Abstract
Scyliorhinin I, a linear decapeptide, is the only known tachykinin that shows high affinity for both NK-1 and NK-2 binding sites and low affinity for NK-3 binding sites. As a first step to understand the structure-activity relationship, we report the membrane-induced structure of scyliorhinin I with the aid of circular dichroism and 2D-1H NMR spectroscopy. Sequence specific resonance assignments of protons have been made from correlation spectroscopy (TOCSY, DQF-COSY) and NOESY spectroscopy. The interproton distance constraints and dihedral angle constraints have been utilized to generate a family of structures using DYANA. The superimposition of 20 final structures has been reported with backbone pairwise root mean-square deviation of 0.38 ± 0.19 Å. The results show that scyliorhinin I exists in a random coil state in aqueous environments, whereas helical conformation is induced toward the C-terminal region of the peptide (D4-M10) in the presence of dodecyl phosphocholine micelles. Analysis of NMR data is suggestive of the presence of a 310-helix that is in equilibrium with an α-helix in this region from residue 4 to 10. An extended highly flexible N-terminus of scyliorhinin I displays some degree of order and a possible turn structure. Observed conformational features have been compared with respect to that of substance P and neurokinin A, which are endogenous agonists of NK-1 and NK-2 receptors, respectively.
INTRODUCTION
Scy I is a linear decapeptide with amino acid sequence Ala-Lys-Phe-Asp-Lys-Phe-Tyr-Gly-Leu-Met-NH2. On the basis of its abilities to contract isolated longitudinal smooth muscle from the guinea pig ileum, Scy I was first purified from an extract of the intestine of an elasmobranch fish, Scyliorhinus canicula (European common dogfish; Conlon et al., 1986). Due to the presence of a highly conserved C-terminal amino acid sequence, Phe-Xaa-Gly-Leu-Met-NH2, Scy I joins the tachykinin family of neuropeptides, which represents one of the largest peptide families described in the animal kingdom. The earliest known members of this family are the mammalian tachykinins SP, NKA, and NKB followed by tachykinins from nonmammalian sources physalaemin, eledoisin, and others. The primary structure of Scy I has 50% identity to physalaemin (seven residues), NKA (six residues), and SP (five residues). These similarities are concentrated mainly on the C-terminal part, “message domain” of the peptides that are considered to be important in receptor interaction. SP: R P K P Q Q F F G L M NH2; NKA: H K T D S F V G L M NH2; Phys: B A D P N K F Y G L M NH2; Scy I: A K F D K F Y G L M NH2.
In mammals, tachykinins have been shown to elicit a wide array of activities such as powerful vasodilatation, hypertensive action, and stimulation of extravascular smooth muscle and are known to be involved in variety of clinical conditions, including chronic pain, Parkinson's disease, Alzheimer's disease, depression, rheumatoid arthritis, irritable bowel syndrome and asthma (Khawaja and Rogers, 1996). This broad spectrum of action of tachykinins is attributed to the lack of selectivity of tachykinins to their receptors. Three distinct G-protein coupled receptor subtypes have been cloned and characterized for tachykinins (Hanley and Jackson, 1987; Masu et al., 1987; Nakanishi, 1991) designated as NK1, NK2, and NK3. All tachykinins interact with all the three receptor subtypes with SP preferring NK1, NKA preferring NK2, and NKB preferring NK3. Considerably more is known about the actions of Scy I in mammalian systems than in their own species. Competitive binding studies have shown that Scy I is a high-affinity agonist for both the mammalian NK1 and NK2 tachykinin receptors and possess 50–250 times reduced affinity for NK3 receptor. The binding affinity of Scy I is as high as that of endogenous ligands SP and NKA for NK1 and NK2, respectively (Beaujouan et al., 1988; Buck and Krstenansky, 1987). This property of Scy I makes it a valuable tool for further research on tachykinin receptors. Elucidation of the membrane bound conformation of this dual selective agonist is expected to be helpful in understanding the structure-activity relationship and developing efficient drugs.
Bioactive conformation of tachykinin neuropeptides has been extensively investigated using high-resolution NMR, CD, and infrared spectroscopy. Solution structure for SP, NKA, NKB, physalaemin, eledoisin, and various naturally derived or synthetic analogs has been reported in various membrane mimetic solvents (Ananthanarayanan and Orlicky, 1992; Chandrashekar and Cowsik, 2003; Chandrashekar et al., 2004; Convert et al., 1988, 1991; Cowsik et al., 1997; Grace et al., 2001, 2003; Horne et al., 1993; Mantha et al., 2004; Seelig, 1992; Whitehead et al., 1998). Only a few studies have been reported on the structure of Scy I. A solution conformational study for Scy I and its analogs with conformational constraints has been reported in water and DMSO-d6 using a combination of CD, 2D NMR, and theoretical conformational analysis (Klaudel et al., 2000; Rodziewicz-Motowidlo et al., 2000). Their results showed that the 3D structure of Scy I was rather flexible, whereas the presence of a local constraint (MePhe7) significantly rigidified the whole molecule. To date, none of the reported data deals with the high-resolution structure of Scy I. Binding of Scy I to its receptor occurs in the membrane environment. Membrane is proposed to induce a specific conformation to the peptide backbone of Scy I before interacting with its receptor, and this conformational alteration should be an essential step for the recognition by the receptor.
In this study, we report the 3D structure of Scy I in DPC, one of the well-characterized model membrane systems (Opella, 1997), using 2D NMR spectroscopy. CD spectroscopy has been used to explore secondary structural features of Scy-I in different membrane mimetic environments, including 2, 2, 2-TFE, TFE/water mixtures, SDS, DPC micelles, and in the presence of calcium ions. We also discuss the mechanism of interaction of Scy I with membranes and the structural requirements for its function.
