Abstract
The alpha-fetoprotein gene (Afp) is a member of a multigenic family that comprises the related genes encoding albumin, alpha-albumin, and vitamin D binding protein. The biological role of this major embryonic serum protein is unknown although numerous speculations have been made. We have used gene targeting to show that AFP is not required for embryonic development. AFP null embryos develop normally, and individually transplanted homozygous embryos can develop in an AFP-deficient microenvironment. Whereas mutant homozygous adult males are viable and fertile, AFP null females are infertile. Our analyses of these mice indicate that the defect is caused by a dysfunction of the hypothalamic/pituitary system, leading to anovulation.
Alpha-fetoprotein (AFP) is a serum glycoprotein produced at high levels during fetal life by the liver and the visceral endoderm of the yolk sac and at lower levels by the developing gastrointestinal tract (1, 2). The protein expressed by the embryo is transferred to the maternal blood circulation, and abnormal levels of embryonic AFP in the maternal serum are indicative of spina bifida or Down's syndrome in the fetus (3, 4). The synthesis of AFP decreases dramatically after birth, and only trace amounts are detected in the adult (2). AFP expression has been shown to be regulated by transcriptional mechanisms involving a relatively large promoter (P1) and three distant enhancers (for reviews see refs. 5 and 6). More recently it has been shown that the first intron of the Afp gene contains an enhancer and an alternative promoter called P2 (7). The genes of the albumin family are linked in the mammalian genomes, and this conserved organization has been proposed to be important for the developmental expression switch of the genes of the family after birth (8). Several hypotheses have been proposed for the physiological function of AFP (for reviews see refs. 6, 9, and 10). Because AFP is synthesized during the cell cycle G1 and S phases, it has been hypothesized that it affects cell growth (11, 12). The observation that AFP is able to bind estrogen led to the suggestion that AFP plays a role in sexual differentiation of the brain by protecting the fetus from the effects of circulating estrogen (13). In addition to binding estrogen, AFP, like albumin, is able to bind other steroids as well as endogenous and exogenous substances such as fatty acids, bilirubin, and various pharmaceutical agents, suggesting that AFP may play a general transportation role (for review see ref. 14). Moreover, because cellular internalization of the protein has been reported, AFP could also interact with cytoplasmic chaperone proteins that normally escort nuclear receptors or transcription cofactors through the cytoplasm toward organelle interfaces (15, 16). AFP has also been proposed to be one protein that protects the embryo against the maternal immune system, on the basis of the observation that addition of purified AFP into the culture of splenic or lymph node mononuclear cells exerts a suppressive effect on antibody synthesis (17, 18). Because of its high level of expression during embryonic development, AFP has been assumed to be essential for mammalian development.
We have generated mice lacking AFP to assess its developmental role and physiological function in vivo. Our results show that AFP is not required for development but plays a critical and nonredundant role in the female reproductive system.
Methods
Generation of Mice Carrying a Germ-Line Mutation in the Afp Gene.
A 16-kb genomic fragment of the mouse Afp gene was isolated from a 129/CGR lambda library by using an Afp promoter fragment as a probe. The genomic insert was subcloned in pKIL-PCR2 (19). The targeting vector pAFP KO1 (pKO1) consists of two recombination arms. The 5′ arm (2.5 kb) was generated by PCR with the PFU polymerase (Stratagene), and the following primers: N-Mer1, agagcggccgcggaagtgacaaagcagaacc, annealing to the MerI sequence of the AFP enhancer 1 (20) and X-exon1, agactcgagggatgagggaagcgggtgtg, complementary to the Afp exon 1. pAFP KO2 (pKO2) differs from pAFP KO1 by the size of the 5′ recombination arm generated by PCR with N-Mer1 and the primer X-exon2, ctcgagtctaacgtggaagctgaaag, complementary to the Afp exon 2. The 3′ arm (used for the generation of both pAFP KO1 and pAFP KO2) was subcloned from lambda into pBSIIKS(+) vector (Stratagene). The 5′ recombination arm was introduced upstream of the 3′ recombination arm. The IRES (Internal Ribosome Entry Site) lacZ/Pgk neo reporter-selective cassette was introduced between the recombination arms. The tk2 negative selective marker was introduced into the SalI site to generate pAFP KO1 and pAFP KO2. These constructions were linearized with NotI and electroporated into E14 embryonic stem (ES) cells. Recombinant ES cells were selected in medium containing G418 and gancyclovir (for ES cell culture see ref. 21). Correctly targeted clones were identified by Southern blot analysis with an external probe from the 5′ region. In each targeting experiment 300 clones were screened; two clones were obtained with pAFP KO1 (Afptm1Ibmm) and three with pAFP KO2 (Afptm2Ibmm).
ES Cell Injections and Animal Genotyping.
Recombinant ES cells carrying the targeted allele were injected into C57BL/6J blastocysts; one clone was transmitted through the germ line for Afptm1Ibmm and three clones for Afptm2Ibmm. Animals were genotyped by Southern blot or PCR (22) using DNA extracted from tails as described (23). Male chimeras were mated with CD1 or C57BL6 females to introduce the mutant alleles onto different genetic backgrounds. Four and nine backcross generations were performed on each genetic background for the lines carrying the Afptm2Ibmm and the Afptm1Ibmm alleles, respectively. A consistent phenotype (see below) was observed for both AFP mutant alleles on all genetic backgrounds tested.
