Abstract
Calsequestrin, the major calcium sequestering protein in the sarcoplasmic reticulum of muscle, forms a quaternary complex with the ryanodine receptor calcium release channel and the intrinsic membrane proteins triadin and junctin. We have investigated the possibility that calsequestrin is a luminal calcium concentration sensor for the ryanodine receptor. We measured the luminal calcium concentration at which calsequestrin dissociates from the ryanodine receptor and the effect of calsequestrin on the response of the ryanodine receptor to changes in luminal calcium. We provide electrophysiological and biochemical evidence that: 1), luminal calcium concentration of ≥4 mM dissociates calsequestrin from junctional face membrane, whereas in the range of 1–3 mM calsequestrin remains attached; 2), the association with calsequestrin inhibits ryanodine receptor activity, but amplifies its response to changes in luminal calcium concentration; and 3), under physiological calcium conditions (1 mM), phosphorylation of calsequestrin does not alter its ability to inhibit native ryanodine receptor activity when the anchoring proteins triadin and junctin are present. These data suggest that the quaternary complex is intact in vivo, and provides further evidence that calsequestrin is involved in the sarcoplasmic reticulum calcium signaling pathway and has a role as a luminal calcium sensor for the ryanodine receptor.
INTRODUCTION
In skeletal muscle, Ca2+ release through the sarcoplasmic reticulum (SR) Ca2+ release channel, the ryanodine receptor (RyR), triggers muscle contraction. The RyR is known to associate with other proteins, such as the FK506-binding protein, DHPR, triadin, junctin, and calsequestrin (CSQ), forming a large macromolecular complex in the junctional SR membrane (Meissner, 2004). CSQ, the major Ca2+ binding protein within the SR lumen is located in the direct vicinity of the RyR1 (Franzini-Armstrong et al., 1987), binds triadin and junctin (Guo and Campbell, 1995; Guo et al., 1994; Jones et al., 1995), and presumably these proteins form a quaternary complex that controls Ca2+ release (Wang et al., 1998; Yano and Zarain-Herzberg, 1994). Both triadin and junctin span the SR membrane and are the only proteins known to associate with both the RyR and CSQ (Brandt et al., 1990; Groh et al., 1999; Guo and Campbell, 1995; Kim and Caswell, 1990; Kobayashi et al., 2000; Zhang et al., 1997), thus providing the physical link between the RyR and CSQ. Although CSQ was thought to have the main role of maintaining Ca2+ homeostasis and providing Ca2+ for release by the RyR, recent reports have demonstrated its regulatory ability on both native RyRs, presumably by binding to coproteins triadin and junctin on the luminal side of the channel complex (Beard et al., 2002; Györke et al., 2004; Wang et al., 2001), and purified RyR channel activity, by binding directly to the luminal portion of the RyR (Szegedi et al., 1999). Therefore, CSQ is ideally placed to act as a sensor for luminal [Ca2+] in the transduction pathway linking RyR activity with store load.
The release of Ca2+ from the SR is strongly dependent on the SR Ca2+ load (Györke et al., 2002; Lamb et al., 2001). This may be in part due to the electrochemical driving force on Ca2+ ions, although this is unlikely to be a major factor because the free [Ca2+] is strongly buffered at ∼1 mM by CSQ (Fryer and Stephenson, 1996). A more important feature is the ability of luminal [Ca2+] to regulate single RyR channel activity (Fill et al., 1990; Herrmann-Frank and Lehmann-Horn, 1996; Ma et al., 1988; Sitsapesan and Williams, 1995; Szegedi et al., 1999; Tripathy and Meissner, 1996), via Ca2+ sensors associated with luminal domains of the RyR (Ching et al., 2000), one of which is likely to be CSQ (Györke and Györke, 1998). However, CSQ can only function in this capacity if it is physically coupled to the RyR at the normal free luminal [Ca2+] of ∼1 mM. CSQ could act as a Ca2+ sensor because many of its properties are Ca2+ dependent. Ca2+ is important in stabilizing both the polymer structure of CSQ and the quaternary complex (Wang et al., 1998), whereas CSQ binding to junctin and triadin is inhibited in the presence of >10 mM Ca2+ (Zhang et al., 1997).
Our recent data suggest that CSQ is associated with the RyR when the free luminal [Ca2+] is 1 mM. Raising luminal Ca2+ from 1 to 13 mM increases RyR activity in two phases (Beard et al., 2002). Firstly, an initial fast phase, which is most likely due to direct Ca2+ activation of the RyR (at cytoplasmic and/or luminal sites). Secondly, a slower phase is likely to be due to CSQ dissociation and hence the removal of CSQs inhibitory effect. These results are in contrast to other studies that suggest that CSQ may be dissociated from triadin and junctin with 1 mM luminal [Ca2+] (Shin et al., 2000). To determine more precisely the luminal [Ca2+] at which CSQ dissociates and whether or not CSQ is associated with the RyR/triadin/junctin (RyR/T/J) complex in working muscle, the effect of varying luminal [Ca2+] between 1 and 5 mM has been examined and is reported here. In addition, we explore the possibility that CSQ communicates changes in luminal [Ca2+] to the RyR. We have also investigated the effects of CSQ phosphorylation on its inhibition of native RyRs (where both triadin and junctin are present) and the interaction with these anchoring proteins, at 1 mM Ca2+. The results show that: a), CSQ remains associated with the junctional face membrane in the presence of 1 and 2 mM Ca2+, but that CSQ is removed from native RyRs when luminal Ca2+ is increased to 4 mM; b), the presence of CSQ amplifies the response of native RyRs to changes in luminal [Ca2+]; c), phosphorylation of CSQ does not influence CSQs ability to inhibit native RyRs at a physiological [Ca2+]; and d), phosphorylation of CSQ does not alter the physical coupling between CSQ and triadin and junctin in an in vivo environment.
MATERIALS AND METHODS
Materials
The polyclonal anti-CSQ antibody was obtained from Swant Chemicals (Bellinzona, Switzerland), whereas both the monoclonal VD111D12 (anti-CSQ) antibody and anti-phosphothreonine antibody was obtained from Bio Scientific (Gymea, Australia). Other chemicals were from Sigma-Aldrich (Castle Hill, Australia).
Methods
SR vesicle preparation
Back and leg muscles were removed from New Zealand white rabbits and SR vesicles prepared using methods of Saito et al. (1984), with minor changes (Ahern et al., 1994).
Junctional face membrane
Junctional face membrane was isolated from either heavy SR (Kim et al., 1983), or SR vesicles as previously described (Costello et al., 1986), with minor changes (Beard, 2003).
Calsequestrin purification from rabbit skeletal muscle
CSQ was purified from RyR-enriched SR, using the methodology reported previously (Costello et al., 1986), except that centrifugation after solubilization was performed at 48,000 × g for 1 h (Professor Cecilia Hidalgo, Instituto de Ciencias Biomedicas, Universidad de Chile, Santiago, Chile, personal communication).
To determine the effect of [Ca2+] on CSQ dissociation from junctional face membrane, the concentration of junctional face membrane was adjusted to 3.3 mg/ml by the addition of 230 mM Cs methanesulfonate (CsMS), 20 mM CsCl, 1 mM CaCl2, 10 mM TES (which corresponds to the solution used in single channel recordings; see below), with 0.5% triton X-100, 0.4 mM benzamidine, and 1 μg/ml leupeptin. After incubation on ice for 30 min and centrifugation at 100,000 × g for 15 min, the solubilized junctional face membrane pellet was suspended at 5 mg/ml, in 230 mM CsMS, 20 mM CsCl, 1 mM CaCl2, 10 mM TES, 0.5% triton X-100, 0.4 mM benzamidine, and 1 μg/ml leupeptin. The final [Ca2+] was adjusted to 1, 2, 3, 4, 5, or 10 mM, by the addition of appropriate aliquots of a 1 M CaCl2 stock (in 10 mM TES), pH 7.4, and after incubation on ice for ∼1 h, the suspension centrifuged as above. The resulting pellets and supernatants were analyzed by electrophoresis and immunoblot.