MATERIALS AND METHODS
Materials
Scy I (AKFDKFYGLM-[NH2]) was custom synthesized by Princeton Biomolecules (Langhorne, PA). The purity of peptide reported by HPLC analysis was >98%. Perdeuterated DPC (d38) was obtained from Cambridge Isotope Laboratories (Andover, MA). NMR reagents were obtained from Aldrich Chemical Company (Milwaukee, WI). TFE, SDS, and CaCl2 were obtained from Sigma (St. Louis, MO) in the highest available purity.
CD spectropolarimetry
CD spectra (190–250 nm) of the peptide were recorded on a Jasco (Tokyo, Japan) J-720 spectropolarimeter. The instrument had been calibrated previously for wavelength using benzene vapor and for optical rotation using d-10-camphorsulphonic acid. A cell with a path length of 1 mm was used. All the experiments were carried out at room temperature. The parameters used were as follows: bandwidth, 1 nm; step resolution, 0.25 nm; scan speed, 50 nm/min; response time, 0.2 s. Each spectrum was obtained after an average of four scans. The peptide concentration was typically 109 μM in DPC, sodium phosphate buffer pH 7.2, various ratios of TFE/water mixture, and SDS micelles. Ca2+ titrations were carried out using aliquots of CaCl2 in TFE solution so as to get molar ratios (Ca2+: peptide conc.) of 1:1, 5:1, 10:1, 15:1, and 20:1. Before calculation of the final ellipticity, all spectra were corrected by subtraction of respective spectra obtained for peptide-free samples (containing only membrane mimetic solvents). Spectra were then smoothened using fast Fourier transform. CD intensity is expressed in terms of molar ellipticity ([θ], expressed in degrees cm2mol−1) according to the following equation,
 |
where θmdeg is the measured ellipticity in millidegrees, l is the cell path length in cm; and C is the molar concentration of the protein or peptide.
Analysis of CD data
Quantitative estimations of the secondary structure contents were made using the CDPRO software package, which includes the programs CDSSTR, CONTINLL, and SELCON 3 (http://lamar.colostate.edu/∼sreeram/CDPro; Sreerama et al., 1999; Sreerama and Woody, 1994). On analysis of the CD spectra with these three programs, CDSSTR gave rise to a best fit with the lowest root mean-square deviation (RMSD) between experimentally derived and calculated points whereas SELCON 3 and CONTINLL gave negative fractions in some calculations. The results tested with a reference set of 42 proteins are reported in Tables 1 and 2. Other reference protein sets, such as 29, 37, 43, and 48 were also tested and gave rise to comparable results.
TABLE 1.
Secondary structural analysis of the Scy I peptide in various membrane-mimicking environments using CDSSTR with a 42-reference protein set*
| Secondary structure (%)*
| |||||||
|---|---|---|---|---|---|---|---|
| Environment | Helix | Strand | Turn | Unrd | RMSD | R1† | R2† |
| Phosphate buffer, pH 7.2 | 0 | 32.8 | 15.9 | 52.7 | 0.134 | 4.67 | −0.11 |
| 90% TFE | 56.8 | 15.8 | 13.4 | 13.4 | 0.100 | −1.01 | 0.85 |
| 64 mM SDS | 68.3 | 11.9 | 12.6 | 6.3 | 0.062 | −1.10 | 0.90 |
| 5 mM DPC | 71.3 | 9.4 | 7.8 | 10.1 | 0.99 | −1.96 | 0.95 |
For convenience of data interpretation, we added the distorted and regular components of both helical and strand secondary-structural elements together to obtain overall helical and strand structures in both Tables 1 and 2.
Here R1 is the ratio of the intensity of the maximum between 190 and 195 nm and the intensity of the minimum between 200 and 210 nm, and R2 is the ratio of the intensity of the minimum near 222 nm and the intensity of the minimum between 200 and 210 nm.
TABLE 2.
Effect of calcium concentration on the conformation of Scy I using CDSSTR with a 42-reference protein set
| Secondary structure (%)
| |||||||
|---|---|---|---|---|---|---|---|
| Environment (Calcium: peptide ratio) | Helix | Strand | Turn | Unrd | RMSD | R1 | R2 |
| 100% TFE | 56.8 | 15.8 | 13.4 | 13.4 | 0.100 | −1.01 | 0.85 |
| 1:1 | 60.2 | 17.3 | 12.7 | 9.5 | 0.083 | −1.30 | 0.865 |
| 5:1 | 62.5 | 14.3 | 10.9 | 12.0 | 0.084 | −1.32 | 0.88 |
| 10:1 | 57.4 | 20.1 | 11.6 | 10.8 | 0.083 | −0.22 | 0.67 |
| 15:1 | 54 | 14.8 | 16.3 | 15.3 | 0.135 | −0.20 | 0.63 |
| 20:1 | 49.8 | 20.8 | 9.0 | 20.5 | 0.141 | 0.32 | 0.20 |
NMR spectroscopy
The NMR samples were prepared by dissolving 3 mg of Scy I in ∼0.5 ml of water (90% H2O, 10% D2O, pH 4.5). The experiments in lipid environment were performed with an identical peptide sample to which 40 mg of perdeuterated DPC was added, yielding in solution a lipid concentration of 200 mM, which is well above the critical micellar concentration (1mM). Some preliminary 1D spectra were recorded at different temperatures (290–320 K).
