Abstract
In this study, we used cholestatrienol (CTL) as a fluorescent reporter molecule to study sterol-rich Lo domains in complex lipid bilayers. CTL is a fluorescent cholesterol analog that mimics the behavior of cholesterol well. The ability of 12SLPC to quench the fluorescence of cholestatrienol gives a measure of the amount of sterol included in Lo domains in mixed lipid membranes. The stability of sterol-rich domains formed in complex lipid mixtures containing saturated sphingomyelins, phosphatidylcholines, or galactosylceramide as potential domain-forming lipids were studied. The amount of sterol associated with sterol-rich domains seemed to always increase with increasing temperature. The quenching efficiency was highly dependent on the domain-forming lipid present in complex lipid mixtures. Sphingomyelins formed stable sterol-enriched domains and were able to shield CTL from quenching better than the other lipids included in this study. The saturated phosphatidylcholines also formed sterol-rich domains, but the quenching efficiency in membranes with these was higher than with sphingomyelins and the domains melted at lower temperatures. PGalCer was not able to form sterol-enriched domains. However, we found that PGalCer stabilized sterol-rich domains formed in PSM-containing bilayers. Using a fluorescent ceramide analog, we also demonstrated that N-palmitoyl-ceramide displaced the sterol from sphingolipid-rich domains in mixed bilayer membranes.
INTRODUCTION
Lipids and proteins are organized in biological membranes to form lateral domains or raft structures. These rafts are of biological significance as they take part in cellular processes, such as signal transduction, membrane trafficking, and protein sorting (for reviews on this subject see Brown and Dobrowsky, 2000; Fielding and Fielding, 2000; Ikonen, 2001; London, 2000; Maier et al., 2001; Simons and Toomre, 2000). Lateral domains form in membranes mainly due to lipid-lipid interactions, which are largely dependent on the structure and biophysical properties of the lipid components. Formation of ordered domains in liquid bilayers is favored by the presence of long-chain saturated sphingolipids and cholesterol (Brown, 1998; London and Brown, 2000; Rietveld and Simons, 1998). Cholesterol is the major neutral lipid constituent of mammalian cell plasma membranes. Cholesterol influences the physical properties of the surrounding phospholipids and is known to cause phase-separation into Ld cholesterol-poor and Lo cholesterol-rich domains in mixed lipid bilayers containing both low and high Tm lipids (Ahmed et al., 1997; de Almeida et al., 2003; Silvius et al., 1996; Xu and London, 2000).
Cholestatrienol (CTL) is a fluorescent cholesterol analog that has been shown to mimic the membrane behavior of cholesterol quite well (Fischer et al., 1984; Hyslop et al., 1990; Scheidt et al., 2003; Schroeder et al., 1988; Yeagle et al., 1990). A recent study that utilized electron paramagnetic resonance, NMR, and fluorescence spectroscopy to investigate the potential of several fluorescent and spin-labeled sterol probes to function as cholesterol analogs found CTL to mimic cholesterol extraordinarily well in orientation and motion, to have similar membrane topology, and to have a comparable condensing effect on surrounding lipids (Scheidt et al., 2003). CTL exerts intrinsic fluorescence due to two additional double bonds compared to cholesterol. Dehydroergosterol (DHE), which is another commonly used fluorescent sterol, has three more double bonds than cholesterol and an additional methyl group in the hydrocarbon tail. It is known that the addition of a methyl group to the sterol side chain affects the membrane and domain properties of the sterol significantly (Halling and Slotte, 2004). CTL therefore seems to be a better choice as a fluorescent cholesterol analog, promoting the formation of sterol-rich Lo domains and having membrane properties that most closely resemble those of cholesterol. The fluorescence properties of CTL have earlier been characterized in POPC vesicles (Schroeder et al., 1988). It was observed that CTL at concentrations below 6 mol % did not self-quench. In complex lipid bilayers, in which lateral domains are expected to be formed, CTL can be used as a fluorescent sterol analog together with a quencher (12SLPC) located outside the Lo domains (Ahmed et al., 1997). The amount of CTL exposed to quenching by 12SLPC thereby gives a measure of CTL distribution between Lo and Ld membrane domains. However, our method does not report whether ordered domains are in the Lo or the gel phase, if such phases were present in the experimental system studied. Glycosphingolipids have long been known to form microdomains in bilayer membranes due to their saturated nature and because of the extensive hydrogen-bonding network formed between the sugar-headgroups (Masserini and Ravasi, 2001; Schroeder et al., 1994). The importance of cholesterol in these domains is yet a debated subject. The formation of domains by cerebrosides has been found to be only weakly influenced by the presence of cholesterol (Xu et al., 2001). It also seems that in the presence of both glycosphingolipids and sphingomyelin, cholesterol prefers to interact with sphingomyelin over other sphingolipids (Ferraretto et al., 1997; Masserini and Ravasi, 2001). Cholesterol (or another cholesterol-like sterol) promotes phase separation of saturated sphingomyelins and formation of Lo phase (Ipsen et al., 1987; Patra et al., 1999; Wolf et al., 2001). Dihydrosphingomyelin, which is a sphingomyelin species that is common in the human eye lens and has a saturated sphinganine base, seems to be more strongly associated with cholesterol than other sphingomyelin species (Kuikka et al., 2001; Nyholm et al., 2003). In this study, we examined how sterol-rich domains formed in complex bilayer membranes based on the fluorescence properties of CTL. We included two domain-forming glycerophospholipids (DPPC and DSPC), two sphingomyelins (PSM and DHPSM), and PGalCer and studied the stability of sterol-rich domains formed as a function of temperature.
Ceramides are intermediates of sphingolipid biogenesis and are involved in cell signaling (Kolesnick et al., 2000; Merrill, 2002). It has recently been suggested that the effect of ceramide as a second messenger may be mediated by the effects of ceramide on membrane structure (van Blitterswijk et al., 2003). Natural ceramide is composed of a long-chain sphingosine base and a long saturated acyl chain. These structural features make ceramide increase the order of the acyl-chain region in bilayer membranes, much as cholesterol does (Holopainen et al., 1998). As other sphingolipids, ceramide may also be involved in hydrogen-bonding interactions with other membrane components. A recent publication suggests that ceramide would compete with cholesterol for association with domain-forming lipids in bilayer membranes (Megha and London, 2004). The ability of PCer to displace cholesterol from ordered domains in our mixed membrane systems was studied using tParCer as a fluorescent ceramide analog.