Embryo Implantations and Ovary Transfers.
Blastocysts were flushed from uteri 3.5 days after fertilization into phosphate-buffered medium 1 (24) and were immediately transferred individually into uteri of pseudopregnant recipients at 2.5 days postcoitum, as described (23). Twelve recipients were used, and nine pups were obtained. Reciprocal ovary transfers were performed by using a modification of the method described (23). Ovaries were carefully dissected from the bursa bilaterally of each female. Dissections were performed on two females simultaneously (one mutant and one wild type of similar age from the same breeding program); extracted ovaries were held in PBS with 10% FCS until required for transfer. Once recovered, the females were caged with wild-type males to test for fertility.
RNA Isolation, Northern Blot Analysis, and Quantitative RT-PCR.
Total RNA was isolated by using Trizol (GIBCO/BRL) extraction according to the manufacturer's instructions. For the Northern analyses 20 μg of total RNA was electrophoresed and transferred to nylon membranes; filters were hybridized with a 630-bp mouse Afp probe as described (22). Quantitative RT-PCR was performed on a Gene Amp 5700 cycler (Perkin–Elmer, Applied Biosystems), and the optimal primer concentrations were established for each transcript according to the TaqMan Universal protocol (Perkin–Elmer, Applied Biosystems). The rodent Gapdh primers and probe (VIC) supplied by the manufacturer were used as internal control for each sample. The primers used were: for Afp, primer 1, cttggtgaagcaaaagcctgaa and primer 2, ggaccctcttctgtgaaacagact; 6-FAM probe, cagccgccagctgctcctctgt; for Alb, primer 1, gcaaggctgctgacaagga and primer 2, ggcgtctttgcatctagtgaca; 6-FAM probe, acctgcttctcgactgagggtccaaac; for Alf, primer 1, cgtatgcgctcccatcagt and primer 2, gtcctccttacgaggctgaca; 6-FAM probe, tccgcacttgtatctgctttgcatacagac.
LacZ Reporter Gene Expression and Histological Analysis of Tissues.
To isolate embryonic stages, natural matings were set up, and the presence of a vaginal plug at noon the following day was taken as 0.5 day of gestation. Staged embryos were stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) as wholemounts as described (25). For cryostat sectioning, tissues were embedded in optimal cutting temperature (OTC) compounds (Miles), and sections stained for X-Gal were counterstained with haematoxylin and eosin and mounted. Tissues were dehydrated in ethanol, cleared in benzene, and finally embedded in paraffin, and sections were stained with haematoxylin and eosin for histological analysis.
Vaginal Smears Analysis, Superovulation, Hormonal Assays, and Injection.
Vaginal smears were taken daily from mutant and control mice over a period of at least 10 days. Ovulation was stimulated by i.p. injections of 5–7.5 units of pregnant mare's serum gonadotropin (Folligon; Intervet, Boxmeer, The Netherlands) followed by 5–7.5 units of human chorionic gonadotropin (Pregnyl, Organon) 48 h later. Serum estradiol and progesterone concentrations were assayed by competitive RIA (26, 27) according to classical procedures slightly modified for small volumes. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) concentrations were assayed with a time-resolved immunofluometric assay validated for mouse sera as published (28). To compare the hormonal levels between the mutant and normal mice, we used the Student's t test.
Results
Generation of Afp Null Alleles.
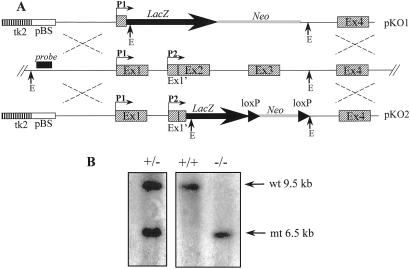
Fig. 1 shows the targeting strategy used to generate two different mutant alleles of the Afp gene. The lacZ reporter gene was introduced into the Afp locus either under the control of the P1 promoter alone (pKO1) or under the control of both P1 and P2 (pKO2) promoters to generate the Afptm1Ibmmand Afptm2Ibmm alleles, respectively (Fig. 1A). Targeting constructs were introduced into ES cells, and resultant G418 resistant colonies were screened by Southern blotting to detect homologous recombination events (Fig. 1B). ES cells heterozygous for each of the recombined Afp genes were injected into C57BL/6J blastocysts. Chimeric animals were obtained and mated to outbred CD1 mice to test for germ-line transmission and to C57BL/6J mice to maintain the targeted alleles on an inbred genetic background. Heterozygous mice were generated [identified by Southern blot (Fig. 1B) or PCR] and were viable and fertile (Table 1). The reporter gene activity was analyzed in heterozygous embryos, and identical expression patterns were observed with both mutant alleles. β-Galactosidase activity was detected in the expected embryonic tissues, namely liver and yolk sac visceral endoderm. In the adult, β-galactosidase activity was detected in a few rare cells of the liver and in some cells of the gut (data not shown). These animals may therefore be a useful model system for studying Afp gene regulation in vivo without the complicating position effects often observed with standard transgenic approaches (for review see ref. 29).
