Abstract
Catastrophic population collapses such as observed in many exploited fish populations have been argued to result from depensatory growth mechanisms (i.e., reduced reproductive success at low population densities, also known as Allee effect). Empirical support for depensation from population-level data is, however, hard to obtain and inconclusive. Using a size-structured, individual-based model we show that catastrophic population collapses may nonetheless be an intrinsic property of many communities, because of two general aspects of individual life history: size- and food-dependent individual growth and individual mortality decreasing with body size. Positive density dependence, characteristic for depensatory growth mechanisms and catastrophic behavior, results as a direct and robust consequence of the interplay between these individual life-history traits, which are commonly found in many species.
Recent studies on lakes, coral reefs, oceans, forests, and arid lands document drastic changes in the composition of the biological community and the functioning of the ecosystem (1, 2). One view is that these abrupt changes represent catastrophic shifts between contrasting states of the system that are both stable equilibria (1, 2). The collapse of several exploited fish stocks, which fail to recover after over-fishing and a closure of fisheries, could be interpreted as such a shift, if the community state without the exploited fish species represents a stable equilibrium (2–4). If the interpretation in terms of alternative stable equilibria is correct, our view of how these ecological systems function and how to manage them is heavily affected (5). Models of biological communities show that the extinct state may be stable if the exploited species exhibits a reduced population growth capacity at low densities (2, 6). Such depensatory growth (also known as Allee effect, positive or inverse density-dependence) will prevent rebound of the population after a crash. Population-level data do not unambiguously support or refute the presence of depensatory mechanisms in natural systems (6–9). Estimates of spawner abundance and the number of surviving progeny for 128 fish stocks indicated only 3 stocks with significant depensation (7). More advanced statistical testing of these stock-recruitment relations showed that the probability of depensation was nonetheless significant (8). Also, 15 years after a collapse of the population most of the exploited fish stocks have yet to recover. Assuming that management strategies have been implemented to reduce fishing mortality after a collapse, such a lack of recovery indicates a reduced capacity to rebound from low densities (9).
Community models incorporating depensatory mechanisms do so as an assumption (7), which is justified by verbal arguments. For example, it is argued that depensatory growth may result from adult individuals negatively affecting competitors of their own juveniles (6), from reduced cooperative behavior at low densities (10), or predator swamping at high density (11). These arguments all reflect assumptions about processes at the level of ensembles of individuals or the population as a whole. Moreover, their representation in the community model is generally phenomenological. As such, they do not provide a stringent, mechanistic link between depensatory growth and the life history and behavior of single individuals. The extent to which they can inform us about the likelihood of depensatory growth in natural systems is hence limited.
Using a size- and individual-based model, we show that two general characteristics of the individual life history of many consumer species, namely that individual growth depends on body size as well as on the amount of food ingested and that individual mortality decreases with body size, naturally result in depensatory growth of their predators at the population level and thus predict that the possibility of catastrophic population collapses of these predators is the rule rather than the exception. These individual life history traits are very common: Feeding rate is known to affect growth rates and to increase with body size in many species (12, 13). Mortality often decreases with size, with the highest mortality occurring among small or young individuals (so-called Type III survival curves; ref. 14). Type III survival curves are common among fish species that produce millions of eggs of which only few survive to adulthood (15, 16), but are also found in many other species, both aquatic (17) and terrestrial (18). The use of a size- and individual-based model ensures that resulting, population-level phenomena are direct consequences of individual life-history traits. Hence, our results offer an explanation for the occurrence of depensatory growth as opposed to assuming its presence a priori.
The Model
We investigated the consequences of size-dependent foraging and a type III survival pattern in a food chain model consisting of an unstructured predator that forages on a size-structured consumer population, which, in turn, feeds on an unstructured basic resource. This model represents the simplest ecosystem structure, in which the interplay between food-dependent growth and size-dependent mortality of a consumer population can be analyzed. Nonetheless, the results of this simple food chain model are robust and generalize to more complex situations (see below). The model was parameterized to mimic life history characteristics of roach (Rutilus rutilus; ref. 19), a common Eurasian freshwater planktivorous fish species feeding on a cladoceran zooplankton, Daphnia spp. Parameter values for the predator are based on life history relationships for perch (Perca fluviatilis), assuming an average individual length of 200 mm (19). Based on the rich empirical literature on size-dependent mortality in fish (15, 16), we assumed that small consumers experienced an additional, predator-induced mortality risk in addition to a low, size-independent background mortality.