Taken together, our results showed that the saturated lipids used in this study formed sterol-rich domains to varying degrees and that the stability of the domains varied with acyl chain length and headgroup composition of the lipids. We showed that PGalCer, which was not able to form sterol-rich domains to any appreciable degree at physiological temperature, was able to accommodate more sterol at higher temperatures. We also showed that PCer effectively displaced sterols from ordered membrane domains.
EXPERIMENTAL PROCEDURES
Material
PSM was purified from egg yolk sphingomyelin (Avanti Polar Lipids, Alabaster, AL) by reverse-phase HPLC (Supelco Discovery C18-column, dimensions 250 × 21, 2 mm, 5 micron particle size, with 100% methanol as the mobile phase). The identity of the product was verified on a Micromass Quattro II mass spectrometer (Manchester, UK). DHPSM was prepared from PSM by hydrogenation using palladium oxide (Aldrich Chemical, Milwaukee, WI) as the catalyst (Schneider and Kennedy, 1967) and purified as described for PSM. DPPC, POPC, DSPC, and 12SLPC were obtained from Avanti Polar Lipids. PCer and PGalCer were from Larodan Fine Chemicals (Malmö, Sweden), and cholesterol was from Sigma Chemicals (St. Louis, MO). Stock solutions of lipids were prepared in hexane/2-propanol (3:2, vol/vol), stored in the dark at −20°C, and warmed to ambient temperature before use.
Cholestatrienol (CTL) was synthesized using the method published by Fischer et al. (1984). tParCer and tParGalCer were synthesized from trans-parinaric acid (Molecular Probes, Eugene, OR) and D-sphingosine (Sigma Chemicals) or psychosine (Avanti Polar Lipids) through a modified version of the method for synthesis of sphingomyelins by Cohen et al. (1984). Briefly, equimolar amounts of sphingosine or psychosine and trans-parinaric acid were dissolved in argon-saturated dichloromethane containing 9 vol % methanol together with dicyclohexylcarbodiimide as a catalyst. Butylated hydroxytoluene was added to the reaction mixture to prevent oxidation, and molecular sieves (Fluka, Buchs, Switzerland) were added to keep the solvent dry. The reactions were carried out for 3 h at room temperature in the dark under an argon atmosphere. The fluorescent probes were identified by mass spectrometry. The purity was checked by reverse-phase HPLC on a RP-18 column before use, with methanol/acetonitrile (30:70, vol/vol) as eluent for CTL, methanol/H2O (80:20, vol/vol) for tParCer, and 100% methanol for tParGalCer. The fluorescent molecules were stored in the dark at −87°C until solubilized in argon-purged methanol. Stock solutions of fluorescent lipids were stored in the dark at −20°C and used within a week.
Preparation of bilayer vesicles
Bilayer vesicles used in steady-state and time-resolved fluorescence measurements were prepared at a lipid concentration of 50 μM. The lipid mixtures were dried under nitrogen, dispersed in water, and heated above the Tm. The warm samples were vortexed briefly and sonicated for 2 min (20% duty cycle, power output 15 W) with a Branson probe sonifier W-250 (Branson Ultrasonics, Danbury, CT). The water used was purified by reverse osmosis followed by passage through a Millipore UF Plus water purification system to yield a product with a resistivity of 18.2 MΩcm.
CTL properties were studied with steady-state and time-resolved fluorescence spectroscopy in samples composed of phospholipid/cholesterol/CTL at a molar ratio of 95:4:1, where the phospholipid was POPC, DPPC, PSM, or DHPSM. In fluorescence quenching studies, F samples consisted of POPC/12SLPC/variable lipid/cholesterol/CTL (30:30:30:9:1, molar ratio), and POPC replaced 12SLPC in Fo samples. The membrane lipid here was POPC, DPPC, DSPC, PSM, DHPSM, or PGalCer. When the effect of ceramide was examined, PCer replaced half of the domain-forming phospholipid, and the samples were thus made of POPC/12SLPC/phospholipids/PCer/cholesterol (30:30:15:15:10, molar ratio). These samples were studied with CTL or tParCer, which replaced cholesterol or PCer, respectively (final concentration 1 mol %). The fluorescent probes were protected from light during all steps, and all solvents were saturated with argon before being used to minimize the risk of oxidation.
For the DSC experiments, we prepared liposomes of different compositions. The lipids were mixed in hexane/2-propanol after which the solvent was evaporated under a constant flow of N2 and put in vacuum for 1 h. The dry lipids were hydrated in water in a sealed vial above the main phase transition of the lipid with the highest melting temperature and vortexed briefly followed by 2 min of bath sonication at 90°C with a Branson bath sonifier 2510 (Branson Ultrasonics). The final concentration of lipids in the solution was 1 mg/ml. After sonication, the samples were cooled to room temperature and analyzed by DSC.
Steady-state fluorescence measurements
Fluorescence measurements were performed either on a PTI QuantaMaster-1 spectrofluorimeter (Photon Technology International, Lawrenceville, NJ) operating in the T-format or on a PTI QuantaMaster-2 spectrofluorimeter operating in the L-format. Both the excitation and emission slits were set to 5 nm on the former, whereas they were set to 5 nm and 10 nm, respectively, on the other filter-based spectrofluorimeter. The temperature was controlled by a Peltier element, with a temperature probe immersed in the sample solution. All experiments were made in quartz cuvettes, and the sample solutions were kept at constant stirring (260 rpm) during the measurements.
Fluorescence emission intensity of CTL was measured at 25°C and 37°C with excitation and emission wavelengths at 324 nm and 374 nm, respectively. Fluorescence emission of tParCer was detected at 410 nm, whereas excitation occurred at 305 nm. When studying temperature dependence of emission intensity, the samples were heated at a rate of 5°C/min during the measurements.
Quenching of steady-state fluorescence
The quenching of steady-state cholestatrienol fluorescence by 12SLPC was measured on a PTI QuantaMaster 1 spectrofluorimeter, essentially following the procedure described by Ahmed et al. (1997). Briefly, vesicles with a total lipid concentration of 50 μM with 1 mol % CTL were used. F samples contained quencher (12SLPC) and a complex lipid mixture as described above, and POPC replaced 12SLPC in Fo samples. The fluorescence intensity in the F samples was compared to the fluorescence intensity in Fo samples giving the fraction of quenched CTL fluorescence. The temperature in the samples was controlled by a Peltier element with a temperature probe immersed in the sample solution.