Figure 1.
Targeting strategy. (A) Structure of the mouse Afp genomic locus with the representation of the two promoters (P1 and P2) and the targeting vectors pAFP KO1 (pKO1) and pAFP KO2 (pKO2). The Afp exons are shown as dashed boxes. The Afp flanking probe used for Southern blotting is represented (black box). The EcoRI (E) site used to detect the polymorphism generated by the homologous recombination of pAFPKO1 and pAFPKO2 is indicated. LacZ indicates the IRES-LacZ cassette used as reporter, and Neo is the Pgk Neo selectable marker used to select for insertion of the vectors. The LoxP sites flanking the Neo cassette in pAFPKO2 are indicated. pBS represents the plasmidic pBSIIKS+ vector (Stratagene); tk2 is the thymidine kinase marker used to select for homologous recombination. (B) Example of Southern blot analysis of genomic DNA of wild-type, heterozygous (Afptm1Ibmm/+), and mutant Afptm1Ibmm/tm1Ibmm animals: the arrows point to the 9.5-kb wild-type (wt) and the 6.5-kb mutant (mt) bands.
Table 1.
Breeding results from intercrosses
| Strains | Parents
|
Offspring
|
|||
|---|---|---|---|---|---|
| Male | Female | +/+ | +/− | −/− | |
| CD1 | +/− | +/− | 166 (24%) | 374 (53%) | 163 (23%) |
| C57BL/6J | +/− | +/− | 19 (23%) | 43 (54%) | 19 (23%) |
Heterozygous males and females carrying either the Afptm1Ibmm or the Afptm2Ibmm allele were mated to analyze the viability of homozygous animals. This test was performed on two different genetic backgrounds: outbred CD1 and inbred C57BL/6J. The number of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) animals generated is indicated.
AFP Is Not Required for Embryonic Development.
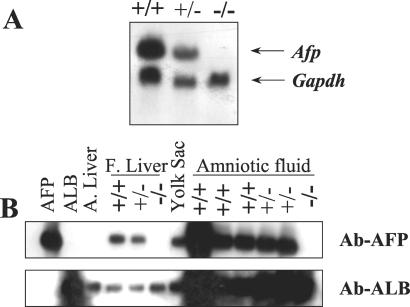
Intercross matings between heterozygotes gave rise to viable, apparently normal, homozygous mutant mice at the expected Mendelian ratio on both the outbred CD1 and the inbred C57BL/6J genetic backgrounds (Table 1). The sex ratio was also normal (784 animals of Table 1). We used both Northern and Western blotting to verify that the targeted insertions had generated null alleles. Afp transcripts were detected in RNA isolated from the liver of wild-type and heterozygous but not homozygous mutant embryos (Fig. 2A). AFP was detected in extracts from embryonic (day 16.5 postcoitum) liver and amniotic fluid of wild type and heterozygotes but not homozyote mutant embryos (Fig. 2B).
Figure 2.
(A) Northern blot analysis of intercross litters. Total RNA from 16.5 E embryos (genotyped by Southern blot) was analyzed by Northern blot. The mouse Afp probe detects a 2.2-kb transcript. A human GAPDH probe was used as loading control. (B) Western blot analysis with protein extracts from different tissues. Pure AFP, albumin (ALB), and adult liver (A. liver) were used as controls. Extracts were from wild-type (+/+), heterozygous (+/−), and Afptm1Ibmm/tm1Ibmm mutant (−/−) mice. Protein from fetal liver (F. Liver), yolk sac, and amniotic fluid were tested with a serum raised against AFP (Ab-AFP). The same blot was tested with a serum raised against albumin (Ab-ALB).
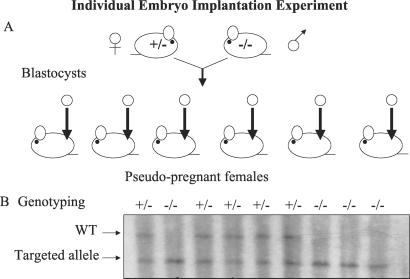
The fact that intercross matings produced homozygous mutant animals at the expected Mendelian ratio indicates that there is no reduction in the viability of Afp knockout mice in these conditions. However, it does not prove that AFP is dispensable for development because in litters derived from intercross matings the homozygous embryos develop in the presence of their AFP-producing wild-type and heterozygote littermates. AFP produced by these embryos is present in the maternal serum and could theoretically rescue the homozygous null embryos. However, AFP was not detected in the amniotic fluid of homozygous embryos (Fig. 2B), indicating that AFP is not efficiently transferred from one embryo to another via the maternal circulation. To determine whether embryonic development could proceed in the complete absence of AFP, homozygous mutant males and females were mated. No pups were ever obtained from these crosses, because, as shown below, the homozygous mutant females were infertile. Therefore, we had to perform single embryo transfer to assess the possible role of AFP in early development. Single blastocysts, which were derived from matings between homozygous mutant males and heterozygous females, were transplanted into the uterus of pseudopregnant females (Fig. 3). We demonstrated that both heterozygous and homozygous mutant male and female offspring could be produced. This finding clearly demonstrates that embryo-derived AFP is not essential for development. The phenotype of the mice obtained from these individual implantations was identical to that of homozygotes obtained from intercrosses (data not shown).