We modeled the life history of individual consumers by specifying their feeding, growth, reproduction, and mortality rate as a function of their length ℓ, the resource biomass density R, and the predator density P. The functions g(R, ℓ), b(R, ℓ), and I(R, ℓ), which depend on consumer length ℓ and resource biomass R, denote growth, reproduction, and feeding rate, respectively. Their functional form is based on a simple budget model describing consumer energetics (20). Derivations of these functions from the budget model have been presented elsewhere (20, 21). In short, individual consumers are born at length ℓb, mature on reaching length ℓj, and may reach the maximum length ℓm under very high food conditions. Resource ingestion of consumers is assumed proportional to their squared length with proportionality constant Im and follows a type II functional response to resource biomass: I(R, ℓ) = Imℓ2R/(Rh + R) with half-saturation constant Rh. A fixed fraction of ingested food is channeled to reproduction, while the remainder is spent on growth plus maintenance. Maintenance takes precedence over growth, which hence follows a von Bertalanffy growth law: g(R, ℓ) = γ(ℓmR/(Rh + R) − ℓ), with ℓm and γ representing the maximum length under very high food densities and the growth rate, respectively. After reaching the maturation length ℓj, individuals produce offspring at a rate b(R, ℓ) = rmℓ2R/(Rh + R) with proportionality constant rm. Consumer mortality equals the sum of the background mortality μ and the predator-induced mortality d(P). Predation mortality is assumed larger than 0 only for individuals below a vulnerability threshold ℓv.
Predators follow simple Lotka-Volterra dynamics, experiencing a background mortality rate δ, while foraging on consumers with length between ℓb and ℓv with attack rate a, handling time Th, and conversion efficiency ɛ. The variable B denotes the biomass of the consumers with length between ℓb and ℓv that are vulnerable to predation. This biomass can be computed as an integral over the consumer size distribution c(t, ℓ), weighted by the consumer length-weight relation:
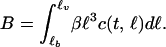 |
1 |
Individual consumer biomass is assumed proportional to cubed length with proportionality constant β. The intake rate of consumer biomass by a single predator individual thus equals aB/(1 + aThB). Likewise, predation mortality of consumers with length between ℓb and ℓv follows:
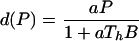 |
2 |
while d(P) = 0 for larger individuals. Total juvenile and adult biomass can also be computed as an integral of the biomass-size distribution βℓ3c(t, ℓ) over the size ranges ℓb to ℓj and ℓj to ℓm, respectively. Resource regrowth is assumed to follow semichemostat dynamics (21, 23) with maximum resource biomass density K and flow-through rate ρ.
All assumptions about consumers, discussed above, pertain to their individual life history. Without making any further assumptions—i.e., without assuming a population-level Allee effect—the size-structured, food chain model can be derived by bookkeeping only (22). It is described by the following set of equations:
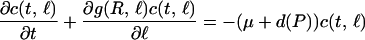 |
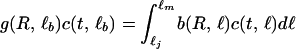 |
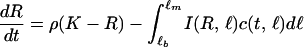 |
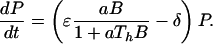 |
[3]
Equilibrium conditions for this size-structured model can be explicitly derived (22) and have been used in numerical computations of the predator density and the biomass densities of resource, juvenile, and adult consumers at equilibrium. Unless stated otherwise, we have used as default parameters: ℓb = 7 mm, ℓv = 27 mm, ℓj = 110 mm, ℓm = 300 mm, Im = 1.0 × 10−4 g/day/mm2, Rh = 1.5 × 10−5 g/liter, rm = 0.003/day/mm2, γ = 0.006/day, μ = 0.01/day, β = 9.0 × 10−6 g/mm3, ρ = 0.1/day, K = 0.0003 g/liter, ɛ = 0.5, a = 5,000 liter/day, Th = 0.1 day/g, and δ = 0.01/day. These parameters mimic the life history characteristics of roach (R. rutilus) (19), feeding on Daphnia spp., while being predated by perch (P. fluviatilis).
