 behavior at an oil-water interface is presented in Fig. 10. SC3 forms a membrane that is permeable to water but excludes molecules >200 Da. During formation there is first movement of soluble-state SC3 to the oil-water interface, then self-association resulting in the β-sheet II state. The driving force for continued insertion once the membrane is formed is the mechanical stirring that results in budding off of SC3-covered vesicles. The oil, as well as the solutes in the oil, will therefore be emulsified by SC3. In the absence of free SC3, the assembled membrane is totally inert without any mass transfer or emulsification ability.
Biological implications
A semipermeable hydrophobin membrane has biological implications. Such membranes covering fungal structures in contact with air (e.g., aerial hyphae, spores, air channels within fruiting bodies) would allow evaporation of water and not protect against desiccation. This is in agreement with preliminary results showing that the rate of dessication of colonies did not increase after deleting the SC3 gene (H. A. B. Wösten, unpublished results). On the other hand, a semipermeable membrane would allow rehydration from a humid atmosphere after a dry period, thus enabling the emergent structure to start up metabolism. Semipermeable hydrophobin membranes would also not impede exchange of metabolic gases. Exchange of such gases is essential for growth of aerial hyphae, fruiting bodies, and lichens. In fact, hydrophobin-coated air channels traverse fruiting bodies and lichens to improve gas exchange (see Wösten, 2001).
A semipermeable hydrophobin membrane would prevent diffusion of small molecules in or out of the cell wall. Such a membrane could be instrumental for infectious propagules (e.g., spores) of pathogenic fungi to evade plant and animal defenses. For instance, it would prevent diffusion of elicitors from the cell wall (Wösten and Wessels, 1997). Moreover, the hydrophobin membrane could shield ligands that are recognized by the immune system of animals. However, swelling of a spore resulting from uptake of water disrupts the hydrophobin membrane. This implies that the hydrophobin membrane would only protect the spore during initial stages of contact. Interestingly, the rodlet layer on conidiospores of Aspergillus fumigatus has indeed been shown to function in early, and not in later, stages of infection, probably by protecting the spores against macrophages and neutrophils (Shibuya et al., 1999; Paris et al., 2003). Disruption of the hydrophobin layer, as occurs upon swelling of spores, could also be beneficial; it would allow uptake of low-molecular-weight nutrients.
A hydrophobin membrane with such unique mass transfer properties might also be intriguing for nanotechnology, for instance, as coatings for drug delivery carriers. The hydrophobin coating would not only miniaturize the oil droplets or the particles of water-insoluble compounds and thus make them soluble in solution, but would also provide a physically stable proteinaceous layer that would allow washing, separation from other components, and probably lyophilization of these particles. In addition, a hydrophobin might improve the bioavailability of drugs (Scholtmeijer et al., 2002).
Acknowledgments
We thank Professor Jos Wessels for discussions about the biological consequences of a semipermeable membrane. We also thank K. Dijkstra and R. M. Scheek for their help with the deuterium diffusion NMR measurement.
References
- Bhattacharyya, J., V. Srinivas, and K. K. Sharma. 2002. Evaluation of hydrophobicity versus chaperonelike activity of bovine αA- and αB-crystallin. J. Protein Chem. 21:65–71. [DOI] [PubMed] [Google Scholar]
- Butko, P., J. P. Buford, J. S. Goodwin, P. A. Stroud, C. L. McCormick, and G. C. Cannon. 2001. Spectroscopic evidence for amyloid-like interfacial self-assembly of hydrophobin SC3. Biochem. Biophys. Res. Commun. 280:212–215. [DOI] [PubMed] [Google Scholar]
- de Vocht, M. L. 2001. Structural changes that accompany the self-assembly of hydrophobins. PhD thesis. University of Groningen, The Netherlands.