Expression of rabbit skeletal recombinant CSQ
Rabbit skeletal calsequestrin was subcloned into a pGEX5x1 vector (BamHI at the 5′ end and XhoI at the 3′ end), containing an N-terminal GST tag. Calsequestrin was expressed as GST fusion proteins in Escherichia coli strain BL21DE3 colonies and purified by glutathione Sepharose 4B chromatography. A single colony was grown at 37°C to an optical density at 600 nm of ∼0.5, and expression was induced by 0.5 mM isopropyl B-d-thiogalactoside. Bacteria were pelleted and resuspended, the cell membrane disrupted by lysozyme and French press, centrifuged, and supernatant incubated with glutathione Sepharose 4B beads. After incubation, calsequestrin was cleaved from the GST-glutathione Sepharose 4B complex by incubation with Factor Xa for 4 h at 25°C. CSQ was dialyzed against either 20 mM MOPS, 150 mM KCl, and 1 mM CaCl2 (pH 7.4) or against cis solutions (see Single Channel Recording and Analysis below).
Electrophoresis and immunoblot
SDS-PAGE was performed using the Laemmli buffer system (Laemmli, 1970), with 10% polyacrylamide gels, whereas immunoblot was as per Towbin et al. (1992).
31P-NMR spectroscopy
CSQ phosphorylation status was determined using 31P-NMR spectroscopy. All spectra were acquired on a Varian-Inova 500 spectrometer (Palo Alto, CA), using a spectral width of 15,000 Hz, a pulse width of ∼15 μs, a spectral frequency of 202,421 MHz, and an acquisition time of 0.33 s. Samples were kept at a constant temperature of 5°C. CSQ samples for NMR spectroscopy were prepared at a concentration of ∼0.17 mM in an H2O solution containing 10% D2O/90% H2O. Phosphoric acid was used as an internal standard (0 ppm).
Acid phosphatase and casein kinase II treatment
Dephosphorylation (by acid phosphatase treatment) and phosphorylation (by casein kinase II) of recombinant CSQ (coupled to a GST-glutathione Sepharose matrix) were undertaken as previously described (Cala and Jones, 1991). To remove enzymes, samples were washed with 10 vol of 20 mM MOPS, 1 mM Ca2+, and 150 mM KCl.
GST fusion protein affinity chromatography
To determine whether triadin and junctin can bind to both phosphorylated and dephosphorylated CSQ, GST fusion protein affinity chromatography was undertaken as described by Shin et al. (2000), with the following changes. Solubilized junctional face membrane (in 0.1% triton X-100) was added to both phosphorylated and dephosphorylated glutathione Sepharose 4B bound CSQ-GST, in 20 mM MOPS, 150 mM NaCl, and 1 mM CaCl2.
Single channel recording and analysis
Artificial planar bilayers separating two baths (cis and trans) were formed as previously described (Beard et al., 2002; Laver et al., 1995). SR vesicles (50 μg) were added to the cis solution so that the cytoplasmic surface of the SR and RyRs faced the cis solution after incorporation into the lipid bilayer. For SR vesicle incorporation, the solution compositions were as follows: cis, 230 mM CsMS, 20 mM CsCl, 1 mM CaCl2, and 10 mM TES (pH 7.4); and trans, 30mM CsMS, 20 mM CsCl, 1 mM CaCl2, and 10 mM TES (pH 7.4). After incorporation of a channel, trans [Cs+] was raised from 50 to 250 mM with the addition of 200 mM CsMS and the cis solution was altered by the addition of 4.5 mM BAPTA (free [Ca2+] = 100 nM) and 2 mM ATP. Single channel parameters were obtained using the Channel 2 program (developed by P. W. Gage and M. Smith, John Curtin School of Medical Research, Canberra, Australia). Channel activity was assessed from a 30-s record, either from fractional mean current (I′F, which is the average of all data points obtained during a recording period divided by the maximum single channel current), relative mean current (I′t/I′c, which is the fractional mean current under test conditions, divided by the fractional mean current under control conditions), or open probability (Po). All electrical potentials are expressed here using standard physiological convention (i.e., cytoplasmic side relative to the luminal side at virtual ground). Unless otherwise stated, single channel recordings were obtained using a bilayer potential difference of −/+40 mV. Measurements were carried out at 23 ± 2°C. Channel activity is also expressed as relative Po to include data in which activity varied from ∼0.01 to ∼0.6 and would more accurately reflect the population response in the intact fiber (N = 4–14).
Statistics
Average data are presented as mean ± SE. The significance of differences between control and test values was tested using a Student's t-test for paired data or a sign test (Minium et al., 1993), as appropriate. A p-value of <0.05 was considered to be significant.
RESULTS
Effects of varying luminal Ca2+ concentration between 1 and 5 mM
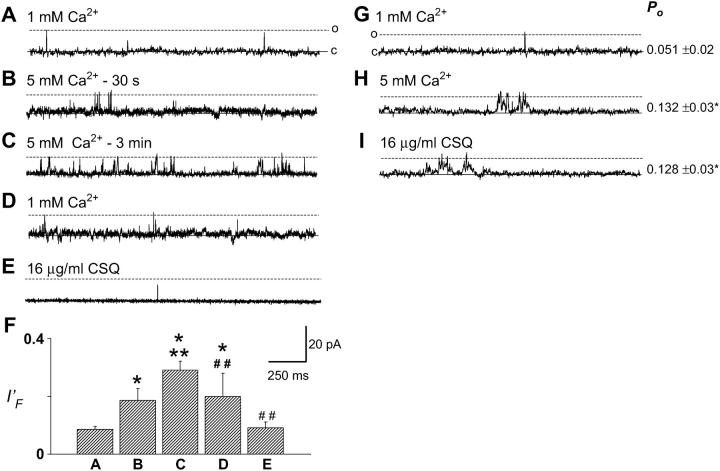
Upon increasing trans Ca2+ from 1 to 5 mM, there was an almost immediate and significant increase in the fractional mean current (I′F) of the channel, from 0.086 (±0.01) to 0.186 (±0.04) in six experiments (Fig. 1, B and F). A secondary rise in channel activity was then observed to always occur within 3 min of exposure to 5 mM trans Ca2+, with I′F increasing to 0.291 (±0.03) (Fig. 1 C). The average dissociation time due to 5 mM Ca2+ (110 ± 17 s) was consistent with the time course of high Cs+ dissociation of CSQ (89 ± 15 s) (Beard et al., 2002). Perfusion of the trans chamber to lower Ca2+ from 5 to 1 mM Ca2+ reduced channel activity somewhat, but not to control levels, despite observing channel activity for up to 12 min. Subsequent addition of a final concentration of 16 μg/ml trans CSQ reduced channel activity to a level that was not significantly different from control (Fig. 1 E). Furthermore, 0.5 μg/ml trans polyclonal anti-CSQ antibody (which inhibits RyR activity only when CSQ is present) (Beard et al., 2002), was added to one channel after CSQ addition and further reduced channel activity (data not shown). These results are consistent with firstly, an initial direct activation of the RyR by luminal Ca2+ when it was increased from 1 to 5 mM, and a further activation when the 5 mM trans Ca2+ dissociated CSQ from the RyR/T/J complex. Secondly, lowering [Ca2+] from 5 to 1 mM reversed the direct activation of the RyR, and addition of 16 μg/ml trans CSQ resulted in a further decline of activity, presumably by allowing reassociation of CSQ with the RyR/T/J complex (possible once trans Ca2+ had been restored to 1 mM). Although data are only shown at +40 mV, similar effects were obtained at −40 mV in all experiments.
FIGURE 1.
Increasing trans Ca2+ from 1 to 5 mM resulted in a biphasic RyR activation. (A–E) Records of 2 s of single channel activity. Single channel opening is upward from zero current (solid line) to maximum open conductance (dashed line). (A) Control, with 1 mM trans Ca2+; (B) 30 s after increasing trans Ca2+ to 5 mM; (C) 3 min after increasing trans Ca2+ to 5 mM; (D) after perfusing the trans chamber with 1 mM Ca2+; (E) addition of 16 μg/ml CSQ; (F) average data (n = 5–6) for fractional mean current (I′F) for conditions shown in panels A–E. (G) Control, with 1 mM trans Ca2+; (H) after perfusing the trans chamber with 5 mM Ca2+, after CSQ dissociation; (I) 5 min after addition of 16 μg/ml CSQ. Average Po (n = 5) is given to the right of panels G–I. Asterisks (*) indicate average values significantly (p < 0.05, t-test) different from control, and double asterisk (**) and double cross-hatch (##) indicate average values significantly different (p < 0.05, t-test and sign test, respectively) from the previous condition. Vm-ECs+ was +40 mV for all records presented in Figs. 1, 2, 4, and 5, except where indicated.