Figure 3.
Single embryo implantations. (A) Heterozygous Afptm1Ibmm/+ females and homozygous Afptm1Ibmm/tm1Ibmm mutant males were mated. Blastocysts were collected and implanted individually into pseudopregnant females. (B) Southern blot analysis of mice obtained from different females.
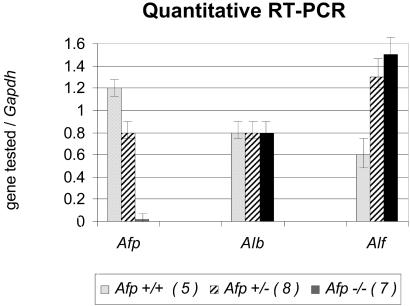
We then investigated whether AFP function during development could have been compensated in mutants, at least in part, by a higher level of expression of alpha-albumin or albumin, the most closely related members of the albumin family. We performed quantitative RT-PCR experiments on RNA samples from wild-type or mutant (Afptm1Ibmm/tm1Ibmm) embryonic liver and the albumin (Alb), alpha-albumin (Alf), and Afp transcripts (Fig. 4). We observed that the level of Alf transcript was increased in heterozygous and mutant samples, indicating that alpha-albumin could compensate for the absence of AFP during embryonic development and suggesting a possible dosage effect. However, this predicted compensation is not complete as the Afp mutant females are infertile, as discussed below.
Figure 4.
Quantitative RT-PCR on the different genes of the albumin family. Embryos (15.5 E) from intercrosses matings were dissected and genotyped, and total RNA from their livers was extracted. The concentration of Gapdh transcript was measured for each sample tested, and the ratio of the tested transcript [Afp, albumin (Alb), alpha-albumin (Alf)] on the Gapdh transcript was calculated. The relative amounts the three mRNAs tested is given for wild-type embryos (Afp+/+), heterozygous (Afp+/−), or homozygous (Afp−/−). The number of each sample tested for each genotype is indicated in parentheses.
Afp Null Females Are Infertile.
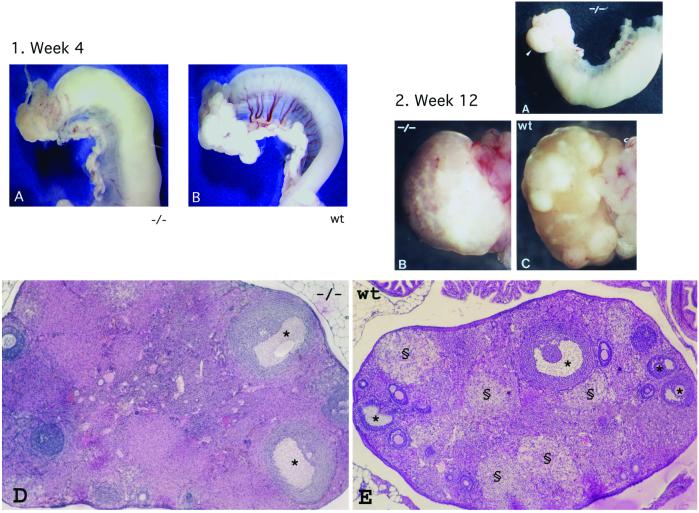
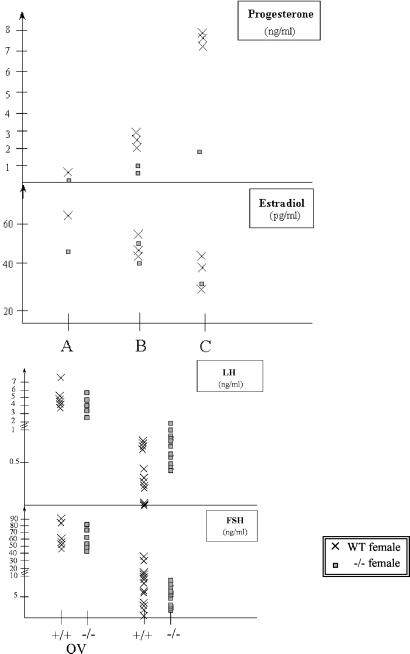
Homozygous mutant males or females were mated with wild-type animals. Males homozygous for an Afp disrupted allele were fertile and sired offspring. In contrast, no offspring were produced from homozygous females (Table 2), indicating that AFP is essential for the female reproductive system. No vaginal plugs were detected in mutant females, indicating that the origin of the observed infertility is caused by the absence of mating, possibly because of a deficit in the estrus cycle of the mutant females. Cytological analysis of vaginal smears was performed that indicated that mutant females had an abnormal estrus cycle (data not shown). In addition, a clear anatomical difference was detected between the ovaries of mutant and wild-type females (Fig. 5, see 2B and 2C): the smooth appearance of the surface of mutant ovaries suggested that they had not ovulated. This hypothesis was confirmed by the lack of corpora lutea in mature ovaries of mutant mice (Fig. 5, see 2D), and the absence of progesterone in these animals (Fig. 6). However, mutant ovaries did contain follicles at the different stages of maturation showing that the lack of AFP during development has no detrimental effect on female gametogenesis. The presence of preovulatory Graafian follicles (Fig. 5, see 2D) and the fact that estradiol levels were normal (Fig. 6) suggested that the pituitary gonadotropins FSH and LH are present.