Time-resolved fluorescence measurements
Time-resolved fluorescence measurements were performed on a PTI TimeMaster fluorimeter, with an N2 laser as the light source. The temperature of the sample was controlled by a Peltier element, with a temperature probe immersed in the sample solution. All experiments were made in quartz cuvettes, and the sample solutions were kept at constant stirring (240 rpm) during the measurements. Data analysis was performed with the software (TimeMaster 1.2) supplied by the instrument manufacturer. The fit was determined from the reduced χ2, Durbin-Watson, z value, and the weighted residuals.
Unilamellar phospholipid vesicles containing POPC, DPPC, PSM, or DHPSM together with cholesterol and CTL (molar ratio 95:4:1, total lipid concentration 50 μM) were used. The samples were excited at 337 nm, the wavelength generated by the N2 laser, and the emission was measured at 370 nm. The slits were set at 8 nm for all time-resolved measurements.
Differential scanning calorimetry
DSC measurements were performed in a Calorimetry Sciences (Provo, UT) Nano II DSC. Liposomes of equimolar mixtures of PSM and PGalCer (with varying amounts of cholesterol) and of PSM and PCer were prepared as described above. The samples were heated and cooled at a rate of 0.5°C/min. Sequential up and down scans between 15°C and 100°C were performed to study the reversibility of the melting process.
RESULTS
Steady-state and time-resolved fluorescence for CTL in various phospholipid environments
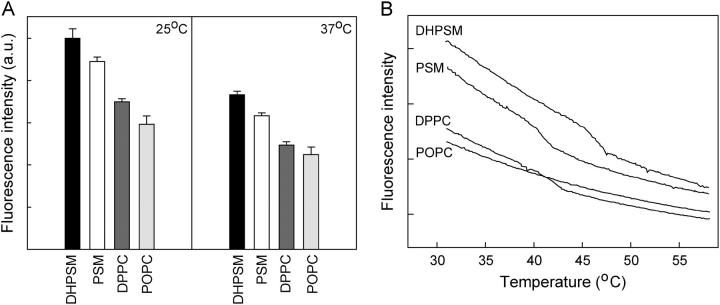
To study whether the fluorescence emission intensity of CTL varied in different phospholipid bilayer membranes, unilamellar vesicles of phospholipid/cholesterol/ CTL (95:4:1, molar ratio) were prepared by probe sonication and the steady-state fluorescence intensity measured at 25°C and 37°C (Fig. 1 A). CTL showed a fluorescence emission that was dependent on the membrane environment at both temperatures. The fluorescence intensity was always higher in DHPSM and PSM than in DPPC or POPC bilayers. A difference was also seen between the two phosphatidylcholines at these temperatures, with the CTL fluorescence intensity being higher in the DPPC environment. The effect of the surrounding phospholipids on the fluorescence lifetimes of CTL was also studied. The time-resolved fluorescence data are presented in Table 1. A model containing two exponential decays gave the best fits in the analysis. The shorter lifetime component had a decay time of 0.5–0.8 ns for all membrane systems studied, whereas the decay time for the longer component varied more. Fluorescence lifetimes for CTL in POPC bilayers have been reported before (Schroeder et al., 1988). The values for the longer lifetime (τ2) given by us (1.5–2.8 ns) differ somewhat from those reported earlier, which varied between 3 and 3.7 ns. This may be a consequence of vesicle size and curvature due to different procedures for preparing the liposomes, i.e., probe- versus bath sonication, of which we prefer the former. From our results, we can see that the differences in fluorescence intensity detected by steady-state fluorescence measurement were not a consequence of differences in fluorescence lifetimes but rather of shifts in the fractional intensities of the two lifetime components, which were present for CTL in all phospholipid environments studied. In POPC vesicles, the shorter lifetime (τ1) dominated to ∼90%, whereas in sphingomyelin bilayers at 25°C only 25%–30% of the fluorescence intensity came from the shorter lifetime component. In DPPC, membranes at 25°C ∼50% of the steady-state fluorescence originated from the shorter lifetime component. At 37°C, the fraction of the shorter lifetime component increased for all lipids studied.
FIGURE 1.
Fluorescence emission intensity of CTL in phospholipid bilayers. (A). Samples consisted of phospholipids/cholesterol/CTL (95:4:1, molar ratio), and the intensity was measured at 25°C and 37°C. The figure shows the average ± range for duplicates of representative samples. (B). Fluorescence emission intensity of CTL in phospholipid bilayers as a function of temperature. The samples consisted of phospholipids/cholesterol/CTL (95:4:1, molar ratio) and were heated at a constant rate of 5°C/min.
TABLE 1.
Time-resolved fluorescence parameters of CTL in various phospholipid bilayers
| Preexponentials
|
Fractional intensities
|
Lifetimes (ns)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Phospholipid | T (°C) | α1 | α2 | F1 | F2 | τ1 | τ2 | χ2 |
| POPC | 25 | 1.0 | 0.1 | 0.9 | 0.1 | 0.7 | 2.0 | 0.9 |
| POPC | 37 | 1.0 | 0.0 | 0.9 | 0.1 | 0.5 | 2.8 | 1.0 |
| DPPC | 25 | 0.7 | 0.3 | 0.5 | 0.5 | 0.8 | 1.8 | 1.0 |
| DPPC | 37 | 0.9 | 0.1 | 0.9 | 0.1 | 0.7 | 2.1 | 1.0 |
| PSM | 25 | 0.5 | 0.5 | 0.3 | 0.7 | 0.7 | 1.7 | 1.0 |
| PSM | 37 | 0.8 | 0.2 | 0.6 | 0.4 | 0.6 | 1.5 | 1.0 |
| DHPSM | 25 | 0.5 | 0.5 | 0.3 | 0.7 | 0.7 | 1.8 | 1.1 |
| DHPSM | 37 | 0.7 | 0.3 | 0.6 | 0.4 | 0.8 | 1.7 | 1.0 |
The samples used contained unilamellar vesicles of phospholipid/cholesterol/CTL (95:4:1, molar ratio).
All data in this table are averages of at least three different experiments.
The standard deviations were below 5% for the longer and below 10% for the shorter lifetimes.
When the fluorescence intensity is measured as a function of temperature for the above samples (as shown in Fig. 1 B), we can see that the CTL fluorescence reported the gel-to-liquid phase transitions for the three saturated lipids studied, i.e., DHPSM, PSM, and DPPC at ∼46, 40, and 41°C, respectively, which are the temperatures given by previous DSC results for bilayers containing 5 mol % of cholesterol (McMullen et al., 1993; Nyholm et al., 2003). The increased diffusional rate in membranes at Tm most likely results in reduced CTL fluorescence emission due to an increased susceptibility to quenching.