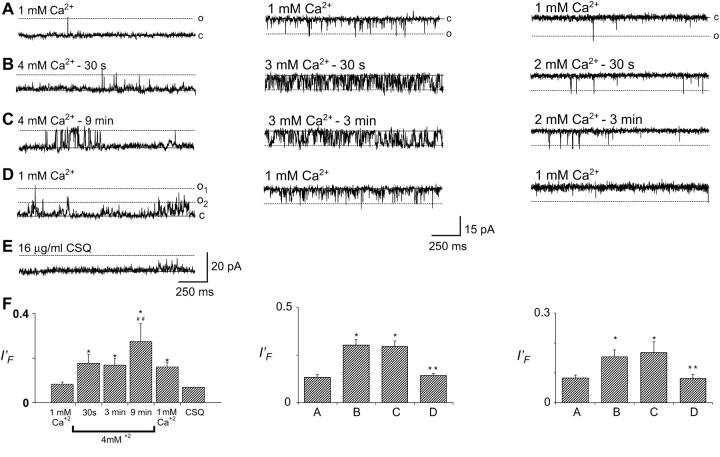
In a second set of experiments CSQ was dissociated from the RyR/T/J complex, after incorporation, by either increasing [Cs+] to 500 mM or increasing trans Ca2+ to 5 mM. A late irreversible increase in activity was seen with CSQ dissociation using both Cs+ and Ca2+ dissociation. There was also an immediate increase in activity with 5 mM Ca2+ (data not shown). The trans chamber was then perfused with solution containing 5 mM Ca2+ to remove dissociated CSQ from the chamber (Fig. 1 H). Subsequent addition of 16 μg/ml exogenous CSQ did not significantly reduce channel activity, despite incubation for 7 min (Fig. 1 I). These data illustrate that 5 mM Ca2+ can both dissociate and prevent reassociation of CSQ to the RyR/T/J complex. CSQ slowly dissociated from channels exposed to 4 mM trans Ca2+. If channels were maintained in 4 mM trans Ca2+, (Fig. 2, left panels), the immediate Ca2+ activation was followed in five out of six channels, by a late activation observed after ∼9 min (where sI′F rose from 0.147 ± 0.05 to 0.285 ± 0.04) (Fig. 2, C and F, left panels). Unlike 5 mM Ca2+ dissociation (Fig. 1), no secondary change in channel activity was observed within 3 min (Fig. 2 F, left panel). Replacement of control trans Ca2+ (1 mM) could only partially remove the activation induced by prolonged exposure to 4 mM Ca2+, and in one out of one channel, 16 μg/ml exogenous CSQ returned channel activity to levels similar to control (Fig. 2, D and E, left panels).
FIGURE 2.
Extended exposure to trans Ca2+ of 4 mM, and not 2 or 3 mM, resulted in a biphasic RyR activation. (A–E) Records of 2 s of single channel activity. Single channel opening is upward from zero current (solid line) to maximum open conductance (dashed line) for the left panels, and downwards from zero current (solid line) to maximum open conductance (dashed line) for the middle and right panels. (A) Control, with 1 mM trans Ca2+; (B) 30 s after increasing trans Ca2+ to 4 mM (left panel), 3 mM (middle panel), and 2 mM (right panel); (C) 3 min (middle and right panels) and 9 min (left panel) after increasing trans Ca2+ to 4, 3, or 2 mM; (D) after perfusing trans chamber with 1 mM Ca2+; (E) addition of 16 μg/ml CSQ; (F) average and individual data (n = 4–8 for A–D and n = 1 for E) for fractional mean current (I′F), at [Ca2+]s listed under the graph (left panel) or for conditions shown in panels A–D (middle, right panels). Asterisks (*) indicate average values significantly (p < 0.05, t-test) different from control, and double asterisk (**) and double cross-hatch (##) indicate average values significantly different (p < 0.05, t-test and sign test, respectively) from the previous condition. Vm-ECs+ was +40 mV for left panel and −40 mV for middle and right panels.
Exposing RyRs to 2 or 3 mM trans Ca2+ resulted in a fast phase of RyR activation, but not the late phase within the time frame of the experiment (Fig. 2, middle and right panels). I′F increased almost immediately from control levels (I′F = 0.1323 ± 0.02 to 0.3017 ± 0.03; Fig. 2 F, middle panel) with no further increase during incubation in 3 mM Ca2+ for up to 18 min (Fig. 2, A–C, middle panel). Similarly, upon exposure to 2 mM trans Ca2+, channel activity rose immediately from control levels (I′F = 0.083 ± 0.01 to 0.153 ± 0.02; Fig. 2 F, right panel). No further rise in activity was observed, despite incubation in 2 mM Ca2+ in one channel for up to 15 min. Reperfusion of the trans chamber with 1 mM Ca2+ restored activity to control levels in both experiments (I′F = 0.081 ± 0.01 and 0.140 ± 0.02 for channels previously exposed to 2 and 3 mM Ca2+, respectively; Fig. 2, D and F). This shows that the activity increase upon raising [Ca2+] to 2 or 3 mM was fully reversible (Fig. 2 D, middle and right panels), and suggested that 2 or 3 mM Ca2+ did not dissociate a significant proportion of CSQ from the RyR/T/J complex within 15–18 min.
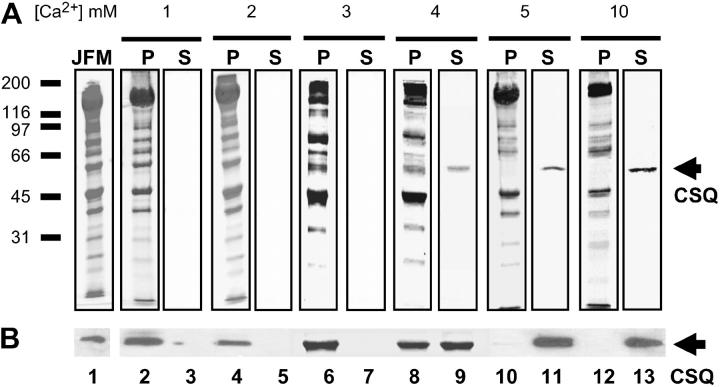
The luminal Ca2+ dependence of CSQ dissociation from junctional face membrane was followed using SDS-PAGE. Comparison of native SR vesicles (used in bilayer experiments) and solubilized junctional face membrane (used in this section) have shown that the relative amounts of RyR, CSQ, triadin, and junctin are the same in both preparations (Beard, 2003). Thus solubilization per se does not dissociate the quaternary complex. Exposure of junctional face membrane to 1 or 2 mM Ca2+ did not detach CSQ (Fig. 3, A and B, lanes 2–5), shown by Coomassie stain (Fig. 3 A), and immunoblot with monocolonal VIIID12 anti-CSQ antibody (Fig. 3 B). There was a significant band at 55 kDa (the apparent molecular weight of CSQ) in the original junctional face membrane (Fig. 3, A and B, lane 1) and in the pellet, but not in the supernatant, from the solubilized fractions after exposure to either 1 or 2 mM Ca2+ [Ca2+] (Fig. 3, A and B, lanes 2–5). In contrast, 4, 5, and 10 mM Ca2+ resulted in significant dissociation of CSQ from the junctional face membrane, indicated by increasing amounts of CSQ in the supernatant (Fig. 3, A and B, lanes 9, 11, and 13), and reduced (Fig. 3, A and B, lane 8) or undetectable amounts in the membrane pellet (Fig. 3, A and B, lanes 10 and 12). CSQ dissociated by 3 mM Ca2+ was undetectable (Fig. 3, A and B, lane 7), with the profile of the JFM and membrane pellet essentially identical (Fig. 3, A and B, lanes 1 and 6). It is unlikely that a small dissociation that might occur with 3 mM Ca2+ would alter the regulatory effect on RyR channels in lipid bilayer experiments (Fig. 2). This dissociation data confirmed our interpretation of the single channel data, i.e., that CSQ was attached to RyRs in the lipid bilayers under control conditions (1 mM Ca2+), that 2 and 3 mM Ca2+ did not induce a CSQ dissociation-dependent RyR activation, and that ≥4 mM Ca2+ can dissociate significant amounts of CSQ from the RyR/T/J complex. From both biochemical and lipid bilayer data, it appears that 4 mM Ca2+ is the minimal Ca2+ concentration required for CSQ dissociation and that dissociation critically depends on a [Ca2+] in the vicinity of 4 mM.