Results
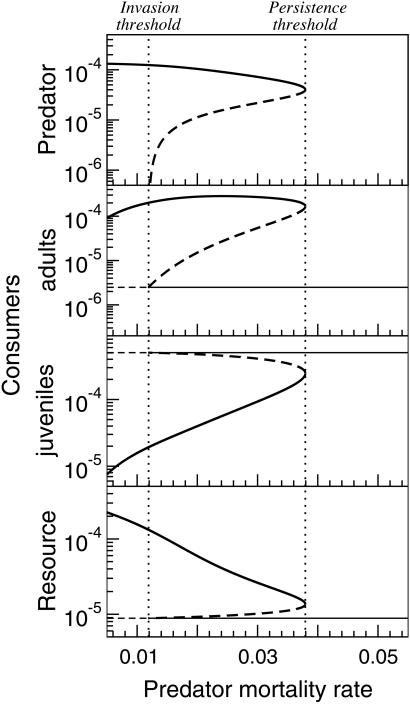
We computed the equilibria of the size-structured model (Eq. 3) for different values of the predator mortality δ (see Fig. 1). Two types of equilibria occur: consumer–resource equilibria without predators and equilibria in which all three species have nonzero densities. The curve, representing consumer–resource equilibria without predators, is independent of predator mortality. The curve relating the three-species equilibrium to predator mortality turns out to be folded and intersects the curve of the consumer–resource equilibria at a particular value of δ (roughly 0.012 for the default parameters used in Fig. 1). This value of δ we will refer to as the “invasion threshold,” because it marks the critical value of mortality, below which predators can invade and establish themselves in an equilibrium consumer–resource system, irrespective of their initial density. The consumer–resource equilibrium thus represents a stable equilibrium for δ values above the invasion threshold, while it is unstable for δ values below it. From the invasion threshold, where the predator density in the three-species equilibrium approaches 0, the three-species equilibrium curve folds to higher values of δ, reaching a turning point at a value of δ = 0.038 for the default parameters used in Fig. 1. This critical value of δ we will refer to as the “persistence threshold,” because it marks the boundary between values of δ, for which a three-species equilibrium is possible or not. For δ values between the invasion and the persistence threshold both a three-species equilibrium with a low and with a high predator density occur. This range of δ values will be referred to as the “bistability range.” Numerical simulations have shown that only the high-predator equilibrium is stable and that large-amplitude, predator–prey cycles or other types of complex dynamics do not occur because of the size-refuge from predation for large consumers (results not shown).
Figure 1.
Variation in predator density (top panel; individuals per liter), adult and juvenile consumer biomass (second and third panels; g/liter), and resource biomass (bottom panel; g/liter) as a function of predator mortality rate (day−1) in the size-structured food chain model. Thin lines, equilibria with only consumers and basic resource; thick lines, equilibria with all three trophic levels; solid lines, stable equilibria; dashed lines, unstable equilibria. Alternative, stable equilibria with and without predators occur between the invasion and persistence threshold (vertical dotted lines).
Fig. 1 clearly illustrates the consequences of size-dependent consumer growth and size-specific predation mortality for the equilibria that can occur in the size-structured food chain. It could represent the response of the ecological system to increased harvesting or fishing intensity on the predator species. At one and the same predator mortality rate within the bistability range, the system can either be in a consumer–resource equilibrium or in a three-species equilibrium. This co-occurrence of stable equilibria with and without predators is possible over a considerable range of mortalities. At the persistence threshold a small increase in predator mortality causes a catastrophic extinction of the predator population without any chance of recovery, as well as a substantial decrease in adult consumer biomass, while juvenile consumer biomass substantially increases. Predators hence significantly increase adult and decrease juvenile consumer biomass. For mortality rates within the bistability range, the predator exhibits an Allee effect, because initial predator populations of low abundance cannot invade the consumer–resource community while more abundant ones do. The predator persistence threshold occurs at a mortality rate that is more than three times higher than its invasion threshold. Once present, predators can thus persist at mortality rates far above their invasion threshold. In addition, if predators have gone extinct because of too high a mortality rate, this rate has to be decreased substantially for predators to reestablish themselves in the system. Fig. 1 shows that an Allee effect (or depensatory growth) and the possibility of a catastrophic population collapse follows directly from the interplay of the individual life-history characteristics of size-dependent consumer growth and size-selective predation mortality without making an explicit assumption about positive density dependence to occur at low population densities.