- de Vocht, M. L., I. Reviakine, W.-P. Ulrich, W. Bergsma-Schutter, H. A. B. Wösten, H. Vogel, A. Brisson, J. G. H. Wessels, and G. T. Robillard. 2002. Self-assembly of the hydrophobin SC3 proceeds via two structural intermediates. Protein Sci. 11:1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vocht, M. L., I. R. Reviakine, H. A. B. Wösten, A. Brisson, J. G. H. Wessels, and G. T. Robillard. 2000. Structural and functional role of the disulfide bridges in the hydrophobin SC3. J. Biol. Chem. 275:28428–28432. [DOI] [PubMed] [Google Scholar]
- de Vocht, M. L., K. Scholtmeijer, E. W. van der Vegte, O. M. H. de Vries, N. Sonveaux, H. A. B. Wösten, J. M. Ruysschaert, G. Hadziioannou, J. G. H. Wessels, and G. T. Robillard. 1998. Structural characterization of the hydrophobin SC3, as a monomer and after self-assembly at hydrophobic/hydrophilic interfaces. Biophys. J. 74:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, O. M. H., M. P. Fekkes, H. A. B. Wösten, and J. G. H. Wessels. 1993. Insoluble hydrophobin complexes in the walls of Schizophyllum commune and other filamentous fungi. Arch. Microbiol. 159:330–335. [Google Scholar]
- LeVine, H. D. 1993. Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 2:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugones, L. G., J. F. de Jong, O. M. H. de Vries, R. Jalving, J. Dijksterhuis, and H. A. B. Wösten. 2004. The SC15 protein of Schizophyllum commune mediates formation of aerial hyphae and attachment in the absence of the SC3 hydrophobin. Mol. Microbiol. 53:707–716. [DOI] [PubMed] [Google Scholar]
- Mackay, J. P., J. M. Matthews, R. D. Winefield, L. G. Mackay, R. G. Haverkamp, and M. D. Templeton. 2001. The hydrophobin EAS is largely unstructured in solution and functions by forming amyloid-like structures. Structure. 9:83–91. [DOI] [PubMed] [Google Scholar]
- Paris, S., J. P. Debeaupuis, R. Crameri, M. Carey, F. Charles, M. C. Prevost, C. Schmitt, B. Phillipe, and J. P. Latge. 2003. Conidial hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 69:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtmeijer, K., M. I. Janssen, B. Gerssen, M. L. de Vocht, B. M. van Leeuwen, T. G. van Kooten, H. A. B. Wösten, and J. G. H. Wessels. 2002. Surface modification using engineered hydrophobins. Appl. Environ. Microbiol. 68:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, A. C., and B. Chakrabarti. 1990. Proximity of sulfhydryl groups in lens proteins. J. Biol. Chem. 265:14277–14284. [PubMed] [Google Scholar]
- Shibuya, K., M. Takaoka, K. Uchida, M. Wakayama, H. Yamaguchi, K. Takahashi, S. Paris, J. P. Latge, and S. Naoe. 1999. Histopathology of experimental invasive pulmonary aspergillosis in rats: pathological comparison of pulmonary lesions induced by specific virulent factor deficient mutants. Microb. Pathog. 27:123–131. [DOI] [PubMed] [Google Scholar]
- Wang, X., M. L. de Vocht, J. de Jonge, B. Poolman, and G. T. Robillard. 2002. Structural changes and molecular interactions of hydrophobin SC3 in solution and on a hydrophobic surface. Protein Sci. 11:1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., J. F. Graveland-Bikker, C. G. de Kruif, and G. T. Robillard. 2004a. Oligomerization of hydrophobin SC3 in solution: from soluble state to self-assembly. Protein Sci. 13:810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., H. P. Permentier, A. P. Bruins, R. Rink, B. Poolman, and G. T. Robillard. 2004b. Probing the self-assembly on a hydrophobic surface and structural changes of hydrophobin SC3 by mass spectrometry. Biophys. J. 87:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels, J. G. H. 1994. Developmental regulation of fungal cell wall formation. Annu. Rev. Phytopathol. 32:413–437. [Google Scholar]
- Wessels, J. G. H. 1997. Hydrophobins: proteins that change the nature of a fungal surface. Adv. Microb. Physiol. 38:1–45. [DOI] [PubMed] [Google Scholar]
- Wösten, H. A. B. 2001. Hydrophobins: multipurpose proteins. Annu. Rev. Microbiol. 55:625–646. [DOI] [PubMed] [Google Scholar]
- Wösten, H. A. B., and M. L. de Vocht. 2000. Hydrophobins, the fungal coat unraveled. Biochim. Biophys. Acta. 1469:79–86. [DOI] [PubMed] [Google Scholar]
- Wösten, H. A. B., O. M. H. de Vries, H. C. van der Mei, H. J. Busscher, and J. G. H. Wessels. 1994b. Atomic composition of the hydrophobic and hydrophilic sides of self-assembled SC3p hydrophobin. J. Bacteriol. 176:7085–7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H. A. B., O. M. H. de Vries, and J. G. H. Wessels. 1993. Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell. 5:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H. A. B., T. G. Ruardy, H. C. van der Mei, H. J. Busscher, and J. G. H. Wessels. 1995. Interfacial self-assembly of a Schizophyllum commune hydrophobin into an insoluble amphipathic membrane depends on surface hydrophobicity. Colloids Surf. B. 5:189–195. [Google Scholar]
- Wösten, H. A. B., F. H. J. Schuren, and J. G. H. Wessels. 1994a. Interfacial self-assembly of a hydrophobin into an amphipathic membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 13:5848–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H. A. B., and J. G. H. Wessels. 1997. Hydrophobins, from molecular structure to multiple functions in fungal development. Mycoscience. 38:363–374. [Google Scholar]