FIGURE 3.
Only [Ca2+] ≥4 mM can dissociate CSQ from the solubilized junctional face membrane. (A) Ten percent SDS polyacrylamide gel showing the original junctional face membrane pellet (JFM), insoluble junctional face membrane pellet (P), and solubilized supernatant (S), after the junctional face membrane was exposed to 1, 2, 3, 4, 5, or 10 mM Ca2+. CSQ was absent in the solubilized sample (S, lanes 3 and 5) after exposure to 1 or 2 mM Ca2+, with the gel profile of the insoluble pellet (P, lanes 2 and 4) being identical to the original junctional face membrane sample (JFM, lane 1). Only trace amounts of CSQ were found in the solubilized sample (S, lane 7) after incubation with 3 mM Ca2+, leaving the profiles of the original junctional face membrane and the insoluble pellet (compare lanes 1 and 6) virtually identical. In contrast, increasing amounts of CSQ were dissociated from the original junctional face membrane sample by exposure to 4, 5, and 10 mM Ca2+ (S, lanes 9, 11, and 13) with significantly reduced (P, lane 8) or undetectable levels of CSQ observed in the insoluble pellet (P, lanes 10 and 12). (B) Immunoblot of protein products shown in panel A, immunoprobed with VIIID12 monoclonal anti-CSQ antibody. Approximate position of the molecular weight markers are indicated to the left of lane 1 in panel A, with CSQ indicated by the arrows. As [Ca2+] was kept at 1 mM throughout the CSQ purification procedure, no additional Ca2+ was added to the 1-mM sample. The following amount of protein was added to the appropriate lanes; 50 μg original junctional face membrane, 40 μg pellet, and 10 μl solubilized supernatant (which equates to 2.7 μg in lane 9, 3.3 μg in lane 11, and 4.7 μg in lane 13). As a protein concentration cannot be determined for the supernatants obtained after 1, 2, and 3 mM Ca2+ extraction, an equivolume (10 μl) of all supernatant fractions was loaded for both the Coomassie stained gel and the immunoblot.
Changes in RyR activity caused by varying luminal Ca2+ between 1 and 5 mM
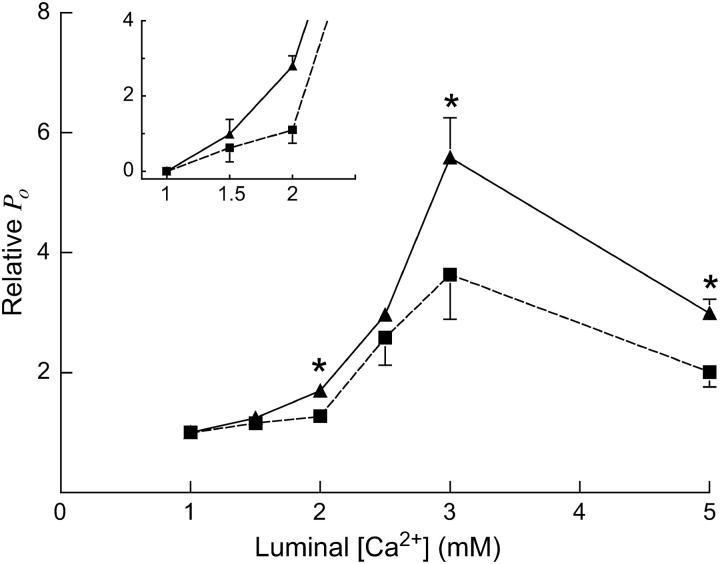
Native RyRs were incorporated into bilayers in the presence of 1 mM trans Ca2+, [Ca2+] was then increased to 1.5, 2, 2.5, 3, and 5 mM in a stepwise fashion (Fig. 4), and channel activity was followed at both positive and negative potentials.
FIGURE 4.
RyR responses to changes in luminal [Ca2+] between 1 and 5 mM, in the presence and absence of CSQ. Experimental conditions: cis (mM) 250 Cs+, 2 ATP (activating), and 1 nM Ca2+(subactivating); trans (mM) 250 Cs+, 1–5 Ca2+. Trans [Ca2+] was altered by aliquot additions of 200 mM stock Ca2+ ([Ca2+] determined by a Ca2+ electrode). CSQ was dissociated from RyRs before increasing [Ca2+] by exposure to 500 mM Cs+, or to high [Ca2+] (in the case of the data obtained at 5 mM Ca2+). Each data point is the mean relative open probability (relative Po), which is test Po (2–5 mM Ca2+) relative to control Po (1 mM), for RyRs in the absence (CSQ(−); ▪) and presence (CSQ(+); ▴) of CSQ. The bars are means ± SE for n = 4–14. Absolute mean Po values for control activities are listed in the Results section. The inset chart shows relative Po changes at luminal Ca2+ from 1 to 2.0 mM Ca2+ in more detail. Asterisks (*) indicate average values significantly different from control (p > 0.05, t-test).
For CSQ-attached (CSQ(+)) RyRs, currents were analyzed within 1 min of exposure to the increased [Ca2+] to avoid any effects of CSQ dissociation. However, in agreement with results presented above (Fig. 2), channels exposed to 1.5–3 mM Ca2+ did not show a secondary increase in activity under these conditions for up to 18 min, again indicating that 1–3 mM luminal Ca2+ did not dissociate CSQ during this period. For experiments examining increases in luminal Ca2+ to 1.5–3 mM, CSQ-depleted (CSQ (−)) channels were obtained increasing trans ionic strength before trans [Ca2+] was raised. The increase in ionic strength effectively dissociates CSQ from the RyR (Beard et al., 2002). The trans Cs+ was increased from 250 to 500 mM and maintained at that concentration until an increase in channel activity was observed (89 ± 15 s), indicating CSQ dissociation (Beard et al., 2002). Once activity increased and remained stable for 1 min, trans [Cs+] was restored to 250 mM by perfusion. It should be noted that returning to 250 mM Cs+ (control conditions) did not restore control RyR activity, as expected if CSQ had been dissociated from the RyR/T/J complex. For experiments with 5 mM Ca2+, the high trans Ca2+ itself was used to dissociate CSQ, and activity after the secondary increase (analyzed 3 min after exposure to 5 mM Ca2+), was used as the measure of the response of channel activity in the absence of CSQ. Channel activity is reported as relative Po, i.e., Po under test conditions (2–5 mM) relative to Po under control (1 mM Ca2+) conditions. Removal of CSQ made RyRs less responsive to increasing trans [Ca2+] at each [Ca2+] tested (Fig. 4), with significant differences in relative Po seen at 2, 3, and 5 mM trans Ca2+. It should be noted that although absolute channel Po under control conditions was higher for CSQ(−) RyRs than for CSQ(+) RyRs (0.14 ± 0.02 and 0.09 ± 0.01, respectively), due to the removal of CSQs inhibiting effect, CSQ(+) channels were more responsive to changes in [Ca2+] from 1 mM than CSQ(−) RyRs (Fig. 4). The apparently smaller increase in channel activity at 5 mM Ca2+ compared with 3 mM Ca2+in both CSQ(+) and CSQ(−) channels was not explored further, but is indicative of a small decline in activation seen at higher Ca2+ concentrations and is perhaps due to a separate inhibitory mechanism (Ching et al., 2000; Tripathy and Meissner, 1996). Together, the data clearly demonstrate that CSQ amplified the activating effect of luminal [Ca2+] between 1 and 5 mM.