Table 2.
Infertility phenotype of the Afp mutant females
| Parents
|
Offspring
|
|||
|---|---|---|---|---|
| Male | Female | +/+ | +/− | −/− |
| −/− | −/− (4) | 0 | 0 | 0 |
| −/− | +/+ (6) | 0 | 71 | 0 |
| +/+ | −/− (38) | 0 | 0 | 0 |
Afptm1Ibmm/tm1Ibmm or Afp Afptm2Ibmm/tm2Ibmm (−/−) homozygous animals were mated but no offspring were obtained. To test the fertility of Afp knockout (−/−) males and females, homozygous mutant males and females were mated with wild-type (+/+) animals. The number of matings for each test is indicated in parentheses.
Figure 5.
Anatomical and histological analysis of Afp mutant (Afptm1Ibmm/tm1Ibmm) ovaries and uteri of preburtal (week 4) and adult (week 12) mice. (1A) Uterine horn and ovary of a 4-week-old Afptm1Ibmm/tm1Ibmm mutant female. (1B) Uterine horn and ovary of a wild-type 4-week-old female. (2A) Uterine horn and ovary (arrowhead) of an adult Afptm1Ibmm/tm1Ibmm mutant female. (2B) Ovary from a 12-week-old Afptm1Ibmm/tm1Ibmm mutant female. (2C) Ovary from a 12-week-old wild-type female. The surface distortions caused by large corpora lutea are not observed in the Afptm1Ibmm/tm1Ibmm mutant female. (2D) General histological structure of an Afptm1Ibmm/tm1Ibmm mutant ovary (section from a 16-week-old female) showing that mature Graafian follicles (*) are present. (2E) At the same age, wild-type ovaries exhibit large corpora lutea (§), indicative of successful ovulation (these structures were never found in Afptm1Ibmm/tm1Ibmm mutant ovaries). (Magnifications: 1A and 2A, ×2.5; 2B and 2C, ×10; and 2D and 2E, ×25.)
Figure 6.
Hormonal levels. Each point corresponds to a single mouse. (Upper) Results of progesterone and estradiol assays in Afptm1Ibmm/tm1Ibmm mutant females and controls. Assays were performed on serum from different batches of females maintained for at least 6 weeks in three different cages (A, B, C). Note the lack of progesterone in the mutant mice; the difference with the control mice is significant (P = 0.05). (Lower) Results of gonadotropin (LH and FSH) measurements in wild-type and Afptm1Ibmm/tm1Ibmm mutant females ovarectomized (OV: first two series) or not ovarectomized (last two series). The difference in the LH levels is significant (P = 0.01), whereas that in the FSH levels is not (P = 0.16).
The anovulatory syndrome of the mutant females could be caused by either the lack of a signal needed to trigger ovulation or an intrinsic developmental or functional defect of the ovary. An equivalent number of blastocysts were observed in the uterus of mutant and wild-type littermates after artificial ovulation induction with gonadotropins (Table 3), indicating that the anovulation is not attributable to a defect at the ovarian level. However, this hormonal stimulation was not sufficient to restore fertility, as injected Afp−/− females mated with males never produced offspring despite the presence of vaginal plugs. We observed that the uterine horns of Afp−/− mice were swollen, suggesting that this tissue is sensitive to estrogen stimulation (Fig. 5, see 1A and 2A). This abnormality was detected both in prepubertal (4 weeks old) and adult (12 weeks old or more) mutant mice. Histological examination showed endometrial hyperplasia compatible with chronic hyperestrogenism. On the other hand, the uterine horns of 1-week-old mutant females had a normal appearance. AFP has been shown to inhibit the responsiveness of the immature mouse uterus to estrogens (30) and is present in significant amounts (>0.1 mg/ml) in the circulation of young mice up to the age of 2–3 weeks (31). Hyperstimulation of the uterus in the absence of AFP is thus not surprising and likely makes the uterus of mutant mice incompatible for embryo implantation.
Table 3.
Ovulation induction in Afptm1Ibmm/+ and Afptm1Ibmm/tm1Ibmm females
| Mice injected | Average no. of oocytes/mouse |
|---|---|
| Afptm1Ibmm/+ females (9 weeks old) (8) | 37 ± 5 |
| Afptm1Ibmm/tm1Ibmm females (9 weeks old) (10) | 31 ± 7 |
Afptm1Ibmm/tm1Ibmm and Afptm1Ibmm/+ females were hormonally treated to induce ovulation. The average number of postovulation oocytes obtained from females is shown. The number of females tested is indicated in parentheses.