The phase sensitive homonuclear 2D DQF-COSY, TOCSY, NOESY, and ROESY experiments at 315 K were performed using a time-proportional phase incrementation method (Marion and Wüthrich, 1983). For these experiments, 512 transients with 2K complex data points were collected for each of the increments with a relaxation delay of 1.5 s between successive transients, the data along the t1 dimension were zero filled, and the resultant 1K data were subjected to 2D-FT. A TOCSY experiment was performed using 100 ms, MLEV-17 spin-lock mixing pulse. NOESY experiments were performed at mixing times of 100, 150, 200, and 250 ms, and a ROESY experiment at 200 ms mixing time. All NMR spectra were recorded on a Bruker (Zurich, Switzerland) DRX-500 spectrometer. The NMR spectra were processed using XWINNMR software on a Silicon Graphics fuel workstation (SGI, Mountain View, CA).
Structure calculation
Distance constraints were extracted from the NOESY spectra with 150 ms mixing time. All the NOE intensities were classified as strong, medium, and weak and were converted to the corresponding upper bound interproton distance restraints of 2.7, 3.5, and 5.0 Å, respectively. A total of 94 NOE constraints (42 intraresidue, 26 i, i + 1, 15 i, i + 2, 8 i, i + 3, and 3 i, i + 4) were originally applied for structure calculation using DYANA (Güntert et al., 1997). 3JHNα-coupling constants were obtained from high resolution 1D spectra and converted to dihedral angles by applying the Karplus relationship (Pardi et al., 1984). A total of 50 structures were calculated by DYANA out of which 20 conformers with lowest target function value were consequently subjected to restrained energy minimization.
RESULTS
Secondary-structural studies of Scy-I by CD spectropolarimetry
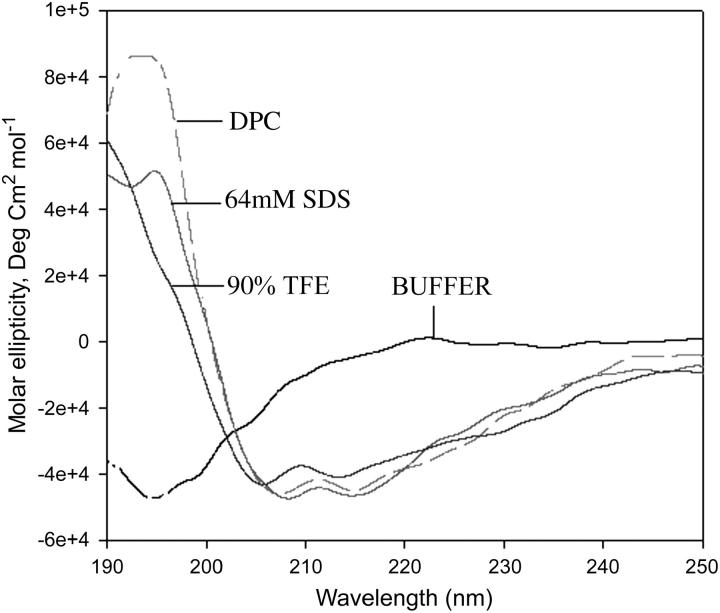
To investigate the secondary structure of Scy I in various membrane mimetic solvents, CD spectra were recorded in phosphate buffer pH 7.2, TFE/water mixture (10%–90%), SDS micelles (4 mM–64 mM) and DPC micelles (5 mM). As shown in Fig. 1, in an aqueous environment, Scy I exhibits a strong negative band at 195 nm and a weak positive band around 218 nm, characteristic of random coil conformation (Woody, 1992). However, Scy I adopts helical conformation with increasing titers of TFE. The helical content is further increased to 68.3% in 64 mM SDS and 71.3% in 5 mM DPC as compared to 56.8% in 90% TFE. The CD data analyzed by using CDPRO (CDSSTR) software is in good agreement with the values of two parameters, R1 and R2, which are independent of inaccuracies in determined peptide concentration, as well as those caused by small shifts in wavelength (Bruch et al., 1991). For a random structure, R1 is positive and R2 is close to zero. On the other hand, in a highly helical state, R1 will be close to −2 and R2 will approach 1 (Bruch et al., 1991; Rizo et al., 1993). Thus the CD results (Table 1) indicate that Scy I associates with TFE, SDS, and DPC micelles undergoing a conformational transition from a prevalently random coil state (in water) to an α-helical state.
FIGURE 1.
CD spectra of Scy I in buffer, 64 mM SDS micelles, 90% TFE and DPC micelles.
The interaction of Ca2+ with Scy I was studied by monitoring changes in CD spectra of the peptide in a 190–250 nm range to observe alteration in the secondary structure of the peptide. CD spectral features of Scy I indicate no appreciable secondary structure in water (Fig. 1). No significant change was observed even on addition of over 2 molar excess of Ca2+ to a solution of Scy I in water (data not shown). However, a substantial change in CD occurred on treating Scy I with Ca2+ in TFE. As seen in Fig. 2, there is an increase in the helical content on addition of Ca2+ (up to a ratio of 5:1) as compared to 100% TFE. There is a subsequent decrease on further addition of calcium. This effect is nonspecific and is perhaps due to the destabilization of the structure by the high ionic strength of calcium solution used. Secondary structural features obtained are summarized in Table 2.
FIGURE 2.
CD spectra of calcium titration of Scy I. Different spectra in the figure represent various ratios of calcium to peptide A: 100% TFE; B: 1:1; C: 5:1; D: 10:1; E: 15:1; and F: 20:1, respectively.
Resonance assignments
All proton resonances were assigned using the standard sequential assignment strategy (Wüthrich, 1986). TOCSY and DQF-COSY spectra were used to assign spin systems of most of the amino acid residues. Aromatic residues were identified using NOE connectivities between Cβ protons and aromatic ring protons. Complete sequence specific assignments were obtained by direct comparison of TOCSY and NOESY spectra (Figs. 3 and 4).