Determination of CSQs phosphorylation status
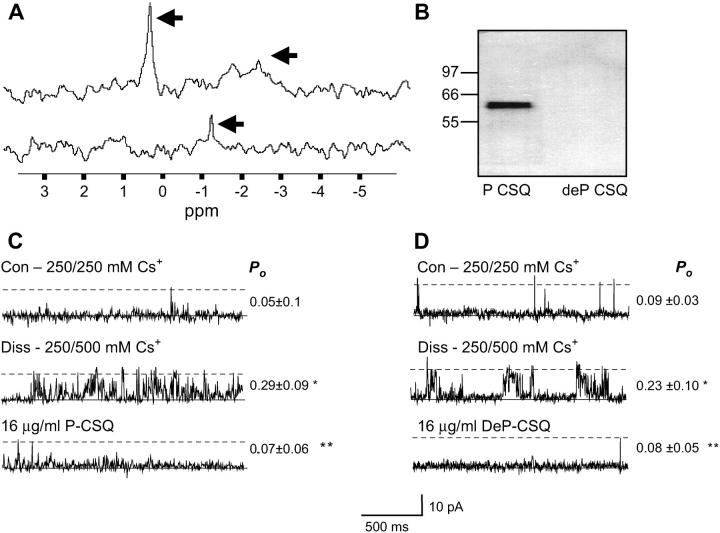
The phosphorylation status of the isolated CSQ was determined by both 31P-NMR analysis and immunoblot with anti-phosphothreonine. The 31P-NMR analysis indicated that CSQ from rabbit skeletal muscle was phosphorylated (Fig. 5 A, top trace). In the spectra, the horizontal axis corresponds to a chemical shift (ppm), and reflects the environment experienced by the phosphorous atoms. The original CSQ sample (top trace) displays a resonance peak at ∼0.2 ppm, suggesting that CSQ is at least in part phosphorylated. CSQ, therefore, is probably phosphorylated in vivo as it is unlikely that CSQ underwent autophosphorylation during the isolation procedure as ATP was absent from all isolation media.
FIGURE 5.
Phosphorylation status of CSQ in skeletal muscle; both exogenous phosphorylated and dephosphorylated CSQ inhibit CSQ(−) RyRs. (A) 31P-NMR spectrum. The resonances for phosphorylated (P) CSQ, from the original CSQ sample are indicated with arrows in the top trace, whereas residual unbound phosphorous in the dephosphorylated (deP) CSQ, is indicated with an arrow in the bottom trace; (B) Immunoblot of 20 μg P-CSQ and deP-CSQ, immunoprobed with polyclonal antibody anti-phosphothreonine, with the approximate position of molecular weight markers indicated next to the immunoblot; (C and D) changes in CSQ(−) RyR activity after addition of exogenous P-CSQ (C) and deP CSQ (D). In panels C and D, records of 2 s of single channel activity where channel opening is upward from zero current (solid line) to maximum open conductance (dashed line). The top traces show control (Con) activity with 250 mM trans Cs+; the middle traces shows activity after increasing trans Cs+ to 500 mM with CsMS (Diss); the bottom trace shows addition of 16 μg/ml of P-CSQ (C) or DeP-CSQ (D), after trans perfusion with 250 mM Cs+. Average channel Po values (with means ± SE) are given to the right of traces in panel C (n = 6) and panel D (n = 5). Asterisks (*) indicate average values significantly different from control and double asterisks (**) indicate values different from the previous condition (p < 0.05, t-test).
The appearance of a broad resonance peak, at between −1.5 and −2.8 ppm (arrow, top trace) in the phosphorylated sample, suggests that the phosphorous atoms may reside in an aggregated form of CSQ. Indeed, protein precipitation was observed after NMR analysis, suggesting that some CSQ aggregated during the 24-h testing period. This NMR data show clearly that CSQ was phosphorylated but does not allow determination of the number of phosphorylated residues.
After dephosphorylation by acid phosphatase treatment and subsequent dialysis to remove free phosphorous, the peaks observed in the phosphorylated sample of CSQ at ∼0.2 and between −1.5 and −2.8 ppm (Fig. 5 A, arrows, top trace) disappeared. The appearance of a small peak near −1.2 ppm (arrow, bottom trace), may be explained by residual (free) phosphorus atoms in the sample, most probably due to incomplete dialysis of the CSQ sample after dephosphorylation. Nevertheless, the dephosphorylated CSQ sample contained at least 10 times less phosphorus than the phosphorylated CSQ sample (indicated by the area under each spectrum).
As Thr353 is phosphorylated in rabbit skeletal CSQ, (Cala and Jones, 1991) both the original and acid phosphatase-treated CSQ samples were probed with a polyclonal anti-phosphothreonine antibody. Only the native CSQ and not the dephosphorylated CSQ could be detected by this antibody (Fig. 5 B). This observation confirms the NMR data showing that isolated CSQ is in a phosphorylated form, and can be significantly dephosphorylated by acid phosphatase treatment.
Native RyR regulation by phosphorylated and dephosphorylated CSQ
The actions of both phosphorylated and dephosphorylated CSQ on native skeletal RyRs were examined under physiological Ca2+ conditions (1 mM trans Ca2+). RyRs were exposed to 500 mM trans Cs+ to dissociate endogenous CSQ, and this procedure resulted in a rise in Po at positive potentials (Fig. 5, C and D, middle panel). After subsequent perfusion of the trans chamber with 250 mM Cs+, 16 μg/ml of phosphorylated CSQ was added (Fig. 5 C, bottom panel). As seen previously (Figs. 1 and 2), phosphorylated CSQ significantly reduced channel Po (Fig. 5 C). Similarly, reassociation of 16 μg/ml of dephosphorylated CSQ also reduced channel activity to control (predissociation) levels (Fig. 5 D). Similar inhibitory effects of phosphorylated and dephosphorylated CSQ were seen at −40 mV. To ascertain whether the addition of exogenous CSQ buffered [Ca2+]free (and lower it significantly from 1 mM), the [Ca2+] free was measured in the absence and presence of CSQ using a Ca2+ electrode. No significant difference in [Ca2+]free was detected.
The phosphorylation status of CSQ did not influence its ability to inhibit native RyRs at 1 mM trans Ca2+. Phosphorylated CSQ decreased I′F relative to postdissociation activity to 0.51 ± 0.10 (N = 16), whereas dephosphorylated CSQ decreased relative I′F to 0.43 ± 0.15 (N = 8). Apparently, altering the phosphorylation status of exogenous CSQ did not alter its functional interaction with the native RyR/T/J complex, or the degree of inhibition it imposed.
CSQ interactions with triadin and junction
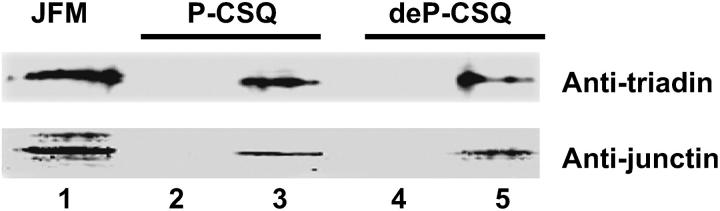
Determination of whether phosphorylation of CSQ altered its binding to triadin and junctin was carried out using GST fusion protein affinity chromatography. GST-tagged CSQ was bound to glutathione Sepharose 4B matrix, and was experimentally phosphorylated or dephosphorylated (see Methods) in situ. Only the phosphorylated CSQ sample was detectably phosphorylated, as determined by anti-phosphothreonine immunoblot (see Methods; data not shown). Solubilized junctional face membrane (in 0.1% triton X-100) was applied to either phosphorylated or dephosphorylated GST-tagged CSQ under near physiological conditions (1 mM CaCl2, 150 mM NaCl), and after a 2-h incubation, unbound proteins were washed and separated from the protein-bound CSQ-GST-glutathione Sepharose complex. This complex was subsequently collected by centrifugation. Junctional face membrane proteins that interacted with GST-tagged CSQ were separated by SDS-PAGE and immunoprobed with anti-triadin and anti-junctin after Western blot (Fig. 6). The results indicated that CSQ will still form a complex with triadin and junctin regardless of its phosphorylation status, as both triadin and junctin interacted with both phosphorylated and dephosphorylated CSQ (Fig. 6).
FIGURE 6.
Interactions of triadin and junctin with CSQ are independent of phosphorylation status. Western blot, after 10% SDS-PAGE, of the GST-CSQ coupled to glutathione Sepharose 4B before (lanes 2 and 4) and after (lanes 3 and 5) exposure to solubilized junctional face membrane (JFM; lane 1). Blot was immunoprobed with anti-triadin and anti-junctin. Both phosphorylated (P-CSQ; lanes 2 and 3) and dephosphorylated (deP-CSQ; lanes 4 and 5) CSQ could bind triadin and junctin (lanes 3 and 5) under close to physiological conditions (1 mM Ca2+, 150 mM NaCl).