The “extra-gonadal” cause of the infertility in Afp−/− females was then confirmed by reciprocal ovary transplantation experiments between wild-type and homozygous mutant females and subsequent matings with wild-type males. Whereas mutant females (n = 10) transplanted with wild-type ovaries did not produce offspring, wild-type females containing mutant ovaries (four of eight) gave birth to live pups. All offspring from these matings were genotyped as heterozygous, confirming that they indeed originated from the transplanted ovaries. These results demonstrated that the ovaries of Afp−/− females are functional but lack a signal required for ovulation. We therefore tested the levels of pituitary gonadotropins in mutant animals. LH and FSH are both present, but the LH/FSH balance is slightly abnormal in Afp−/− females; indeed the LH level is significantly higher compared with the controls (P = 0.008) (Fig. 6). Gonadotropin production is regulated by a negative feedback mechanism involving ovary-derived estrogen. This control is presumably absent in ovariectomized animals, leading to an increase of the gonadotropins in the serum (Fig. 6). Both FSH and LH levels increased in ovariectomized Afp−/− mice, indicating that the pituitary-hypothalamic system of the mutant mice is sensitive to the ovary negative feedback control.
Histological analysis of the pituitary-hypothalamic axis was performed. As expected, no gross morphological difference was observed between mutated and wild-type females (data not shown). We considered whether expression of the Afp gene could be detected in the hypothalamus or the pituitary and tested isolated wild-type tissues for the presence of Afp mRNA by RT-PCR, using primers for the two forms of AFP (8). No PCR product could be detected (whereas strong signals were obtained with the yolk sac or the fetal liver; data not shown), in agreement with earlier molecular hybridization results showing that AFP is not produced in the brain (32).
Injection of exogenous AFP (10 mg) into newborn (2, 3, or 4 days old) or adult mutant mice did not rescue the fertility defect (data not shown). Although it is possible that exogenous AFP cannot reach its target tissue or is not present in sufficiently high concentration levels, this result further supports the hypothesis that AFP is required for the functional development of the female reproductive system either prenatally or perinatally.
Discussion
The abundance of AFP during fetal life has led to the speculation that this serum protein is essential for mammalian embryo development and/or sexual differentiation. Surprisingly, homozygous Afp knockout mice are viable, and single blastocyst implantation experiments demonstrated that an embryo can indeed develop in the complete absence of embryonic AFP. An increase in alpha-albumin transcript in the Afp null animals supports the previously suggested compensation mechanisms between members of the albumin family (8). However, even if this is the case, alpha-albumin cannot completely compensate the lack of AFP as female Afp−/− mice are sterile.
The infertility phenotype is characterized by the absence of ovulation. Absence of ovulation has been observed in different gene knockout mice, and most often results from mutations affecting genes that are expressed in female reproductive tissues (for review see ref. 33). It has been proposed to divide these mouse models into two categories on the basis of whether prenatal or postnatal ovarian function is affected. Postnatal ovarian defects are further categorized on the basis of whether they are affected in initiation of follicle growth, preantral follicle growth, or ovulation and corpus luteum formation. Afp−/− females belong to the latter group. Ovary transplantation experiments clearly demonstrated that the anovulation of Afp−/− females is caused by an extra-gonadal defect. The relatively higher LH/FSH ratio indicates that these females suffer from a defect in the hypothalamic-pituitary system although no morphological differences in these tissues were observed. Among the various properties ascribed to AFP, its capacity to bind estrogen (30, 34) is obviously relevant to the observed phenotype. Early exposure to estrogen results in defeminization of female animals (such as female rats), characterized by anovulatory sterility associated with altered neuroendocrine production of gonadotropin (13, 35, 36). It is classically assumed that AFP protects the female fetusal brain from the effects of circulating estrogen (for review see ref. 15). An alternative view is that AFP has more than just a neuroprotective role and possibly serves as a specific estrogen transporter that could protect estrogen from degradation and/or actively transport estrogen into brain cells (32, 37). This hypothesis is partially based on the observation that estrogen antagonists can induce anovulatory sterility in postnatally treated female rats (37–39). Receptor-mediated endocytosis could allow cell type-specific delivery of AFP, in a similar manner to the renal uptake of the complex formed by the vitamin D binding protein (which is related to AFP) and vitamin D3 (40). AFP receptors have been described (41, 42) and specific uptake of AFP by some cells, especially developing neurons, has been reported (43, 44). This uptake probably explains the presence of AFP in neurons (45, 46), because AFP is not synthesized in the brain (ref. 32 and our results). Neonatal intracranial injections of AFP antibodies have been reported to abolish ovulation in the adult female mouse (47), suggesting that AFP is required within the brain to ensure female fertility (37, 39). In the absence of in situ synthesis, AFP must be internalized (44) in neurons, thereby importing estrogens. Although our study does not discriminate between the classical and alternative hypotheses, it demonstrates definitively that AFP is required for sexual differentiation of the female hypothalamic-pituitary axis. It is most likely that AFP acts by virtue of its capacity to bind estrogen, because the infertility phenotype of the Afp knockout mice resembles that of female animals exposed perinatally to estrogens or estrogen antogonists (13, 32, 38). As previously suggested (34, 37, 38), estrogen may be required for female sexual differentiation of the brain, and this requirement may be quantitative rather than qualitative. AFP may ensure this quantitative requirement by providing an “on call” source of estrogens (38).