Stability of sterol-rich domains in complex lipid bilayers
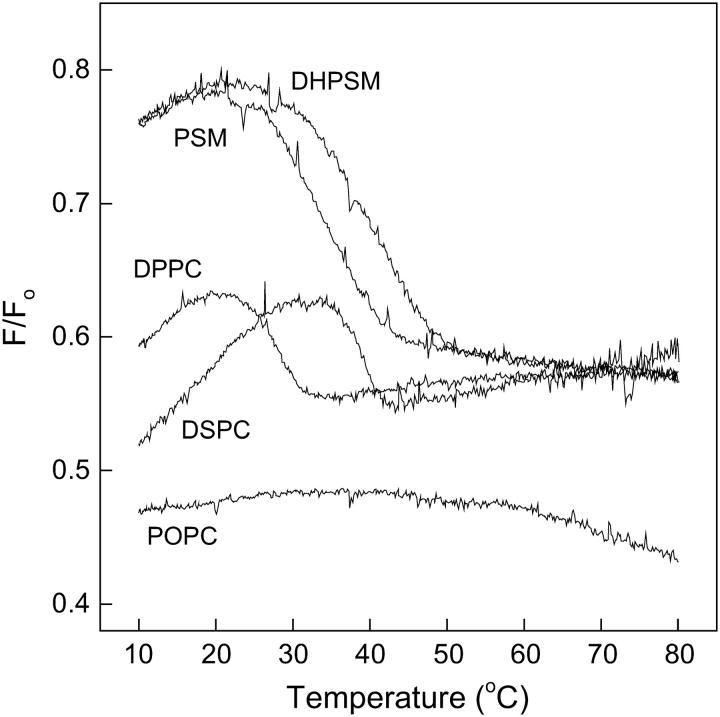
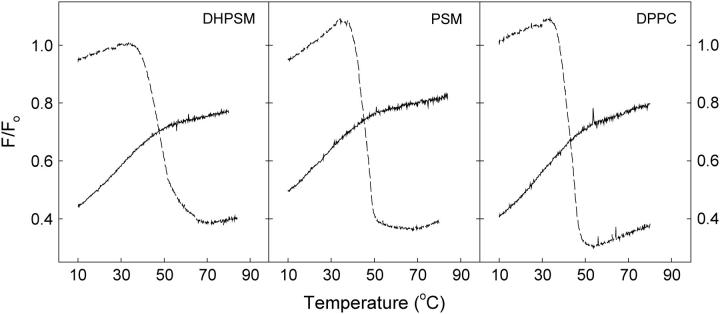
The stability of sterol-enriched domains in mixed lipid bilayers was studied by determining the fraction of CTL emission that was quenchable by 12SLPC (a quencher located outside the Lo domains) as a function of temperature. Fluorescence emission intensity was measured in F samples consisting of POPC/12SLPC/phospholipid/cholesterol/CTL (30:30:30:9:1, molar ratio) and compared to Fo samples, in which 12SLPC was replaced by POPC. The ratio F/Fo is plotted as a function of temperature in Fig. 2. The melting of sterol-rich domains formed with DPPC, DSPC, PSM, and DHPSM was clearly detected. The sterol-rich domains formed with the DHPSM were the most stable because they melted at higher temperatures than those formed with the other phospholipids. Relatively low melting temperatures for the sterol-rich domains in phosphatidylcholine-containing membranes were detected, compared to the DSC-derived Tm values of ∼40°C and 54°C, respectively, for DPPC and DSPC liposomes containing 10 mol % cholesterol (McMullen et al., 1993; McMullen and McElhaney, 1997). This indicates a larger effect of POPC on these domains than on the sterol-rich domains formed with sphingomyelins. No melting of sterol-enriched domains was detected in POPC bilayers in the temperature range studied.
FIGURE 2.
Quenching of CTL emission by 12SLPC in phospholipid bilayers as a function of temperature. Emission intensities were measured in F and Fo samples, composed of POPC/(12SLPC or POPC)/phospholipids/cholesterol/CTL (30:30:30:9:1, molar ratio) heated by 5°C/min, and the F/Fo ratio was calculated. Temperature was increased by 5°C/min.
The quenching resistance of CTL fluorescence before domain melting was also higher in sphingomyelin-containing membranes than with the phosphatidylcholines. In POPC membranes, where no sterol-rich domain melting could be detected, the quenching efficiency was ∼50% at all temperatures studied. Just below the domain-melting temperature, the quenching efficiency in DPPC- and DSPC-containing membranes was between 35% and 40%, whereas the sphingomyelins were able to shield CTL from quenching better so that the corresponding value was only ∼20%. Above the domain-melting temperatures, the quenching efficiency was almost equal (∼45%) in DPPC-, DSPC-, PSM-, and DHPSM-containing membranes.
Another interesting feature seen in Fig. 2 is the apparent increased association of sterols with increasing temperature seen as an increase in shielding of CTL from quenching before domain melting.
Formation of ordered domains in the presence of galactosylceramide
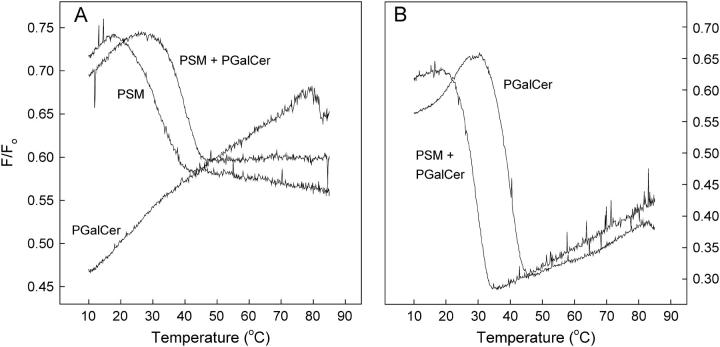
The fluorescence-quenching assay described above was used to study formation of sterol-enriched domains in bilayers containing PGalCer. The bilayer membranes contained PGalCer (30 mol %), PSM (30 mol %), or an equimolar mixture of the two (15 mol % each), in addition to POPC, 12SLPC, and sterols (1 mol % CTL and 9 mol % cholesterol). The ratio of the quenched and unquenched emission (F/Fo) is plotted as a function of temperature in Fig. 3 A. It can be seen that some of the CTL associated with PGalCer as temperature was increased and detected a melting at ∼80°C. The addition of an equimolar amount of PGalCer into PSM-containing bilayers led to the formation of sterol-rich domains which were somewhat more thermostable than the sterol-rich domains formed with PSM alone. The fluorescent reporter molecule tParGalCer detected domain melting at ∼40° in PGalCer-containing mixed membranes containing 29 mol % PGalCer and 1 mol % tParGalCer, POPC, 12SLPC, and 10 mol % cholesterol (Fig. 3 B). tParGalCer also detected the melting of domains in membranes where an equimolar mixture of PGalCer and PSM represented the sphingolipid fraction. These domains melted at ∼30°C, which is a little lower than the domain-melting temperature reported by CTL for similar mixed membranes, indicating that the fluorescent probes are sensing different microenvironments in the membranes.