DISCUSSION
Novel observations on the Ca2+ dependence of the association between CSQ and the RyR, and the effect of CSQ on the luminal Ca2+ dependence of RyR activity, are described. Using two different techniques, we show that CSQ is associated with the RyR when the luminal [Ca2+] is maintained at the physiological level of ∼1 mM. Furthermore, CSQ remains associated with the RyR/T/J complex when luminal Ca2+ is increased from 1 to 3 mM, but dissociates at 4 and 5 mM (and cannot reassociate at 5 mM Ca2+). In addition, this is the first report of the effects of high luminal [Ca2+] on skeletal RyR activity under conditions in which CSQ would have remained associated with the RyR/T/J complex after RyRs were incorporated into bilayers. We show that under these conditions, channel activity increases as luminal Ca2+ is increased and that the response of the RyR to small increases in luminal [Ca2+] from 1 to 5 mM is amplified by CSQ. Finally, we show that CSQ in native SR is phosphorylated and that both a physical and functional coupling of CSQ with native RyR channels and with triadin and junctin is independent of CSQ phosphorylation.
Is CSQ associated with the RyR/T/J complex under physiological conditions?
If CSQ plays an active role in communicating luminal [Ca2+] to the RyR in vivo, it must be physically coupled either directly or indirectly to the RyR under normal physiological conditions. Using both biochemical (Fig. 3) and electrophysiological techniques (Figs. 1 and 2), we show that CSQ is associated with the junctional face membrane when the luminal [Ca2+] is ∼1 mM, i.e., the free [Ca2+] that is considered to exist in the lumen of the SR in vivo (Fryer and Stephenson, 1996). In addition, electron micrographs of terminal cisternae, and electron tomography of frozen hydrated triad junctions from skeletal muscle (fixed under physiological conditions), show CSQ located close to the RyR, suggesting that under physiological conditions, CSQ is associated with the RyR (Franzini-Armstrong, 1973; Wagenknecht et al., 2002). This is not unexpected because CSQ association with triadin and junctin in skeletal muscle requires Ca2+ (Guo and Campbell, 1995; Wang et al., 1998). However, other conflicting evidence suggests that binding of solubilized skeletal SR to a GST-CSQ fusion protein is gradually reduced as Ca2+ increased from 0 to 5 mM, with maximal binding occurring at 0 mM Ca2+, leading to the conclusion that CSQ may not associate with the skeletal RyR/T/J complex in the presence of physiological (1 mM) Ca2+ (Shin et al., 2000). It should be noted that data presented by Shin et al. (2000) show significant CSQ association with the solubilized SR at 1 mM Ca2+. In the light of our data (Figs. 1–3 and 6) and those of Guo and Campbell (1995) and Costello et al. (1986), skeletal CSQ association with triadin and junctin under physiological [Ca2+] seems to be likely.
How does high Ca2+ dissociate CSQ from the RyR/T/J complex?
It is likely that the ability of CSQ to associate with and dissociate from triadin and junctin at different [Ca2+], depends on the structure of the CSQ protein, which is strongly Ca2+ dependent. It is not clear how high [Ca2+] dissociates CSQ from the RyR/T/J complex. At low [Ca2+], CSQ assumes a mostly random coil structure, with α-helical content increasing as Ca2+ binds (Ikemoto et al., 1972, 1974; Ostwald et al., 1974). To form dimers and polymers, both skeletal and cardiac CSQ require Ca2+, presumably above 10 μM. High [Ca2+] (≥5 mM) dissociates CSQ from the junctional face membrane (this study) and has been successfully used to selectively elute recombinant CSQ from phenyl-Sepharose affinity matrix (Cala and Jones, 1983). Recent studies with the cardiac RyR show that exposure of the reconstituted quaternary complex to 5 mM luminal Ca2+ resulted in channel activation due to CSQ dissociation (Györke et al., 2004). Additionally, increasing [Ca2+] to ≥5 mM decreases the stokes radius and increases the apparent compaction of CSQ (Cozens and Reithmeier, 1984), and results in CSQ aggregation (N. A. Beard and A. F. Dulhunty, unpublished data; Park et al., 2003). As the [Ca2+] rises, hydrophobic side chains are buried within the polymer, reducing the ability of CSQ to bind to other proteins (Mitchell et al., 1988). This is suggestive of a somewhat “supercompacted” CSQ within the polymer (Beard et al., 2004). Supercompaction of CSQ upon exposure to increasing luminal [Ca2+] may disrupt the interactions with triadin and junctin (and indeed the RyR), allowing selective dissociation of CSQ.
Role for CSQ in regulating RyRs at 1–5 mM luminal Ca2+
Ikemoto et al. (1972) and Ostwald et al. (1974) reported changes in CSQ conformation occurring within the range of [Ca2+] tested here. Alterations in protein conformation with luminal [Ca2+] changes between 0.1 and 3 mM have been postulated to increase SR Ca2+ release rate constants (Donoso et al., 1995). In this study, the channel response to varying [Ca2+] between 1 and 5 mM was amplified in the presence of CSQ. The change in activity observed upon exposure to 2 mM Ca2+ cannot be attributed to an effect of CSQ dissociation, as exposure of junctional face membrane to 2 mM Ca2+ does not dissociate CSQ (Fig. 3). It is possible that regulation of RyRs by luminal Ca2+ is partly due to Ca2+-dependent changes in CSQ conformation that do not result in CSQ dissociation (He et al., 1993; Ikemoto et al., 1989).
In cardiac muscle, increasing luminal [Ca2+] induced an increase in activity in native, but not purified RyRs (Györke et al., 2004). Only restoration of the quaternary complex, by adding exogenous triadin, junctin, and CSQ (but not by adding triadin and junctin alone), could restore RyR responsiveness to luminal Ca2+ (Györke et al., 2004). Although in the absence of CSQ, increasing luminal [Ca2+] resulted in RyR activation, we show that in skeletal muscle, RyR responsiveness to increased luminal [Ca2+] was substantially augmented by the presence of CSQ. Both studies (Fig. 4; Györke et al., 2004) provide evidence for the role of CSQ (as part of an intact quaternary complex) as a luminal Ca2+ sensor.
CSQ activation versus CSQ inhibition of RyRs
The reported effects of CSQ on RyRs vary between laboratories and preparations, as seen with the response of the RyR to phosphorylated and dephosphorylated CSQ. With physiological luminal [Ca2+], the ability of CSQ to inhibit native skeletal RyRs was independent of phosphorylation (Fig. 5). In contrast, Szegedi et al. (1999) showed that CSQ activates purified skeletal RyRs only when it is dephosphorylated and we have confirmed these findings in our laboratory (N. A. Beard, unpublished data). Thus, the differences in RyR regulation observed in these two studies can be explained by the different preparations used; the 3-[(3-Cholamidopropyl)Dimethyl-Ammonio]-1-Propanesulfonate solubilized and purified RyR (with presumably no significant amounts of accessory proteins triadin and junctin present) in Szegedi's study (and our observations), and the native RyR, with triadin and junctin present (in this study). Although we cannot discount that another unknown accessory protein found within the SR lumen may be responsible for anchoring CSQ to the RyR in the native preparation, the only two proteins known to bind both CSQ and the RyR in the lumen are triadin and junctin.
In addition, the reported differences in CSQ regulation of RyRs support the hypothesis that CSQ modulates RyR activity via two mechanisms; firstly, by inducing RyR inhibition through interactions with triadin and junctin in a phosphorylation-independent manner (this study), and secondly, by binding directly to the RyR (Herzog et al., 2000) and activating the channel in a phosphorylation-dependent manner, as shown by Szegedi et al. (1999). Whether or not both mechanisms of CSQ regulation operate in native RyRs in vivo is unknown. If so, the reported CSQ activation induced by a direct RyR-CSQ interaction is overshadowed by the triadin/junctin mediated CSQ inhibition, seen as an overall inhibition of the channel.
Phosphorylation as an in vivo modulator of CSQ regulation of RyRs?