Our results also show that the responses of the hypothalamic-pituitary axis either to the stimulatory (positive feedback) or the inhibitory (negative feedback) effect of estrogen are independently controlled. Indeed, the hypothalamic-pituitary axis of female Afp knockout mice is unable to display adequate gonadotropin production in response to circulating estrogen: estradiol levels are normal in mutant mice, the uterus of which is chronically stimulated, whereas the ovary is not. On the other hand, these mutant mice exhibit a normal rise of gonadotropin (LH and FSH) level in response to ovarectomy (Fig. 6 Lower), thereby showing that in these mice the hypothalamic-pituitary axis is sensitive to the negative feedback exerted by ovary-derived circulating estrogen. The two responses to estrogen are thus uncoupled in the Afp knockout mice, indicating that distinct mechanisms must operate in the female brain to control the positive and negative feedback exerted by ovary-derived estrogen, and that AFP is involved only in the former one.
In the polycystic ovary syndrome in humans, anovulation occurs with an associated increase in serum LH level and concomitantly reduction in FSH levels, which results in arrest of folliculogenesis at the preovulatory antral stage. The controversy in setting the diagnostic criteria for this syndrome is linked to the difficulties in pinpointing the pathophysiology of this type of anovulatory disorder, which could be of primary or secondary ovarian origin (48). Part of the syndrome could be originating from a deregulation at the level of the hypothalamo-pituitary axis by inappropriate steroid feedback regulation (49). The phenotype of the Afp mutant mouse suggests an additional line of investigation.
Acknowledgments
We are grateful to A. J. H. Smith for advice on targeting strategy and the selective cassette used in this work, K. Newton for ES cell injection, P. Delahaut and M. Dubois for steroid hormone assays, F. Van Laethem for the statistical analysis, and D. Peddie and M. Rivière for genotyping. We also acknowledge J. Balthazart and J. Bakker for thoughtful reading of the manuscript and suggestions. This work was supported by the Département des Relations Internationales, the Communauté Française de Belgique (ARC 00/05), the Région Wallonne (First Spin-Off 991/3942), the British Medical Research Council (to L.F. and A.W.), and the Biotechnology and Biological Science Research Council. T.V.R. was a fellow of the Fonds pour la Recherche et l'Industrie Agroalimentaire. C.S. is a Research Director of the Fonds National de la Recherche Scientifique.
Abbreviations
- AFP
alpha-fetoprotein
- ES
embryonic stem
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Andrews G K, Dziadek M, Tamaoki T. J Biol Chem. 1982;257:5148–5153. [PubMed] [Google Scholar]
- 2.Tilghman S M, Belayew A. Proc Natl Acad Sci USA. 1982;79:5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leighton P C, Kitau M J, Chard T, Gordon Y B, Leek A E. Lancet. 1975;7943:1012–1015. doi: 10.1016/s0140-6736(75)90295-0. [DOI] [PubMed] [Google Scholar]
- 4.Cuckle H S, Wald N J, Lindenbaum R H. Lancet. 1984;8383:926–929. doi: 10.1016/s0140-6736(84)92389-4. [DOI] [PubMed] [Google Scholar]
- 5.Tilghman S M. In: Oxford Survey on Eukaryotic Genes. Maclean N, editor. Oxford: Oxford Univ. Press; 1985. pp. 160–206. [PubMed] [Google Scholar]
- 6.Chen H, Egan J O, Chiu J-F. Crit Rev Eukaryotic Gene Expression. 1997;7:11–41. doi: 10.1615/critreveukargeneexpr.v7.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 7.Scohy S, Gabant P, Szpirer C, Szpirer J. Nucleic Acids Res. 2000;28:3743–3751. doi: 10.1093/nar/28.19.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camper S A, Tilghman S M. Genes Dev. 1989;3:537–546. doi: 10.1101/gad.3.4.537. [DOI] [PubMed] [Google Scholar]
- 9.Deutsch H F. Adv Cancer Res. 1991;56:253–312. doi: 10.1016/s0065-230x(08)60483-2. [DOI] [PubMed] [Google Scholar]
- 10.Abelev G I, Eraiser T L. Semin Cancer Biol. 1999;9:95–107. doi: 10.1006/scbi.1998.0084. [DOI] [PubMed] [Google Scholar]
- 11.Leffert H L, Sell S J. Cell Biol. 1974;61:823–829. doi: 10.1083/jcb.61.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sell S, Skelly H, Leffert H L, Muller-Eberhard U, Kida S. Ann NY Acad Sci. 1975;259:45–58. doi: 10.1111/j.1749-6632.1975.tb25401.x. [DOI] [PubMed] [Google Scholar]
- 13.MacLusky N J, Naftolin F. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie J R, Uversky V N. Biochim Biophys Acta. 2000;1480:41–56. doi: 10.1016/s0167-4838(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 15.Mizejewski G J. In: Alpha-Fetoprotein and Congenital Disorder. Mizejewski G J, Porter I H, editors. Orlando, FL: Academic; 1985. pp. 5–34. [Google Scholar]