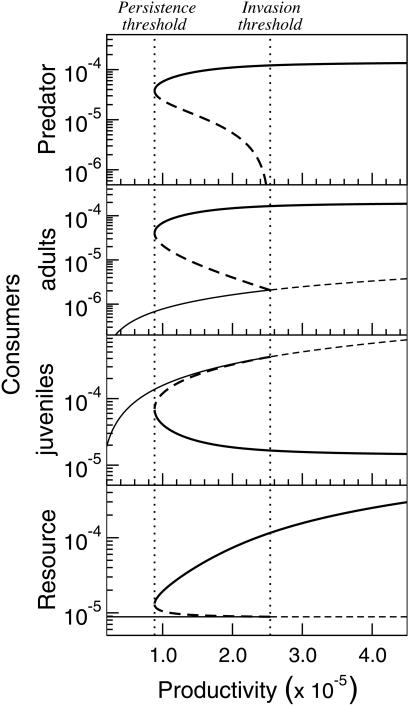
The bistability also occurs for a fixed predator mortality rate when the productivity of the system, defined as the product ρK, is varied (Fig. 2). The bistability regime spans a significant range of productivity levels, again bounded by the predator invasion and persistence threshold. Now, predators can always invade a consumer–resource equilibrium if the system productivity is above the invasion threshold and will always go extinct if it is below the persistence threshold (Fig. 2). The predator persistence threshold is located at a productivity level much smaller (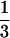 ) than the productivity above which predators can invade the consumer–resource equilibrium. As before, with predators present the biomass density of adult consumers is more than an order of magnitude larger than in their absence, whereas juvenile consumer biomass is significantly lower. This pattern contrasts with predictions of classical food chain models, which only account for the number or biomass of individuals and which form the basis of most current theories about biological communities. Such unstructured models do not allow for alternative equilibria and predict that the number of trophic levels changes only at invasion thresholds, where food density for a higher trophic level becomes sufficiently abundant to persist (24, 25). Therefore, in such models the invasion and persistence thresholds coincide and predators are hence predicted to be always present above their invasion threshold and always go extinct below it (24, 25). Moreover, beyond its invasion threshold the abundance of the highest trophic level is predicted to increase monotonically, as opposed to the jump in abundance occurring at the invasion threshold in the size-structured model. By varying both productivity and predator mortality we found that the range of mortalities for which bistability occurs expands proportionally with increasing resource productivity. The likelihood that alternative stable states occur because of size-selective predation may therefore be significantly higher in more productive systems.
) than the productivity above which predators can invade the consumer–resource equilibrium. As before, with predators present the biomass density of adult consumers is more than an order of magnitude larger than in their absence, whereas juvenile consumer biomass is significantly lower. This pattern contrasts with predictions of classical food chain models, which only account for the number or biomass of individuals and which form the basis of most current theories about biological communities. Such unstructured models do not allow for alternative equilibria and predict that the number of trophic levels changes only at invasion thresholds, where food density for a higher trophic level becomes sufficiently abundant to persist (24, 25). Therefore, in such models the invasion and persistence thresholds coincide and predators are hence predicted to be always present above their invasion threshold and always go extinct below it (24, 25). Moreover, beyond its invasion threshold the abundance of the highest trophic level is predicted to increase monotonically, as opposed to the jump in abundance occurring at the invasion threshold in the size-structured model. By varying both productivity and predator mortality we found that the range of mortalities for which bistability occurs expands proportionally with increasing resource productivity. The likelihood that alternative stable states occur because of size-selective predation may therefore be significantly higher in more productive systems.
Figure 2.
Variation in predator density (top panel; individuals per liter), adult and juvenile consumer biomass (second and third panels; g/liter), and resource biomass (bottom panel; g/liter) as a function of system productivity (g/liter/day) in the size-structured food chain model. Line styles as in Fig. 1.