FIGURE 3.
The NH-α,β,γ region of the 500 MHz NOESY spectrum of Scy I in the presence of membrane mimetic solvent (DPC), recorded with a mixing time of 150 ms.
FIGURE 4.
The NH-NH region of the 500 MHz phase-sensitive NOESY spectrum of Scy I in the presence of membrane mimetic solvent (DPC), recorded with a mixing time of 150 ms.
On the basis of observation of a relatively small number of interresidue crosspeaks in the ROESY spectra, it was concluded that Scy I essentially adopted a random coil conformation in water. Also the CD results indicated that Scy I was unstructured in aqueous media. Hence no further analysis was carried out in water.
Structure calculations in DPC micelles
A summary of the interresidue sequential and medium-range NOEs is provided in Fig. 5. The presence of several NH-NH connectivities, together with αH-NHi+3 and αH-βHi+3 connectivities suggests the presence of some helical elements from residue 2–10. Medium range NOE connectivities, 5 dNN (i, i + 2), 8 dαN (i, i + 2), along with dαN (i, i + 3) support the presence of a 310-helix from residue 4–10, which is also supported by the negative chemical shift index (Wishart et al., 1992) and values of coupling constants for these residues. The presence of some population of α-helix is supported by the observation of dαN (i, i + 4) connectivity between residues F3-Y7, K5-L9, and F6-M10, but the relative strengths of the sequential NOEs, particularly near both termini, are not representative of a well-defined α-helix. For the region near the N-terminus, the possibility of a β-turn seems to be more pronounced, as seen by the absence of dαβ (i, i + 3) crosspeaks. This is further supported by the presence of Asparagine at Position 4, which has the helix breaking tendency. Analysis of NMR data is suggestive of the presence of a 310-helix that is in equilibrium with an α-helix in this region from residue 4 to 10.
FIGURE 5.
The NOEs that are important to characterize the secondary structure of Scy I in the presence of DPC micelles. Solid triangles refer to a coupling constant of 4 to 5 Hz and solid circles to 6 Hz and above.
All measurable 3JHNα-coupling constants for Scy I are in the range of 3–6 Hz, with the exception of L9 and M10 (8 and 9.2 Hz, respectively). Higher values of 3JHNα (>6 Hz) for L9 and M10 can be attributed to the fraying of the helix at the terminus and susceptibility of short, linear peptides to undergo conformational averaging (Dyson and Wright, 1991). Estimates of the fraction of molecules possessing folded conformation can be obtained by the analysis of αH-NHi + 1 and NH-NH NOE intensities using the procedure of Bradley et al. (1990). In this analysis, we use the term “folded” in preference to “helix” since reverse turn and helix structures can produce similar NOE intensities. A calculation based on NOE intensities confirms a high helical content (84%) over residues 4–10. The value is somewhat higher than that indicated by the CD data (71%), but this may be rationalized by potential difficulties in the CD spectra of small peptides, where dichroism of aromatic amino acids can mask the CD signal of “structured” conformers, especially for short helices.
Generation of the 3D structure
A 3D structure of Scy I was obtained using a torsion angle dynamics algorithm for NMR applications, DYANA (Güntert et al., 1997). A total of 91 NOE distance constraints and angle constraints from measured 3JHNα were used as input. Initially 50 structures were generated, out of which 20 conformers with the lowest target function values (i.e., least violations of experimental restraints and van der Waals distances) were chosen for further restrained energy minimization. The resulting structures are shown in Fig. 6, after superimposing the backbone atoms. The backbone pairwise RMSD for all residues is 0.38 Å with standard deviation of 0.19 Å and for all heavy atoms is 1.99 Å with standard deviation of 0.40 Å. The lowest RMS differences are seen for residues 5–9, where the folded structures determined from the NOE data are best defined.
FIGURE 6.
Stereo view showing the superimposition of the backbone atoms of Scy I for 20 structures generated by DYANA.
The Ramachandran plot for all 20 refined structures (included in the supplementary material) indicates that the backbone dihedral angles consistently lie in the α-region of the plot and are within the allowed ranges. The average values of Φ and Ψ angles for the helix region are −74°(±9°), −27°(±7°) and for the turn are −79°(±6), −22°(±4). The shifted backbone conformation for the C-terminal residue (Φ, Ψ angles: −90°, 0°) compared to the rest of the helix is a characteristic feature of 310-helices. The deviation can be attributed to the release of electrostatic repulsion between the carbonyl oxygen atoms at the two C-terminal residues and further stabilization (due to a more linear geometry) of an intrahelical hydrogen bond. Measurements of C=Oi, NHi + 3 versus C=Oi, NHi + 4 distances along the stretch of residue 4–10 were made, and the i, i + 3 distances were <3.0 Å in the majority (>75%) of structures as compared to i, i + 4 distances, suggesting that Scy I has a preference for a 310-helix over a regular helix.
Results from this study indicate that the Scy I prefers to be in a random coil conformation in aqueous solution, whereas part of the N-terminus address domain and the whole of the C-terminal message domain are folded. The NMR data suggest the presence of equilibrium between the 310-helix and the α-helix along the residues 4–10 of Scy I, preceded by a turn over residues 1–3 of Scy I. In DPC, the structural equilibrium is biased toward a 310 helix though a small population of regular α-helix cannot be excluded in the solution ensemble. The 310-helix exists as the intermediate between the folding pathway of helices (random coil ↔ nascent helix ↔ 310-heilx ↔ α-helix; Millhauser, 1995). A recent study on the analysis of the shortest helices explains the formation of the β-turn might be correlated to the nucleation step for the formation of both α- and 310-helices (Pal et al., 2003).