- 16.Mizejewski G J. Life Sci. 1995;56:1–9. doi: 10.1016/0024-3205(94)00401-d. [DOI] [PubMed] [Google Scholar]
- 17.Belanger L, Waithe W I, Daguillard F, Larochelle J, Dufour D. In: Alpha-Fetoprotein. Masseyeff R, editor. Paris: Institut National de la Santé et de la Recherche Médicale; 1974. pp. 423–440. [Google Scholar]
- 18.Tomasi T B. Annu Rev Med. 1977;28:453–465. doi: 10.1146/annurev.me.28.020177.002321. [DOI] [PubMed] [Google Scholar]
- 19.Gabant P, Dreze P-L, Van Reeth T, Szpirer J, Szpirer C. BioTechniques. 1997;23:938–941. doi: 10.2144/97235pf01. [DOI] [PubMed] [Google Scholar]
- 20.Godbout R, Ingram R S, Tilghman S M. Mol Cell Biol. 1988;8:1169–1178. doi: 10.1128/mcb.8.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith A G. J Tissue Culture Methods. 1991;13:89–94. [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 23.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 24.Whittingham D G, Wales R G. Aust J Biol Sci. 1969;22:1065–1068. doi: 10.1071/bi9691065. [DOI] [PubMed] [Google Scholar]
- 25.Forrester L M, Nagy A, Sam M, Watt A, Stevenson L, Bernstein A, Joyner A L, Wurst W. Proc Natl Acad Sci USA. 1996;93:1677–1682. doi: 10.1073/pnas.93.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ectors F, Beckers J F, Ballman P, Derivaux J. C R Acad Sci Paris. 1975;281:1257–1260. [PubMed] [Google Scholar]
- 27.Backers J F, Ballman P, Ectors F, Derivaux J. C R Acad Sci Paris. 1975;280:335–338. [PubMed] [Google Scholar]
- 28.Van Casteren J I, Schoonen W G, Kloosterboer H J. Biol Reprod. 2000;62:886–894. doi: 10.1095/biolreprod62.4.886. [DOI] [PubMed] [Google Scholar]
- 29.Spear B T. Semin Cancer Biol. 1999;9:109–116. doi: 10.1006/scbi.1998.0087. [DOI] [PubMed] [Google Scholar]
- 30.Mizejewski G J, Vonnegut M, Jacobson H I. Proc Natl Acd Sci USA. 1983;80:2733–2737. doi: 10.1073/pnas.80.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson M, Lindahl G, Ruoslahti E. J Exp Med. 1977;145:819–827. doi: 10.1084/jem.145.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toran-Allerand C D. Progr Brain Res. 1984;61:63–98. doi: 10.1016/s0079-6123(08)64429-5. [DOI] [PubMed] [Google Scholar]
- 33.Elvin J A, Matzuk M M. Rev Reprod. 1998;3:183–195. doi: 10.1530/ror.0.0030183. [DOI] [PubMed] [Google Scholar]
- 34.Uriel J, Buoillon D, Aussel C, Dupiers M. Proc Natl Acad Sci USA. 1976;73:1452–1456. doi: 10.1073/pnas.73.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorski R A. J Anim Sci. 1985;61:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- 36.Crowley W R, Kalra S P. Brain Res. 1994;663:257–265. doi: 10.1016/0006-8993(94)91271-8. [DOI] [PubMed] [Google Scholar]
- 37.Döhler K D. Int Rev Cytol. 1991;131:1–57. doi: 10.1016/s0074-7696(08)62016-1. [DOI] [PubMed] [Google Scholar]
- 38.Döhler K D, Hancke J L, Srivastava S S, Hofmann C, Shryne J E, Gorski R A. Progr Brain Res. 1984;61:99–117. doi: 10.1016/S0079-6123(08)64430-1. [DOI] [PubMed] [Google Scholar]
- 39.Döhler K D, Coquelin A, Davis F, Hines M, Shryne J E, Sickmoller P M, Jarzab B, Gorski R A. Neuroendocrinology. 1986;42:443–448. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- 40.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen E I, Willnow T E. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 41.Torres J M, Laborda J, Naval J, Darracq N, Calvo M, Mishal Z, Uriel J. Mol Immunol. 1989;26:851–857. doi: 10.1016/0161-5890(89)90141-7. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Zeng C Q, Alpert E. J Clin Invest. 1992;90:1530–1536. doi: 10.1172/JCI116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uriel J, Villacampa M J, Moro R, Naval J, Failly-Crepin C. Cancer Res. 1984;44:5314–5319. [PubMed] [Google Scholar]
- 44.Toran-Allerand C D. Neurosci Lett. 1987;83:35–40. doi: 10.1016/0304-3940(87)90212-6. [DOI] [PubMed] [Google Scholar]
- 45.Trojan J, Uriel J. Oncodev Biol Med. 1980;1:107–111. [PubMed] [Google Scholar]
- 46.Toran-Allerand C D. Nature (London) 1980;286:733–735. doi: 10.1038/286733a0. [DOI] [PubMed] [Google Scholar]
- 47.Mizejewski G J, Vonnegut M. Teratology. 1982;25:351–360. doi: 10.1002/tera.1420250312. [DOI] [PubMed] [Google Scholar]
- 48.Dewailly D. Hum Fertil. 2000;3:73–76. doi: 10.1080/1464727002000198721. [DOI] [PubMed] [Google Scholar]
- 49.Lobo R A, Granger L, Goebelsmann U, Mishell D R., Jr J Clin Endocrinol Metab. 1981;52:156–158. doi: 10.1210/jcem-52-1-156. [DOI] [PubMed] [Google Scholar]