FIGURE 3.
Domain formation in sphingolipid/cholesterol bilayers detected by 12SLPC quenching of CTL or tParGalCer as a function of temperature. (A). Fluorescence emission intensities were measured in F and Fo samples composed of POPC/(12SLPC or POPC)/sphingolipid/cholesterol/CTL (30:30:30:9:1, molar ratio), where the sphingolipid was PSM, PSM, and PGalCer (1:1) or PGalCer. (B). Fluorescence emission intensities were measured in F and Fo samples composed of POPC/(12SLPC or POPC)/sphingolipid/tParGalCer/cholesterol (30:30:29:1:10, molar ratio), where the sphingolipid was PSM and PGalCer (1:1) or PGalCer.
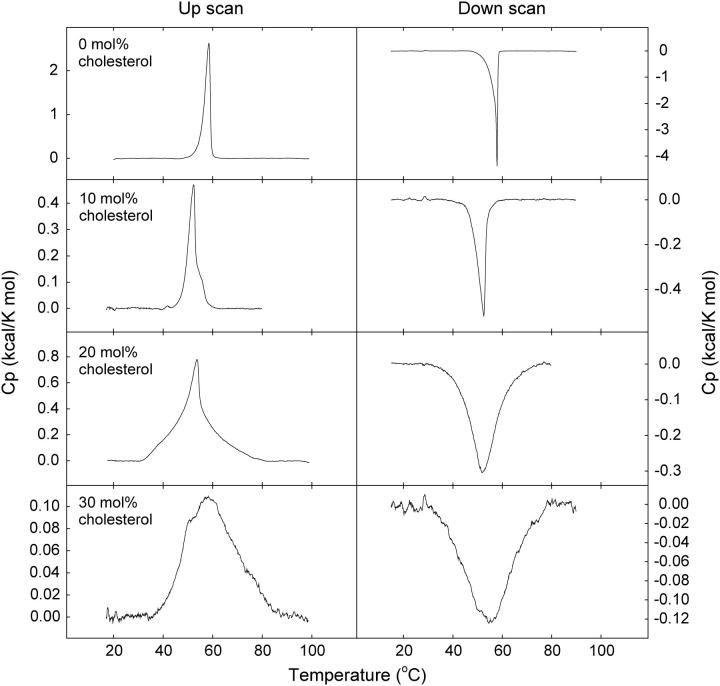
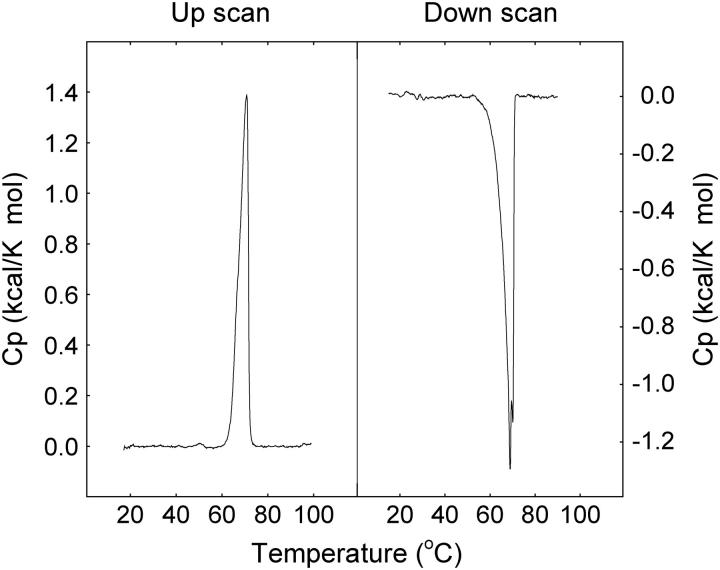
The mixing of cholesterol into an equimolar mixture of PSM and PGalCer was further studied by DSC. The thermograms recorded with PSM/PGalCer bilayers at different sterol concentrations are shown in Fig. 4. The PSM and PGalCer were completely miscible without sterol, since separate transitions for PSM (Tm of 41°C; Bar et al., 1997; Ramstedt and Slotte, 1999a) or PGalCer (Tm of 82°C; Curatolo and Jungalwala, 1985; Ruocco et al., 1981, 1983) could not be detected. Addition of low concentrations of sterol gave a bimodal transition and shifted the Tm toward a lower temperature. These DSC data suggest the presence of a sterol-rich and a sterol-poor component in the membranes and are fully consistent with the data reported by CTL in complex membranes containing both PGalCer and PSM. On the basis of DSC results we can, however, not rule out the possibility that sterol could be present in both phases.
FIGURE 4.
Representative thermograms of PSM/PGalCer bilayers with cholesterol. Equimolar mixtures of PSM and PGalCer containing 0–30 mol % cholesterol were heated and cooled at a rate of 0.5°C/min. Upscans are shown to the left, downscans to the right. Sterol concentrations are indicated in the figure.
Effect of ceramide on phospholipid/cholesterol domains detected by quenching of CTL and tParCer
A recent publication suggests that ceramide has a tendency to displace cholesterol from ordered membrane domains (Megha and London, 2004). We synthesized and used tParCer, a ceramide with an amide-linked trans-parinaric acid, as a fluorescent reporter molecule for ceramide-rich domains. Again, F samples with quencher and Fo samples without it were prepared. The bilayers contained POPC/(12SLPC or POPC)/phospholipid/PCer/cholesterol (30:30:15:15:10, molar ratio), and the quenching resistance was examined both with CTL (solid line) and tParCer (dashed line), replacing 1 mol % of cholesterol or PCer, respectively. The emission ratio F/Fo as a function of temperature is shown in Fig. 5. The results show that ceramide excluded the sterol from the ordered domains effectively, since no transition for sterol-rich domains can be detected in the CTL-containing samples (cf. Fig. 2). TParCer-containing bilayers, however, showed a dramatic decrease in fluorescence intensity with all phospholipid environments studied as the ceramide-enriched domains melted. The thermograms (up- and downscans) for equimolar mixtures of PCer and PSM as reported by DSC are shown in Fig. 6. The thermograms suggest a fairly good miscibility of these two sphingolipids, since separate transitions for PSM (Tm of 41°C; Bar et al., 1997; Ramstedt and Slotte, 1999a) or PCer (Tm over 90°C; Shah et al., 1995; Sot et al., 2005) could not be detected. Our DSC results are consistent with the results presented in Fig. 5, indicating the existence of ceramide/sphingolipid domains.