DISCUSSION
Although only a few studies have been reported on the structure of Scy I, dual equipotent selectivity of Scy I toward NK1 and NK2 receptors makes it a particularly interesting peptide for studying the structure-activity relationship. Important information available on tachykinins is distinctly different structural and functional behavior of N- and C-terminals. Available structure-activity relationship studies indicate that the N-terminal domains of tachykinin peptides affect the desensitization but not the agonist activities of the peptides (Cascieri et al., 1992; Vigna, 2001; Zboinska et al., 1993). On examination of the ability of Scy I to cause rapid homologous desensitization of the NK1 receptor, it was found that Scy I caused desensitization similar to that of SP, and the N-terminal domain of Scy I affected both receptor signaling and desensitization (Vigna, 2003).
It has been reported that the action of tachykinins is dependent on extracellular calcium concentration (O'Riordan et al., 2001; Pernow, 1983) and that the calcium bound conformation of tachykinins is required for receptor activation (Ananthanarayanan and Orlicky, 1992). Ananthanarayanan and co-workers studied conformational determinants for Ca2+ binding by short synthetic peptides in nonpolar solvents (Qi et al., 2000) and reported that even in the absence of acidic side-chain groups, cation binding to the polar groups in the backbone was a facile process in low dielectric medium. The main conformational requirement appeared to be the ability to adopt a folded structure like a helix or turn in nonpolar medium. Many tachykinins including Scy I fulfill this criterion. Structural studies on Scy I suggested induction of folded conformation in TFE, SDS, and DPC micelles. We have in this study made use of these observations as the basis for examining the structure of Scy I in terms of its interaction with calcium in TFE using CD.
The results of our preliminary study demonstrate a specific interaction of Scy I with calcium in TFE. CD studies on calcium titrations show that the helicity increases with increasing concentrations of calcium and that the calcium bound conformation of Scy I is considerably different from that of a free Scy I. It is of interest to note that a similar situation exists in the case of SP, a tachykinin, and two other peptide hormones—insulin and glucagons (Ananthanarayanan et al., 1997; Brimble and Ananthanarayanan, 1992, 1993; Qi et al., 2000). This suggests that specific constraints may be imposed on the Scy I structure on its interaction with calcium at low concentrations, which may play a role in the bioactive conformation of the peptide and its interaction with the receptor. At higher concentrations, calcium seems to be having a nonspecific destabilizing effect on the structure of the peptide. The destabilizing effects of calcium at high ionic strength have also been observed in some studies (Pastrana-Rios et al., 2002).
Previous studies have indicated that diverse hormones such as glucagons, SP, NPG, Bombesin, and GnRh (Ananthanarayanan et al., 1998; Qi et al., 2000) have the ability to bind divalent cation in lipid mimetic solvents and to transport calcium across the synthetic liposomes in aqueous buffers. The ionophoretic ability seems to reside in the C-terminal segment containing the Met-Leu sequence. In light of a proposal on the importance of calcium in dictating the bioactive conformation of the peptide hormones (Ananthanarayanan, 1991; Ananthanarayanan et al., 1997, 1998; Pernow, 1983), in general we would expect the C-terminal of Scy I to insert into membrane with the bound calcium and interact with the receptor to form a ternary complex between hormone, receptor, and cation. The specificity of binding can be determined only after a detailed study with different analogs has been undertaken. More work is needed before the relevance of these observations to the in vivo action of the peptide is understood.
Summarizing the NMR-derived structure, the amidated C-terminus of Scy-1 in DPC comprises a 310-helix or turn-like elements with some possible fraying of the helix terminus. The “address” segment of Scy I, although undergoing greater conformational averaging than the “message” domain, also retains substantial conformational order in DPC. This order may be interpreted as a loosely defined turn or an unstable continuation of the 310-helix along the message domain. The presence of a turn in the N-terminus preceding the helical core correlates well with the conformational features adopted by NK2 agonists like NKA, NPγ, eledoisin, and Kassinin (Chandrashekar and Cowsik, 2003; Chandrashekar et al., 2004; Grace et al., 2001, 2003). It has been proposed that selective agonists for the NK3 receptor prefer to adopt an α-helical conformation (Mantha et al., 2004; Wilson et al., 1994). The presence of a turn preceding the helical core explains the reduced affinity of Scy I toward the NK3 receptor.
The solution conformation of Scy I bound to DPC micelles determined in this study supports various structure-activity and receptor-ligand interaction data available on NK1/NK2 agonists. (Cascieri et al., 1992; Patacchini et al., 1993a,b; Seelig, 1992; Seelig et al., 1996; Whitehead et al., 1998). As seen in Fig. 7, the C-terminus of Scy I presents two different faces to the receptor: a hydrophobic face comprised of Phe 6, Leu 9, Met 10 side chains on one side and a hydrophilic face comprised of Lys 5, Tyr 7, and Gly 8 side chains on the other. This amphipathic nature of the helix at the C-terminus is characteristic of NK-1 receptor agonists (Seelig, 1992; Seelig and Macdonald, 1989; Seelig et al., 1996). Moreover, the presence of an aromatic residue at Position 7 from the N-terminus appears to determine or modify selectivity of the peptide for the NK1 receptor (Severini et al., 2002). In support of this is the observation that the Tyr residue at Position 7 is important in determining the potency and selectivity of Scy I at the tachykinin receptors, and the introduction of Phe in Scy I provides the molecule with higher selectivity for the NK1 receptor (Patacchini et al., 1993a,b). Scy I contains two Lys residues that would result in a more favorable interaction with the anionic fixed-charge compartment of the plasma membrane and greater access to the NK-1 and NK-2 receptors (Schwyzer, 1987). It can be proposed that the “nonpolar” nature of the Tyr side chain at Position 7 of Scy I confers selectivity for NK2 receptor binding, whereas the aromatic ring of Tyr is important for NK-1 binding. Absence of a Pro residue at Position 4 from the N-terminus (as present in the majority of NK1 receptor agonists), adjacent to the crucial neutral or basic residue occupying Position 3 (“Lys” in SP, NKA, and Scy I), can be considered favorable for the binding of NK2 receptors. Aspartate residue in Scy I, analogous to residue 5 in SP and residue 4 in NKA, may be involved in an ionic interaction, thus contributing to the binding energy as proposed by Cascieri and Buck for NK2 agonists (Buck and Shatzer, 1988; Cascieri et al., 1992). We propose that the charged amino acid residues, Lys 2 and Asp 4, of the N-terminus region act as anchoring points, maintaining peptide conformation and positioning the C-terminus within the transmembrane region.