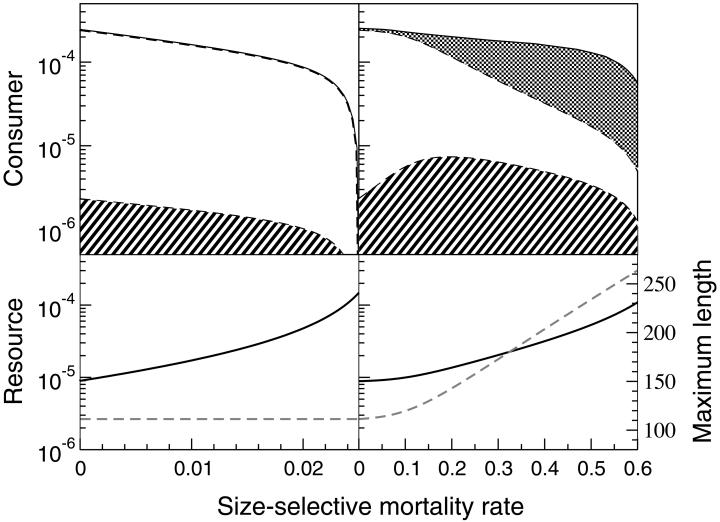
We further studied the mechanisms behind the bistability by analyzing the consumer–resource system without the predator, in which d(P) was replaced by a constant mortality rate v for consumers with length between length at birth ℓb and the vulnerability threshold ℓv in addition to the low, size-independent background mortality that all consumers are subjected to. Increasing the additional mortality rate v of small individuals relieves the competitive pressure among consumers, such that resource levels, maximum individual size, and adult biomass increase, whereas the biomass of nonvulnerable juveniles strongly decreases (Fig. 3). Interestingly, the additional mortality on small, vulnerable juveniles significantly increases their total biomass. The faster individual growth and increased fecundity, which results from the higher resource levels, more than compensate for the additional mortality. If a constant length–age relation is assumed and higher resource levels do not increase individual growth, the maximum size-selective mortality that the consumer population can sustain, is more than an order of magnitude lower than in the presence of a growth response (Fig. 3). Furthermore, without a growth response vulnerable juvenile and adult biomass only decrease with increasing size-selective mortality. For the increase in vulnerable juvenile biomass with increasing size-selective mortality, a resource-dependent growth response is therefore a prerequisite.
Figure 3.
Biomass variation in the size-structured consumer–resource system as a function of size-selective mortality rate (day−1). (Upper) Total consumer biomass (g/liter) and its subdivision over vulnerable juveniles (hatched), invulnerable juveniles (white) and adults (speckled). (Lower) Resource biomass (g/liter; solid line) and maximum individual length (mm; dashed line). (Left) Results for the case of a constant length-age relation, in which increases in resource density do not increase individual growth and the maximum length thus remains constant. (Right) Results for the case of a resource-dependent length–age relation, in which increases in resource densities induce increases in both individual growth and maximum length. K = 0.00015 g/liter; all other parameters have their default value.
This analysis makes clear that at predator densities close to 0 a positive relationship will result between the abundance of predators and the abundance of their food, yielding a higher per-capita growth performance, as long as an increasing number of predators induce higher size-selective consumer mortality. Any predator that selectively exploits small prey individuals may thus increase the availability of its preferred prey by just feeding on them. Note that the occurrence of this Allee effect is independent of the type of predator functional response—i.e., how d(P) changes with prey density. Qualitatively, the Allee effect will be present irrespective of predators having a type I, type II, or type III functional response, as long as d(P) increases with P at low predator density. Quantitatively, the predator functional response does have a considerable influence on the extent of the bistability region: a type I predator functional response (setting Th equal to 0 in Eqs. 2 and 3) yields results that are graphically indistinguishable from those presented in Fig. 1. On the other hand, with a type III functional response we have generally found a much larger region of bistability with the persistence threshold occurring at a mortality level that is roughly an order of magnitude higher than the invasion threshold. For example, with the functional response following aB2/(1 + aThB2), a = 1.0 × 109 liters2/g/day, and Th = 0.1 day/g the invasion threshold occurs at δ ≈ 0.012, as in Fig. 1, but the persistence threshold occurs at δ ≈ 0.12. A type III functional response hence significantly increases the extent of the Allee effect, even though it is not crucial or responsible for its occurrence. We have obtained comparable results in models, involving a size-structured consumer and a size-structured predator with predation risk determined by the ratio between predator and prey body size, which shows that our findings also do not crucially depend on the assumption of a step function in predation vulnerability, used in this study for simplicity. The bistability and the associated catastrophic behavior are therefore general and robust consequences, resulting solely from the interplay of size-/food-dependent growth and inverse size-dependent predation mortality of any consumer species.