FIGURE 5.
Effect of ceramide on phospholipid/cholesterol domains detected by 12SLPC quenching of CTL and tParCer. F and Fo samples consisted of POPC/(12SLPC or POPC)/phospholipids/PCer/cholesterol (30:30:15:15:10, molar ratio). The effect was examined both with CTL (solid line) and tParCer (dashed line), which replaced 1 mol % of cholesterol or PCer, respectively.
FIGURE 6.
Representative thermograms of equimolar mixtures of PCer and PSM. The samples were heated and cooled at a rate of 0.5°C/min.
DISCUSSION
In this study, we used CTL as a fluorescent reporter molecule to study sterol partitioning between bulk lipid and lateral Lo domains in complex lipid bilayers. The stability of sterol-rich domains formed in complex lipid mixtures containing saturated sphingomyelins, phosphatidylcholines, or galactosylceramides as potential domain-forming lipids were studied. For this purpose, the fluorescence properties of CTL in different phospholipid environments were also studied by both steady-state and time-resolved fluorescence spectroscopy, and a quenching assay for the domain residing CTL was developed.
Fluorescence properties of CTL in different phospholipid environments
The CTL fluorescence emission was sensitive to the phospholipid environment in bilayer membranes, showing higher fluorescence intensity in sphingomyelin than in phosphatidylcholine bilayers. The fluorescence intensity differences might arise from differences in packing or in the ability of the matrix phospholipids to shield the probe from interaction with water. The fluorescence intensity for CTL was higher in lipid bilayers than in all neat solvents examined (results not shown). At low mol % (as used in this study) CTL has previously been found to be sensitive to dielectric effects (Schroeder et al., 1988). All conditions that might give larger exposure of CTL to water also gave a reduction in fluorescence intensity. A reduction in CTL fluorescence was also observed at the gel-to-liquid phase transition in DPPC, PSM, and DHPSM bilayers, again suggesting sensitivity to changes in packing density or penetration of water into the membrane. This would also explain the apparent difference in CTL fluorescence in the more ordered DPPC versus the less ordered POPC membranes. Cholesterol desorption from mixed monolayers to β-cyclodextrin in the subphase is a convenient tool to measure how well a phospholipid interacts with cholesterol, since the desorption rate is influenced by packing and interactions with surrounding membrane components that the sterol experiences at the surface of the donor membrane (Ohvo and Slotte, 1996). Cholesterol desorption to β-cyclodextrin from monolayers with DPPC, PSM, and DHPSM has been measured in our previous studies and shows good correlation with the fluorescence intensities seen in this study, with the lowest rates of desorption from DHPSM-containing monolayers and the highest desorption rates from DPPC (Kuikka et al., 2001; Ramstedt and Slotte, 1999b). This might again suggest that the exposure of the sterol at the interface, which is influenced by the interaction of the sterol with the other lipids in the bilayer, also is of importance for the detected CTL fluorescence.
Analysis of the time-resolved fluorescence data gave the best fits with a model containing two exponential decays. At 25°C in POPC vesicles the shorter lifetime component (τ1) dominated to ∼90%, whereas only 25–30% of the fluorescence intensity in sphingomyelin bilayers originated from the shorter lifetime component. In DPPC membranes at 25°C, ∼50% of the steady-state fluorescence came from the shorter lifetime component. The two lifetime components might originate from CTL molecules at different depths in the membrane, much as has been seen for some other fluorescent membrane probes (Klymchenko et al., 2004). The shorter lifetime component would then originate from less shielded CTL molecules, whereas the longer lifetime component could originate from a more shielded population. The CTL fluorescence in sphingomyelin bilayers might be affected by possible hydrogen bonding in the headgroup region of these bilayers (Mombelli et al., 2003; Niemela et al., 2004; Talbott et al., 2000). Sphingomyelins can function as both hydrogen-bond donors and acceptors, which might lead to hydrogen bonding between the probe and sphingomyelins; however, at present we cannot determine whether or not such hydrogen bonding takes place. A recent molecular dynamics simulation study showed clearly slower rotational and lateral diffusion in PSM bilayers compared to DPPC bilayers, most likely due to the differences in hydrogen-bonding patterns in the interfacial area (Niemela et al., 2004). The fluorescence intensity also decreases with higher temperature in all phospholipid bilayers studied. An increase in diffusional rates in the membrane decreases the CTL fluorescence intensity most likely due to increased quenching.
Domain formation studied by quenching of CTL fluorescence
The fraction of CTL emission that was quenchable by 12SLPC was larger in phosphatidylcholine bilayers than in sphingomyelin bilayers, indicating a higher affinity of the sterol for the latter and/or a higher packing density in the sterol-rich domains formed with sphingomyelins. The Lo domains formed in sphingomyelin-containing bilayers were recently compared by atomic force microscopy to sterol-rich domains formed with DPPC in a DOPC matrix (van Duyl et al., 2003). The size and shape of the Lo domains in these two systems differed markedly, with bigger Lo domains in the sphingomyelin-containing membranes. Based on those findings, it is possible that some of our results also may depend on domain morphology, since CTL at the domain interface may be susceptible to quenching by 12SLPC. The small differences seen between DHPSM and PSM can originate from known differences in hydrogen-bonding patterns in the interfacial region in membranes formed by these two lipids (Ferguson-Yankey et al., 2000; Talbott et al., 2000).
The effect of temperature on the formation of sterol-rich domains in complex lipid mixtures
CTL reported the melting of sterol-rich domains formed with DPPC, DSPC, PSM, and DHPSM clearly. Increasing the temperature increased the fraction of CTL that was shielded from quenching in all our lipid mixtures below the domain melting temperature. The domain-forming lipids were probably able to accommodate more sterol as the packing in the domains gradually became less restricted at higher temperatures.