FIGURE 7.
A graphic representation of the lipid bound conformation of Scy I compared with SP and NKA. The peptide backbone is shown as a ribbon tube (blue). Ionic residues are colored red, polar residues are colored purple, and the hydrophobic residues are colored yellow. The helical segment is clearly visible.
Since the structural features of the peptide ligand have been determined in isolation, there could be conformational changes to the structure upon interaction with the receptor. However these structural features, which seem to be essential for the biological activity, will probably be maintained until Scy I approaches its receptor. The elucidation of the 3D structure is particularly important for the development of agonist/antagonists with regard to the NK-1 and NK-2 receptors with potential for synergistic pharmacological effects.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
We gratefully acknowledge the staff of National 500 MHz NMR facility at Sophisticated Instruments Facility, Indian Institute of Science, Bangalore.
Anjali Dike acknowledges the financial support in the form of a Junior Research Fellowship from the Council of Scientific and Industrial Research, India. This work is supported through a grant from the Council of Scientific and Industrial Research, India.
Abbreviations used: Scy I, scyliorhinin I; DPC, dodecylphosphocholine; CD, circular dichroism; TFE, trifluroethanol; SDS, sodium dodecyl sulfate; DQF-COSY, double-quantum filtered correlation spectroscopy; 2D, two-dimensional; NOESY, nuclear Overhauser effect spectroscopy; ROESY, rotating frame Overhauser effect spectroscopy; TOCSY, total correlation spectroscopy; NK, neurokinin; SP, substance P; NKA, neurokinin A; NKB, neurokinin B; NPK, neuropeptide K; NPγ, neuropeptide gamma; NK1, neurokinin-1; NK2, neurokinin-2; nK3, neurokinin-3; DMSO, dimethyl sulfoxide.
References
- Ananthanarayanan, V. S. 1991. Peptide hormones, neurotransmitters and drugs as Ca2+ ionophores: implications for signal transduction. Biochem. Cell Biol. 69:93–95. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan, V. S., M.-P. Belcing, and B. S. Zhorov. 1997. Interaction of oxytocin with Ca2+: II. Proton magnetic resonance and molecular modeling studies of conformations of the hormone and its Ca2+ complex. Biopolymers. 40:445–464. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan, V. S., and S. Orlicky. 1992. Interaction of Substance P and its N- and C-terminal fragments with Ca2+: implications for hormone action. Biopolymers. 32:1765–1773. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan, V. S., O. Salehian, and K. S. Brimble. 1998. Interaction of gonodotropin releasing hormone and its analogs with Ca2+ in a non polar milieu correlation with biopotencies. J. Pept. Res. 52:185–194. [DOI] [PubMed] [Google Scholar]
- Beaujouan, J. C., M. Saffroy, F. Petitet, Y. Torrens, and J. Glowinski. 1988. Neuropeptide K, scyliorhinin I and scyliorhinin II: new tools in the tachykinin receptor field. Eur. J. Pharmacol. 151:353–354. [DOI] [PubMed] [Google Scholar]
- Bradley, E. K., J. F. Thomason, F. E. Cohen, P. A. Kosen, and I. D. Kuntz. 1990. Studies of synthetic helical peptides using circular dichroism and nuclear magnetic resonance. J. Mol. Biol. 215:607–622. [DOI] [PubMed] [Google Scholar]
- Brimble, K. S., and V. S. Ananthanarayanan. 1992. Induction of Ca2+ transport in liposomes by insulin. Biochim Biophys. Acta. 1105:319–327. [DOI] [PubMed] [Google Scholar]
- Brimble, K. S., and V. S. Ananthanarayanan. 1993. Calcium binding and translocation properties of glucagons and its fragments. Biochemistry. 32:1632–1640. [DOI] [PubMed] [Google Scholar]
- Bruch, M. D., M. M. Dhingra, and L. M. Gierasch. 1991. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an alpha-helical region of carboxypeptidase A. Proteins. 10:130–139. [DOI] [PubMed] [Google Scholar]
- Buck, S. H., and J. L. Krstenansky. 1987. The dogfish peptides scyliorhinin I and scyliorhininII bind with differential selectivity to mammalian tachykinin receptors. Eur. J. Pharmacol. 144:109–111. [DOI] [PubMed] [Google Scholar]
- Buck, S. H., and S. A. Shatzer. 1988. Agonists and antagonist binding to tachykinin peptide NK-2 receptors. Life Sci. 42:2701–2708. [DOI] [PubMed] [Google Scholar]
- Cascieri, M. A., R. R. Huang, T. M. Fong, F. M. Cheung, S. Sadowski, E. Ber, and C. D. Strader. 1992. Determination of the amino acid residues of substance P conferring selectivity and specificity for the rat Neurokinin receptors. Mol. Pharmacol. 41:1096–1099. [PubMed] [Google Scholar]