Discussion
We have shown that the interplay between the food-dependent growth and size-selective predation, which characterize the life history of many consumer species, leads to a positive feedback or inverse density-dependence in predator–consumer–resource systems. Size-selective predators induce a shift in the size-structure of the consumers, resulting in higher biomass densities of vulnerable juveniles and adults, whereas densities of intermediately sized individuals decrease strongly (Fig. 3). With predators present, the consumer size-distribution is more bimodal, such that the maximum and average size of adult consumers is higher. Despite the fact that adults are more abundant, the predator-induced shift from invulnerable to vulnerable juveniles leads to a strong (in our model 4-fold) decrease in the average size when measured over all consumers. The positive feedback of the size-selective predation mortality is key to the alternative equilibria occurring in our size-structured food chain model.
In agreement with observations on stunted populations with limited maximum individual length (26, 27), our results suggest that size-selective predation may relieve competition within a population and reduce its stuntedness. However, this effect comes at the price of introducing a breakpoint, where the system exhibits a catastrophic collapse from an equilibrium with predators to an equilibrium without. If predators go extinct because of, for example, overharvesting, mortality may have to be reduced to background levels for them to recover. In case of a type III functional response this might require an order of magnitude reduction in mortality. These model predictions are in agreement with observations on cod and its main prey species, adult capelin (Mallotus villosus), in the Northwest Atlantic. After the collapse of the cod population in the 1990s, dramatic changes in capelin stocks have occurred, including an increased abundance and a reduced individual growth. As a consequence, adult capelin was significantly smaller in the 1990s than in the 1980s, when cod was still present (4). A quantitative analysis has shown that natural mortality due to predation by seals, whales, and piscivorous fish (cod, halibut, plaice, salmon) decreased with the disappearance of cod, while commercial exploitation has not been a serious factor (4). In addition, zooplankton abundance has been lower in the 1990s, suggesting an increase in intraspecific competition among capelin. Altogether the changes in capelin, zooplankton, and also phytoplankton abundance have been attributed to a “trophic cascade.” Four of the observed changes that have occurred after the collapse of the cod population are predicted by the size-structured model analyzed in this paper: the higher capelin abundance, the decrease in zooplankton abundance due to intraspecific competition, and the reductions in individual growth and adult sizes. On the basis of our results, the lack of recovery in the cod population is to be expected if cod mortality is still above background levels because of bycatches. The model suggests the counterintuitive measure of imposing additional mortality on small capelin to promote recovery of the cod population.
Our results may also explain the outcome of predator-removal experiments in which a lack of predator recovery has been attributed to the increase in average consumer size after predators were removed (28, 29). The size-structured food chain model predicts such an increase in average size following predator extinction, when measured over all consumers. As another implication, our findings add new arguments to the debate as to whether top predators should be culled for the sake of fisheries on intermediate consumer species (30). Given the beneficial effect of size-selective predation on consumer size-structure—i.e., the decrease in densities of intermediately sized individuals and the increase in adult consumer biomass—culling a top predator species that is strongly size-selective might only have negative effects on fisheries yield, because it will increase intraspecific competition and hence lead to more stunted consumer populations.
Our results show that two widespread traits of individual life history suggest that catastrophic population collapses may be an intrinsic property of many biological communities. The results provide an individual-level explanation for the depensatory growth mechanism or Allee effect that may be behind the observed catastrophic collapses of top predators (4, 9) and may have prevented the re-invasion of predators in removal experiments (28, 29). In a more general context, they show that a food-web theory, based on individual life history and individual variation, may differ substantially from current theories about biological communities, because accounting for two of the most basic, size-dependent ecological processes already induce qualitatively different community patterns. Most importantly, size-dependent ecological interactions among individuals may substantially increase the likelihood that biological communities occur in alternative stable states with very different size structures of the constituting populations. This, in turn, has far-reaching implications for the dynamics of ecological systems and their management.