Regulation of RyRs by cyclic phosphorylation/dephosphorylation of CSQ in vivo depends on whether CSQ is phosphorylated before luminal segregation, or after targeting to the lumen. This, in turn, depends on the location of specific kinases responsible for CSQ phosphorylation, and whether or not ATP could be transported into the lumen of the SR. It is not known which kinase specifically phosphorylates CSQ in vivo, or whether such kinases are present in the SR lumen (or indeed whether CSQ is phosphorylated inside the lumen). Casein kinase II phosphorylates Thr353 in skeletal CSQ (Cala and Jones, 1991). To date, the presence of casein kinase II within the SR lumen has been inferred, but not proven (Shoshan-Barmatz et al., 1996). Thr353 or another phosphorylatable residue may also be phosphorylated by other kinases, which might be present in the lumen. CSQ has also been identified as a potentially good substrate for casein kinase I and ɛ protein kinase C, but current evidence suggests that these kinases reside only in the cytoplasm and not within the SR lumen (Rodriguez et al., 1999; Salvatori et al., 1994). CSQ is phosphorylated when isolated from muscle homogenates (Fig. 5 B) and this observation shows that CSQ in the SR lumen is phosphorylated. Cala and Jones (1991) found that rabbit skeletal CSQ was not isolated in its phosphorylated form; Campbell and Shamoo (1980) show that CSQ can be phosphorylated in skeletal SR muscle preparations, whereas Varsanyi and Heilmeyer (1980) report that CSQ is capable of autophosphorylation and the isolated calsequestrin from skeletal muscle can be obtained in fully or partially phosphorylated form (Varsanyi and Heilmeyer, 1979). Recently, O'Brian et al. (2002) reported that presumably phosphorylation and glycosylation processes are involved in both common and distinct cellular compartmentation of the calsequestrin isoforms.
Physiological implications
Taken together, these results show that luminal Ca2+ has two actions on RyR channels. Firstly, Ca2+ can bind to activation sites, found on the RyR or an associated protein (when luminal Ca2+ is raised from 1 to ≥4 mM). Secondly, the increase in luminal [Ca2+] can consistently dissociate CSQ, inducing a further significant rise in RyR activity, and 5 mM luminal Ca2+ can prevent reassociation of CSQ with the RyR/T/J complex. It is not likely that the dissociation of CSQ from the junctional face membrane would be caused by normal physiological changes in luminal [Ca2+], and therefore CSQ dissociation-induced changes in RyR activity may not be of physiological importance. However, levels of luminal Ca2+ of ∼10 mM are obtained experimentally when loading the SR to above-normal levels (Lamb et al., 2001), and such loading leads to enhanced Ca2+ release. The contribution of CSQ dissociation from the junctional face membrane in the SR to this increase in activity remains to be investigated.
Although not investigated here, CSQ might influence RyR function in response to changes in total luminal [Ca2+] in a similar manner to calmodulin (associated with cytoplasmic [Ca2+]) (Meissner 1986; Plank et al., 1983; Rodney et al., 2000; Tripathy et al., 1995; Xu and Meissner, 2004). Like calmodulin, the effect of CSQ on RyR function may depend on the Ca2+ binding status of CSQ (and CSQ conformation), which is likely to vary with changes in total luminal [Ca2+] (i.e., store loading). Changes in the amount of Ca2+ bound to CSQ could occur with only minor changes in the free [Ca2+] and might alter CSQ conformation without dissociation, thus causing subtle changes in RyR activity or its response to cytoplasmic ligands such as Ca2+. Indeed, CSQ has been implicated as a major luminal Ca2+ sensor, whose presence is required (along with triadin and junctin), to enhance RyR responsiveness to changes in luminal Ca2+ concentration (this article; Györke et al., 2004).
In conclusion, we find that CSQ is associated with the RyR/T/J complex when free luminal Ca2+ is at a physiological concentration of ∼1 mM, and we provide novel data showing that CSQ dissociates from the skeletal RyR complex when the free [Ca2+] increases to between 3 and 5 mM. The results show that CSQ sensitizes the RyR to changes in free [Ca2+] between 1 and 5 mM. Finally, we show that although CSQ is most likely phosphorylated in vivo, its ability to inhibit RyR activity does not depend on its phosphorylation status. These data provide mounting evidence that an intact quaternary complex between CSQ, triadin, junction, and the RyR forms a signaling pathway that communicates the luminal [Ca2+] to the RyR channel.
Acknowledgments
We acknowledge the kind donation of anti-junctin from Dr. Steven Cala and anti-triadin from Professor Kevin P. Campbell. We thank Suzy Pace and Joan Stivala for help with SR vesicles preparation and Stephanie Cheung for help with expressing recombinant CSQ. We also thank Rolf Thieleczek for helpful comments on the manuscript. Rabbit skeletal muscle calsequestrin cDNA clone were constructed by Oliver Fiebig (Cand.med.). Cloning, gene expression, and purification of the recombinant CSQ were part of his medical doctor thesis.
N.A.B. was supported by the Australian Research Council of Australia (project ID DP0344878), L.W. was supported by an Australian National University PhD Scholarship, and D.R.L. was supported by the National Health & Medical Research Council of Australia (grant No. 234420) and a Professorial Fellowship from the Australian Research Council.
References
- Ahern, G. P., P. R. Junankar, and A. F. Dulhunty. 1994. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 352:369–374. [DOI] [PubMed] [Google Scholar]
- Beard, N. A. 2003. Regulation of the skeletal muscle ryanodine receptor by calsequestrin. PhD thesis. Australian National University, Canberra, Australia.
- Beard, N. A., D. R. Laver, and A. F. Dulhunty. 2004. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog. Biophys. Mol. Biol. 155:33–69. [DOI] [PubMed] [Google Scholar]
- Beard, N. A., M. M. Sakowska, A. F. Dulhunty, and D. R. Laver. 2002. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 82:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, N. R., A. H. Caswell, S. R. Wen, and J. A. Talvenheimo. 1990. Molecular interactions of the junctional foot protein and dihydropyridine receptor in skeletal muscle triads. J. Membr. Biol. 113:237–251. [DOI] [PubMed] [Google Scholar]
- Cala, S. E., and L. R. Jones. 1983. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J. Biol. Chem. 258:11932–11936. [PubMed] [Google Scholar]
- Cala, S. E., and L. R. Jones. 1991. Phosphorylation of cardiac and skeletal muscle calsequestrin isoforms by casein kinase II. Demonstration of a cluster of unique rapidly phosphorylated sites in cardiac calsequestrin. J. Biol. Chem. 266:391–398. [PubMed] [Google Scholar]
- Campbell, K. P., and A. E. Shamoo. 1980. Phosphorylation of heavy sarcoplasmic reticulum vesicles: identification and characterization of three phosphorylated proteins. J. Membr. Biol. 56:241–248. [DOI] [PubMed] [Google Scholar]
- Ching, L. L., A. J. Williams, and R. Sitsapesan. 2000. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 87:201–206. [DOI] [PubMed] [Google Scholar]
- Costello, B., C. Chadwick, A. Saito, A. Chu, A. Maurer, and S. Fleischer. 1986. Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J. Cell Biol. 103:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens, B., and R. A. Reithmeier. 1984. Size and shape of rabbit skeletal muscle calsequestrin. J. Biol. Chem. 259:6248–6252. [PubMed] [Google Scholar]
- Donoso, P., H. Prieto, and C. Hidalgo. 1995. Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys. J. 68:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill, M., R. Coronado, J. R. Mickelson, J. Vilven, J. J. Ma, B. A. Jacobson, and C. F. Louis. 1990. Abnormal ryanodine receptor channels in malignant hyperthermia. Biophys. J. 57:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong, C. 1973. Studies of the triad. IV. Structure of the junction in frog slow fibers. J. Cell Biol. 56:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., L. J. Kenney, and E. Varriano-Marston. 1987. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell Biol. 105:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer, M. W., and D. G. Stephenson. 1996. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J. Physiol. 493:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh, S., I. Marty, M. Ottolia, G. Prestipino, A. Chapel, M. Villaz, and M. Ronjat. 1999. Functional interaction of the cytoplasmic domain of triadin with the skeletal ryanodine receptor. J. Biol. Chem. 274:12278–12283. [DOI] [PubMed] [Google Scholar]