- Chandrashekar, I. R., and S. M. Cowsik. 2003. Three-dimensional structure of the mammalian tachykinin peptide Neurokinin A bound to lipid micelles. Biophys. J. 85:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar, I. R., A. Dike, and S. M. Cowsik. 2004. Membrane induced structure of the mammalian tachykinin neuropeptide gamma. J. Struct. Biol. 148:315–325. [DOI] [PubMed] [Google Scholar]
- Conlon, J. M., C. F. Deacon, L. O'Toole, and L. Thim. 1986. Scyliorhinin I and II: two novel tachykinins from dogfish gut. FEBS Lett. 200:111–116. [DOI] [PubMed] [Google Scholar]
- Convert, O., H. Duplaa, S. Levielle, and G. Chassaing. 1991. Influence of the replacement of amino acid by its D-enantiomer in the sequence of substance P: conformational analysis by NMR and energy calculations. Neuropeptides. 19:259–270. [DOI] [PubMed] [Google Scholar]
- Convert, O., O. Ploux, S. Lavielle, M. Cotrat, and G. Chassaing. 1988. Analysis of tachykinin-binding site interactions using NMR and energy calculation data of potent cyclic analogues of substance P. Biochim. Bhiophys. Acta. 954:287–302. [DOI] [PubMed] [Google Scholar]
- Cowsik, S. M., C. Luke, and H. Ruterjans. 1997. Lipid-induced conformation of Substance P. J. Biomol. Struct. Dyn. 15:27–36. [DOI] [PubMed] [Google Scholar]
- Dyson, H. J., and P. E. Wright. 1991. Defining solution conformation of small linear peptides. Annu. Rev. Biophys. Biophys. Chem. 20:519–538. [DOI] [PubMed] [Google Scholar]
- Grace, C. R., A. M. Lynn, and S. M. Cowsik. 2001. Lipid induced conformation of tachykinin peptide kassinin. J. Biomol. Struct. Dyn. 18:611–625. [DOI] [PubMed] [Google Scholar]
- Grace, C. R., I. R. Chandrashekar, and S. M. Cowsik. 2003. Solution structure of the tachykinin peptide eledoisin. Biophys. J. 84:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntert, P., C. Mumenthaler, and K. Wüthrich. 1997. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273:283–298. [DOI] [PubMed] [Google Scholar]
- Hanley, M. R., and T. Jackson. 1987. Substance K receptor: return of magnificent seven. Nature. 329:766–767. [DOI] [PubMed] [Google Scholar]
- Horne, J., M. Sadek, and D. J. Craik. 1993. Determination of solution structure of neuropeptide K by high-resolution nuclear magnetic resonance spectroscopy. Biochemistry. 32:7406–7412. [DOI] [PubMed] [Google Scholar]
- Khawaja, A. M., and D. F. Rogers. 1996. Tachykinins: receptor to effectore. Int. J. Biochem. Cell Biol. 28:721–738. [DOI] [PubMed] [Google Scholar]
- Klaudel, L., S. Rodziewicz-Motowido, A. Liwo, and K. Rolka. 2000. A comparison of Scyliorhinin I and its analogue with N-Methyl-L-phenylalanine in position 7. Pol. J. Chem. 74:1101–1114. [Google Scholar]
- Mantha, A. K., I. R. Chandrashekar, N. Z. Baquer, and S. M. Cowsik. 2004. Three-dimensional structure of the mammalian tachykinin peptide Neurokinin B bound to lipid micelles. J. Biomol. Struct. Dyn. 22:137–147. [DOI] [PubMed] [Google Scholar]
- Marion, D., and K. Wüthrich. 1983. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurement of 1 H-1 H spin-spin coupling constants in protein. Biochem. Biophys. Res. Commun. 113:967–974. [DOI] [PubMed] [Google Scholar]
- Masu, Y., K. Nakayama, H. Tamaki, M. Harada, Y. Kuno, and S. Nakanishi. 1987. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature. 329:836–838. [DOI] [PubMed] [Google Scholar]
- Millhauser, G. L. 1995. Views of helical peptides: a proposal for the position of 310-helix along the thermodynamic folding pathway. Biochemistry. 34:3873–3877. [DOI] [PubMed] [Google Scholar]
- Nakanishi, S. 1991. Mammalian tachykinin receptors. Annu. Rev. Neurosci. 14:123–136. [DOI] [PubMed] [Google Scholar]
- Opella, S. J. 1997. NMR and membrane proteins. Nat. Struct. Biol. 4(Suppl.):845–848. [PubMed] [Google Scholar]
- O'Riordan, A. M., T. Quinn, J. M. Hyland, D. P. O'Donoghue, and A. W. Baird. 2001. Sources of calcium in Neurokinin A-induced contractions of human colonic smooth muscle in vitro. Am. J. Gastroenterol. 96:3117–3121. [DOI] [PubMed] [Google Scholar]
- Pal, L., P. Chakrabarti, and G. Basu. 2003. Sequence and structure patterns in proteins from an analysis of the shortest helices: implications for helix nucleation. J. Mol. Biol. 326:273–291. [DOI] [PubMed] [Google Scholar]
- Pardi, A., M. Billeter, and K. Wüthrich. 1984. Calibration of the angular dependence of the amide proton-C alpha proton coupling constants, 3JHN alpha, in a globular protein. Use of 3JHN alpha for identification of helical secondary structure. J. Mol. Biol. 180:741–751. [DOI] [PubMed] [Google Scholar]
- Pastrana-Rios, B., W. Ocana, M. Rios, G. L. Vargas, G. Ysa, G. Poynter, J. Tapia, and J. L. Salisbury. 2002. Centrin: its secondary structure in the presence and absence of cations. Biochemistry. 41:6911–6919. [DOI] [PubMed] [Google Scholar]