The sterol-rich domains formed with the sphingomyelins and DSPC were more thermostable than those formed with DPPC. The relatively low melting temperature for the sterol-rich domains in DPPC- and DSPC-containing membranes, compared to what might be expected on the basis of DSC results (McMullen et al., 1993; McMullen and McElhaney, 1997), indicates a larger effect (i.e., miscibility) of POPC on these domains than on the sterol-rich domains formed with sphingomyelins. It is also possible that formation of sterol-rich domains in phosphatidylcholine bilayers actually requires more sterol than in sphingomyelin-containing membranes as recently suggested by Kahya and co-workers (Kahya et al., 2004). No melting of POPC-sterol domains was detected in the temperature range studied, but could probably have been detected at lower experimental temperatures.
In membranes containing PGalCer together with PSM, we could also observe sterol-rich domains. In an equimolar mixture of PSM and PGalCer, stable sterol-rich domains were formed. tParGalCer also detected domain-melting in such membranes although at slightly lower temperatures than CTL, indicating that the probes were sensing different environments to some extent.
Brain galactocerebrosides have been shown (by DSC) to be immiscible with cholesterol in the gel phase (Johnston and Chapman, 1988). According to another study, cholesterol also has only moderate effects on domain formation by cerebrosides (Xu et al., 2001). A small amount of the sterol in our study, however, seemed to be able to associate with PGalCer with increasing temperature, since quenching of CTL fluorescence decreased at higher temperatures. CTL also detected a melting at ∼80°C, which is close to the melting temperature of pure PGalCer, which is 82.3°C (Curatolo and Jungalwala, 1985; Ruocco et al., 1981, 1983).
Including tParGalCer as a fraction of the PGalCer reported domain melting at ∼40°C indicating partial miscibility of POPC in the domains formed by PGalCer. Partial miscibility of POPC with natural cerebrosides has been reported before (Curatolo, 1986). Our own DSC data on POPC/PGalCer mixtures showed complex thermotropic behavior with several transitions, including one at 40°C. The transitions seen appeared to be dependent on the thermal history of the sample (results not shown). From the results obtained regarding PGalCer in this study, we conclude that PGalCer was unable to form sterol-enriched domains to any significant degree when sphingomyelin was not present in the lipid mixture.
Effect of ceramide on phospholipid/cholesterol domains
Ceramides have been shown to stabilize the ordered gel phase in lipid bilayers (Massey, 2001; Xu et al., 2001). The formation of ordered domains in mixed bilayer membranes is also promoted by ceramide (Wang and Silvius, 2003). It was recently suggested that ceramide is able to displace sterols from liquid-ordered domains (Megha and London, 2004). We show that PCer displaced CTL from sterol-rich domains in membranes with DHPSM, PSM, or DPPC. This can be seen from the failure of CTL fluorescence to report a domain melting-induced discontinuity in the quenching susceptibility of PCer-containing membranes. The inclusion of tParCer as a fraction of the ceramide content of similar membranes gave a clear-cut melting of domains with accompanying quenching of the trans-parinaric acid fluorescence at approximately the temperature expected from DSC results. This observation clearly suggests that the ceramide was actually forming domains with the other domain-forming lipids in the bilayers, but the sterols were excluded from these ceramide-enriched domains. It was interesting to find that PCer was able to displace sterols from ordered domains with PSM and DHPSM although both sphingomyelins readily colocalize with cholesterol. The weak discontinuity in the CTL fluorescence curves approximately at the melting temperature for ceramide-rich domains might reflect the changes in packing occurring in the bilayer at this temperature.
CONCLUDING REMARKS
In conclusion, CTL was found to be a good fluorescent cholesterol analog for studying the formation of sterol-rich domains in bilayer membranes. The stability of sterol-rich domains in several lipid environments was studied. The fluorescence quenching efficiency was highly dependent on the domain-forming lipid present in complex lipid mixtures. Sphingomyelins seemed to be able to accommodate most sterol, as the quenching efficiency was lowest in sphingomyelin-containing membranes. Sphingomyelins also formed stable sterol-enriched domains. The saturated phosphatidylcholines also formed sterol-rich domains, but the quenching efficiency in membranes with these was higher than with sphingomyelins. The domains also melted at lower temperatures than expected, most probably indicating a higher miscibility of POPC with the saturated phosphatidylcholines than with sphingomyelins.
Increasing the temperature increased the amount of sterol associated with all sterol-rich domains. PGalCer was, however, not able to form sterol-rich domains although a small amount of sterol associated with PGalCer when temperature was increased. PGalCer was, on the other hand, able to stabilize sterol-rich domains formed in PSM-containing bilayers.
The ability of tParCer to detect domain melting in complex membranes (containing both sterol and ceramide) in which CTL showed no domain melting strongly suggests that the sterols and ceramides resided in different domains in these membranes. The mechanisms and consequences of such molecular competition must await further clarification.
Acknowledgments
We thank Katrin Halling for help with some DSC experiments.
This study was supported by the Academy of Finland, the Sigrid Juselius Foundation, the Magnus Ehrnrooth Foundation, the Oskar Öflund Foundation, and Medicinska Understödsföreningen Liv och Hälsa.
Thomas K. M. Nyholm's present address is Dept. of Biochemistry of Membranes, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands.
Abbreviations used: 12SLPC, 1-palmitoyl-2-stearoyl-(12-DOXYL)-sn-glycero-3-phosphocholine; CTL, cholesta-5,7,9(11)-trien-3-beta-ol; DHE, ergosta-5,7,9(11),22-tetraen-3β-ol; DHPSM, D-erythro-N-palmitoyl-dihydrosphingomyelin; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSC, differential scanning calorimetry; DSPC, 1,2-distearoyl-sn-glycero-3phosphocholine; Ld, liquid-disordered; Lo, liquid-ordered; PC, phosphatidylcholine; PCer, D-erythro-N-palmitoyl-sphingosine; PGalCer, D-erythro-N-palmitoyl-galactosylceramide; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; PSM, D-erythro-N-palmitoyl-sphingomyelin; Tm, mid temperature of the gel to liquid-crystalline phase transition; tParCer, D-erythro-N-trans-parinoyl-sphingosine; tParGalCer, D-erythro-N-trans-parinoyl-galactosylceramide.