- Guo, W., and K. P. Campbell. 1995. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 270:9027–9030. [DOI] [PubMed] [Google Scholar]
- Guo, W., A. O. Jorgensen, and K. P. Campbell. 1994. Characterization and ultrastructural localization of a novel 90-kDa protein unique to skeletal muscle junctional sarcoplasmic reticulum. J. Biol. Chem. 269:28359–28365. [PubMed] [Google Scholar]
- Györke, I., and S. Györke. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke, S., I. Györke, V. Lukyanenko, D. Terentyev, S. Viatchenko-Karpinski, and T. F. Wiesner. 2002. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front. Biosci. 7:d1454–d1463. [DOI] [PubMed] [Google Scholar]
- Györke, I., N. A. Hester, L. R. Jones, and S. Györke. 2004. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z., A. K. Dunker, C. R. Wesson, and W. R. Trumble. 1993. Ca2+-induced folding and aggregation of skeletal muscle sarcoplasmic reticulum calsequestrin. The involvement of the trifluoperazine-binding site. J. Biol. Chem. 268:24635–24641. [PubMed] [Google Scholar]
- Herrmann-Frank, A., and F. Lehmann-Horn. 1996. Regulation of the purified Ca2+ release channel/ryanodine receptor complex of skeletal muscle sarcoplasmic reticulum by luminal calcium. Pflugers Arch. 432:155–157. [DOI] [PubMed] [Google Scholar]
- Herzog, A., C. Szegedi, I. Jona, F. W. Herberg, and M. Varsanyi. 2000. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca2+ release channel/ryanodine receptor with calsequestrin. FEBS Lett. 472:73–77. [DOI] [PubMed] [Google Scholar]
- Ikemoto, N., G. M. Bhatnagar, B. Nagy, and J. Gergely. 1972. Interaction of divalent cations with the 55,000-dalton protein component of the sarcoplasmic reticulum. Studies of fluorescence and circular dichroism. J. Biol. Chem. 247:7835–7837. [PubMed] [Google Scholar]
- Ikemoto, N., B. Nagy, G. M. Bhatnagar, and J. Gergely. 1974. Studies on a metal-binding protein of the sarcoplasmic reticulum. J. Biol. Chem. 249:2357–2365. [PubMed] [Google Scholar]
- Ikemoto, N., M. Ronjat, L. G. Meszaros, and M. Koshita. 1989. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 28:6764–6771. [DOI] [PubMed] [Google Scholar]
- Jones, L. R., L. Zhang, K. Sanborn, A. O. Jorgensen, and J. Kelley. 1995. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J. Biol. Chem. 270:30787–30796. [DOI] [PubMed] [Google Scholar]
- Kim, K. C., and A. H. Caswell. 1990. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry. 29:9281–9289. [DOI] [PubMed] [Google Scholar]
- Kim, D. H., S. T. Ohnishi, and N. Ikemoto. 1983. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J. Biol. Chem. 258:9662–9668. [PubMed] [Google Scholar]
- Kobayashi, Y. M., B. A. Alseikhan, and L. R. Jones. 2000. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J. Biol. Chem. 275:17639–17646. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Lamb, G. D., M. A. Cellini, and D. G. Stephenson. 2001. Different Ca2+ releasing actions of caffeine and depolarization in skeletal muscle fibres of the rat. J. Physiol. 531:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver, D. R., L. D. Roden, G. P. Ahern, K. R. Eager, P. R. Junankar, and A. F. Dulhunty. 1995. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J. Membr. Biol. 147:7–22. [DOI] [PubMed] [Google Scholar]
- Ma, J., M. Fill, C. M. Knudson, K. P. Campbell, and R. Coronado. 1988. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 242:99–102. [DOI] [PubMed] [Google Scholar]
- Meissner, G. 1986. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 25:244–251. [DOI] [PubMed] [Google Scholar]
- Meissner, G. 2004. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 35:621–628. [DOI] [PubMed] [Google Scholar]
- Minium, E. W., B. M. King, and G. Bear. 1993. Statistical Reasoning in Psychology and Education. John Wiley and Sons, New York.
- Mitchell, R. D., H. K. Simmerman, and L. R. Jones. 1988. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J. Biol. Chem. 263:1376–1381. [PubMed] [Google Scholar]
- O'Brian, J. J., M. L. Ram, A. Kiarash and S. E. Cala. 2002. Mass spectrometry of cardiac calsequestrin characterizes microheterogeneity unique to heart and indicative of complex intracellular transit. J. Biol. Chem. 277:37154–37160. [DOI] [PubMed] [Google Scholar]
- Ostwald, T. J., D. H. MacLennan, and K. J. Dorrington. 1974. Effects of cation binding on the conformation of calsequestrin and the high affinity calcium-binding protein of sarcoplasmic reticulum. J. Biol. Chem. 249:5867–5871. [PubMed] [Google Scholar]
- Park, H., S. Wu, A. K. Dunker, and C. Kang. 2003. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 278:16176–16182. [DOI] [PubMed] [Google Scholar]
- Plank, B., W. Wyskovsky, G. Hellmann, and J. Suko. 1983. Calmodulin-dependent elevation of calcium transport associated with calmodulin-dependent phosphorylation in cardiac sarcoplasmic reticulum. Biochim. Biophys. Acta. 732:99–109. [DOI] [PubMed] [Google Scholar]
- Rodney, G. G., B. Y. Williams, G. M. Strasburg, K. Beckingham, and S. L. Hamilton. 2000. Regulation of RYR1 activity by Ca2+ and calmodulin. Biochemistry. 39:7807–7812. [DOI] [PubMed] [Google Scholar]
- Rodriguez, M. M., C. H. Chen, B. L. Smith, and D. Mochly-Rosen. 1999. Characterization of the binding and phosphorylation of cardiac calsequestrin by epsilon protein kinase C. FEBS Lett. 454:240–246. [DOI] [PubMed] [Google Scholar]
- Saito, A., S. Seiler, A. Chu, and S. Fleischer. 1984. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J. Cell Biol. 99:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori, S., S. Furlan, and F. Meggio. 1994. Dual role of calsequestrin as substrate and inhibitor of casein kinase-1 and casein kinase-2. Biochem. Biophys. Res. Commun. 198:144–149. [DOI] [PubMed] [Google Scholar]
- Shin, D. W., J. Ma, and D. H. Kim. 2000. The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin. FEBS Lett. 486:178–182. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz, V., I. Orr, S. Weil, H. Meyer, M. Varsanyi, and L. M. Heilmeyer. 1996. The identification of the phosphorylated 150/160-kDa proteins of sarcoplasmic reticulum, their kinase and their association with the ryanodine receptor. Biochim. Biophys. Acta. 1283:89–100. [DOI] [PubMed] [Google Scholar]
- Sitsapesan, R., and A. J. Williams. 1995. The gating of the sheep skeletal sarcoplasmic reticulum Ca2+-release channel is regulated by luminal Ca2+. J. Membr. Biol. 146:133–144. [DOI] [PubMed] [Google Scholar]
- Szegedi, C., S. Sarkozi, A. Herzog, I. Jona, and M. Varsanyi. 1999. Calsequestrin: more than ‘only’ a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 337:19–22. [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology. 24:145–149. [PubMed] [Google Scholar]
- Tripathy, A., and G. Meissner. 1996. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+release channel. Biophys. J. 70:2600–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy, A., L. Xu, G. Mann, and G. Meissner. 1995. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys. J. 69:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsanyi, M., and L. M. Heilmeyer, Jr. 1979. The protein kinase properties of calsequestrin. FEBS Lett. 103:85–88. [DOI] [PubMed] [Google Scholar]
- Varsanyi, M., and L. M. Heilmeyer, Jr. 1980. Autocatalytic phosphorylation of calsequestrin. FEBS Lett. 122:227–230. [DOI] [PubMed] [Google Scholar]
- Wagenknecht, T., C. E. Hsieh, B. K. Rath, S. Fleischer, and M. Marko. 2002. Electron tomography of frozen-hydrated isolated triad junctions. Biophys. J. 83:2491–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., N. A. Maertz, A. J. Lokua, E. G. Kranias, and H. H. Valdivia. 2001. Regulation of cardiac ryanodine receptors activity by calsequestrin. Biophys. J. 80:590a. (Abstr.). [Google Scholar]
- Wang, S., W. R. Trumble, H. Liao, C. R. Wesson, A. K. Dunker, and C. H. Kang. 1998. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 5:476–483. [DOI] [PubMed] [Google Scholar]
- Xu, L., and G. Meissner. 2004. Mechanism of calmodulin inhibition of cardiac sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor). Biophys. J. 86:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, K., and A. Zarain-Herzberg. 1994. Sarcoplasmic reticulum calsequestrins: structural and functional properties. Mol. Cell. Biol. 135:61–70. [DOI] [PubMed] [Google Scholar]
- Zhang, L., J. Kelley, G. Schmeisser, Y. M. Kobayashi, and L. R. Jones. 1997. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272:23389–23397. [DOI] [PubMed] [Google Scholar]