Acknowledgments
A. Janssen, E. McCauley, M. Sabelis, and S. Carpenter made helpful comments on the manuscript. This research was funded by the Netherlands Organization for Scientific Research, the Swedish Natural Research Council, and the Swedish Council for Forestry and Agricultural Sciences.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Scheffer M, Carpenter S, Foley J A, Folke C, Walker B. Nature (London) 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter S. Ecology. 2002;83:2069–2083. [Google Scholar]
- 3.Post J R, Sullivan M, Cox S, Lester N P, Walters C J, Parkinson E A, Paul A J, Jackson L, Shuter B J. Fisheries. 2001;27:6–17. [Google Scholar]
- 4.Carscadden J E, Frank K T, Leggett W C. Can J Fish Aquat Sci. 2001;58:73–85. [Google Scholar]
- 5.May R M. Nature (London) 1977;269:471–477. [Google Scholar]
- 6.Walters C, Kitchell J F. Can J Fish Aquat Sci. 2001;58:39–50. [Google Scholar]
- 7.Myers R A, Barrowman N J, Hutchings J A, Rosenberg A A. Science. 1995;269:1106–1108. doi: 10.1126/science.269.5227.1106. [DOI] [PubMed] [Google Scholar]
- 8.Liermann M, Hilborn R. Can J Fish Aquat Sci. 1997;54:1976–1984. [Google Scholar]
- 9.Hutchings J A. Nature (London) 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- 10.Courchamp F, Clutton-Brock T, Grenfell B. Trends Ecol Evol. 1999;14:405–410. doi: 10.1016/s0169-5347(99)01683-3. [DOI] [PubMed] [Google Scholar]
- 11.Stephens P A, Sutherland W J. Trends Ecol Evol. 1999;14:401–405. doi: 10.1016/s0169-5347(99)01684-5. [DOI] [PubMed] [Google Scholar]
- 12.Peters R H. The Ecological Implications of Body Size. Cambridge, U.K.: Cambridge Univ. Press; 1983. [Google Scholar]
- 13.Sebens K P. Annu Rev Ecol Syst. 1987;18:371–407. [Google Scholar]
- 14.Pianka E R. Evolutionary Ecology. 4th Ed. New York: Harper & Row; 1987. [Google Scholar]
- 15.Winemiller K O, Rose K A. Can J Fish Aquat Sci. 1992;49:2196–2218. [Google Scholar]
- 16.Rice J A, Crowder L B, Marschall E A. In: Early Life History and Recruitment in Fish Populations. Chambers R C, Trippel E A, editors. London: Chapman & Hall; 1997. pp. 332–356. [Google Scholar]
- 17.Keller G, Ribi G. Oecologia. 1993;93:493–500. doi: 10.1007/BF00328956. [DOI] [PubMed] [Google Scholar]
- 18.Boulton A M, Polis G A. J Arachnol. 1999;27:513–521. [Google Scholar]
- 19.De Roos A M, Persson L. Oikos. 2001;94:51–71. [Google Scholar]
- 20.Kooijman S A L M, Metz J A J. Ecotoxicol Environ Saf. 1984;8:254–274. doi: 10.1016/0147-6513(84)90029-0. [DOI] [PubMed] [Google Scholar]
- 21.De Roos A M, Metz J A J, Evers E, Leipoldt A. J Math Biol. 1990;28:609–643. [Google Scholar]
- 22.De Roos A M. In: Structured-Population Models in Marine, Terrestrial, and Freshwater Systems. Tuljapurkar S, Caswell H, editors. New York: Chapman & Hall; 1997. pp. 119–204. [Google Scholar]
- 23.Persson L, Leonardsson K, Gyllenberg M, De Roos A M, Christensen B. Theor Popul Biol. 1998;54:270–293. doi: 10.1006/tpbi.1998.1380. [DOI] [PubMed] [Google Scholar]
- 24.Oksanen L, Fretwell S D, Arruda J, Niemelä P. Am Nat. 1980;118:240–261. [Google Scholar]
- 25.Leibold M A, Chase J M, Shurin J B, Downing A. Annu Rev Ecol Syst. 1997;28:467–494. [Google Scholar]
- 26.Langeland A, Jonsson B. In: Management of Freshwater Fisheries. van Densen W L T, Steinmetz B, Hughes R H, editors. Wageningen, The Netherlands: Pudoc; 1990. pp. 396–405. [Google Scholar]
- 27.Vanbuskirk J. Ecology. 1993;74:1950–1958. [Google Scholar]
- 28.Paine R H. Ecology. 1976;57:858–873. [Google Scholar]
- 29.Paine R H, Castillo J C, Cancino J. Am Nat. 1985;125:679–691. [Google Scholar]
- 30.Yodzis P. Trends Ecol Evol. 2001;16:78–84. doi: 10.1016/s0169-5347(00)02062-0. [DOI] [PubMed] [Google Scholar]