- Patacchini, R., L. Quartara, P. Rovero, C. Gosos, and C. A. Maggi. 1993a. Role of C-terminal amidation on the biological activity of Neurokinin A derivatives with agonist and antagonist properties. J. Pharmacol. Exp. Ther. 264:17–21. [PubMed] [Google Scholar]
- Patacchini, R., L. Quartara, K. Rolka, J. Zboinska, G. Kupryszewski, and C. A. Maggi. 1993b. Effect of scyliorhinin I and synthetic scyliorhinin I derivatives at mammalian tachykinin NK1, NK2 and NK3 receptors. Eur. J. Pharmacol. 250:311–316. [DOI] [PubMed] [Google Scholar]
- Pernow, B. 1983. Substance P. Pharmacol. Rev. 35:85–141. [PubMed] [Google Scholar]
- Qi, X.-F., B. S. Zhorov, and V. S. Ananthanarayanan. 2000. CD, 1H NMR and molecular modeling studies of the interaction of Ca2+ with Substance P and Ala7-Substance P in a non-polar solvent. J. Pept. Sci. 6:57–83. [DOI] [PubMed] [Google Scholar]
- Rizo, J., F. J. Blanco, B. Kobe, M. D. Bruch, and L. M. Gierasch. 1993. Conformational behavior of Escherichia coli OmpA signal peptides in membrane mimetic environments. Biochemistry. 32:4881–4894. [DOI] [PubMed] [Google Scholar]
- Rodziewicz-Motowidlo, S., A. Legowska, X. F. Qi, C. Czaplewski, A. Liwo, P. Sowinski, W. Mozga, J. Olczak, J. Zabrocki, and K. Rolka. 2000. Solution conformational study of scyliorhinin I analogues with conformational constraints by two-dimensional NMR and theoretical conformational analysis. J. Pept. Res. 56:132–146. [DOI] [PubMed] [Google Scholar]
- Schwyzer, R. 1987. Membrane-assisted molecular mechanism of Neurokinin receptor subtype selection. EMBO J. 6:22–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig, A. 1992. Interaction of a substance P agonist and of substance P antagonists with lipid membranes: a thermodynamic analysis. Biochemistry. 31:2897–2904. [DOI] [PubMed] [Google Scholar]
- Seelig, A., T. Alt, S. Lotz, and G. Holzemann. 1996. Binding of substance P agonists to lipid membranes and to the Neurokinin-1 receptor. Biochemistry. 35:4365–4374. [DOI] [PubMed] [Google Scholar]
- Seelig, A., and P. M. Macdonald. 1989. Binding of a neuropeptide, substance P, to neutral and negatively charged lipids. Biochemistry. 28:2490–2496. [DOI] [PubMed] [Google Scholar]
- Severini, C., G. Improta, G. F. Erspamer, S. Salvadori, and V. Erspamer. 2002. The tachykinin peptide family. Pharmacol. Rev. 54:285–322. [DOI] [PubMed] [Google Scholar]
- Sreerama, N., and R. W. Woody . 1994. Protein secondary structure from circular dichroism spectroscopy. Combining variable selection principle and cluster analysis with neural network, ridge regression and self-consistent methods. J. Mol. Biol. 242:497–507. [DOI] [PubMed] [Google Scholar]
- Sreerama, N., V. S. Yu, and R. W. Woody. 1999. Estimation of the number of α-helical and β-strand segments in proteins using CD spectroscopy. Protein Sci. 8:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna, S. R. 2001. The N-terminal domain of substance P is required for complete homologous desensitization but not phosphorylation of the rat neurokinin-1 receptor. Neuropeptides. 35:24–31. [DOI] [PubMed] [Google Scholar]
- Vigna, S. R. 2003. The role of the amino-terminal domain of tachykinins in Neurokinin-1 receptor signaling and desensitization. Neuropeptides. 37:30–35. [DOI] [PubMed] [Google Scholar]
- Whitehead, T. L., S. D. McNair, C. E. Hadden, J. K. Young, and R. P. Hicks. 1998. Membrane-induced secondary structures of neuropeptides: a comparison of solution conformations adopted agonists and antagonists of the mammalian tachykinin NK1 receptor. J. Med. Chem. 41:1497–1506. [DOI] [PubMed] [Google Scholar]
- Wilson, J. C., K. J. Nielsen, M. J. Mcleish, and D. J. Craik. 1994. A determination of the solution conformation of the nonmammalian tachykinin eledoisin by NMR and CD spectroscopy. Biochemistry. 33:6802–6811. [DOI] [PubMed] [Google Scholar]
- Wishart, D. S., B. D. Sykes, and F. M. Richards. 1992. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 31:1647–1651. [DOI] [PubMed] [Google Scholar]
- Woody, R. W. 1992. Circular dichroism and conformation of unordered polypeptides. Adv. Biophys. Chem. 2:37–79. [Google Scholar]
- Wüthrich, K. 1986. NMR of Proteins and Nucleic Acids. J. Wiley & Sons, New York.
- Zboinska, J., K. Rolka, G. Kupryszewski, K. Golba, P. Imiolek, P. Jana, and Z. S. Herman. 1993. Synthesis and structure-activity relationship of scyliorhinin I analogues modified in position 3, 6, 7, and 8. Collect. Czech. Chem. C. 58:918–924. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.