References
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. [DOI] [PubMed] [Google Scholar]
- Bar, L. K., Y. Barenholz, and T. E. Thompson. 1997. Effect of sphingomyelin composition on the phase structure of phosphatidylcholine-sphingomyelin bilayers. Biochemistry. 36:2507–2516. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- Brown, R. E. 1998. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 111:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, R., Y. Barenholz, S. Gatt, and A. Dagan. 1984. Preparation and characterization of well defined D-erythro-sphingomyelins. Chem. Phys. Lipids. 35:371–384. [DOI] [PubMed] [Google Scholar]
- Curatolo, W. 1986. The interactions of 1-palmitoyl-2-oleylphosphatidylcholine and bovine brain cerebroside. Biochim. Biophys. Acta. 861:373–376. [DOI] [PubMed] [Google Scholar]
- Curatolo, W., and F. B. Jungalwala. 1985. Phase behavior of galactocerebrosides from bovine brain. Biochemistry. 24:6608–6613. [DOI] [PubMed] [Google Scholar]
- de Almeida, R. F., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky, R. T. 2000. Sphingolipid signalling domains floating on rafts or buried in caves? Cell. Signal. 12:81–90. [DOI] [PubMed] [Google Scholar]
- Ferguson-Yankey, S. R., D. Borchman, K. G. Taylor, D. B. DuPre, and M. C. Yappert. 2000. Conformational studies of sphingolipids by NMR spectroscopy. I. Dihydrosphingomyelin. Biochim. Biophys. Acta. 1467:307–325. [DOI] [PubMed] [Google Scholar]
- Ferraretto, A., M. Pitto, P. Palestini, and M. Masserini. 1997. Lipid domains in the membrane: thermotropic properties of sphingomyelin vesicles containing GM1 ganglioside and cholesterol. Biochemistry. 36:9232–9236. [DOI] [PubMed] [Google Scholar]
- Fielding, C. J., and P. E. Fielding. 2000. Cholesterol and caveolae: structural and functional relationships. Biochim. Biophys. Acta. 1529:210–222. [DOI] [PubMed] [Google Scholar]
- Fischer, R. T., F. A. Stephenson, A. Shafiee, and F. Schroeder. 1984. Delta 5,7,9(11)-Cholestatrien-3 beta-ol: a fluorescent cholesterol analogue. Chem. Phys. Lipids. 36:1–14. [DOI] [PubMed] [Google Scholar]
- Halling, K. K., and J. P. Slotte. 2004. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim. Biophys. Acta. 1664:161–171. [DOI] [PubMed] [Google Scholar]
- Holopainen, J. M., M. Subramanian, and P. K. Kinnunen. 1998. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 37:17562–17570. [DOI] [PubMed] [Google Scholar]
- Hyslop, P. A., B. Morel, and R. D. Sauerheber. 1990. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry. 29:1025–1038. [DOI] [PubMed] [Google Scholar]
- Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470–477. [DOI] [PubMed] [Google Scholar]
- Ipsen, J. H., G. Karlstrom, O. G. Mouritsen, H. Wennerstrom, and M. J. Zuckermann. 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 905:162–172. [DOI] [PubMed] [Google Scholar]
- Johnston, D. S., and D. Chapman. 1988. A calorimetric study of the thermotropic behaviour of mixtures of brain cerebrosides with other brain lipids. Biochim. Biophys. Acta. 939:603–614. [DOI] [PubMed] [Google Scholar]
- Kahya, N., D. Scherfeld, K. Bacia, and P. Schwille. 2004. Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy. J. Struct. Biol. 147:77–89. [DOI] [PubMed] [Google Scholar]
- Klymchenko, A. S., G. Duportail, A. P. Demchenko, and Y. Mely. 2004. Bimodal distribution and fluorescence response of environment-sensitive probes in lipid bilayers. Biophys. J. 86:2929–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick, R. N., F. M. Goni, and A. Alonso. 2000. Compartmentalization of ceramide signaling: physical foundations and biological effects. J. Cell. Physiol. 184:285–300. [DOI] [PubMed] [Google Scholar]
- Kuikka, M., B. Ramstedt, H. Ohvo-Rekilä, J. Tuuf, and J. P. Slotte. 2001. Membrane properties of D-erythro-N-acyl sphingomyelins and their corresponding dihydro species. Biophys. J. 80:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London, E., and D. A. Brown. 2000. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta. 1508:182–195. [DOI] [PubMed] [Google Scholar]
- Maier, O., T. Ait Slimane, and D. Hoekstra. 2001. Membrane domains and polarized trafficking of sphingolipids. Semin. Cell Dev. Biol. 12:149–161. [DOI] [PubMed] [Google Scholar]
- Masserini, M., and D. Ravasi. 2001. Role of sphingolipids in the biogenesis of membrane domains. Biochim. Biophys. Acta. 1532:149–161. [DOI] [PubMed] [Google Scholar]
- Massey, J. B. 2001. Interaction of ceramides with phosphatidylcholine, sphingomyelin and sphingomyelin/cholesterol bilayers. Biochim. Biophys. Acta. 1510:167–184. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P., R. N. Lewis, and R. N. McElhaney. 1993. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 32:516–522. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P., and R. N. McElhaney. 1997. Differential scanning calorimetric studies of the interaction of cholesterol with distearoyl and dielaidoyl molecular species of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine. Biochemistry. 36:4979–4986. [DOI] [PubMed] [Google Scholar]
- Megha, and E. London. 2004. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J. Biol. Chem. 279:9997–10004. [DOI] [PubMed] [Google Scholar]
- Merrill, A. H., Jr. 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277:25843–25846. [DOI] [PubMed] [Google Scholar]
- Mombelli, E., R. Morris, W. Taylor, and F. Fraternali. 2003. Hydrogen-bonding propensities of sphingomyelin in solution and in a bilayer assembly: a molecular dynamics study. Biophys. J. 84:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela, P., M. T. Hyvonen, and I. Vattulainen. 2004. Structure and dynamics of sphingomyelin bilayer: insight gained through systematic comparison to phosphatidylcholine. Biophys. J. 87:2976–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm, T. K. M., M. Nylund, and J. P. Slotte. 2003. A calorimetric study of binary mixtures of dihydrosphingomyelin and sterols, sphingomyelin, or phosphatidylcholine. Biophys. J. 84:3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohvo, H., and J. P. Slotte. 1996. Cyclodextrin-mediated removal of sterols from monolayers: effects of sterol structure and phospholipids on desorption rate. Biochemistry. 35:8018–8024. [DOI] [PubMed] [Google Scholar]
- Patra, S. K., A. Alonso, J. L. R. Arrondo, and F. M. Goni. 1999. Liposomes containing sphingomyelin and cholesterol: detergent solubilisation and infrared spectroscopic studies. J. Liposome Res. 9:247–260. [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 1999a. Comparison of the biophysical properties of racemic and d-erythro-N-acyl sphingomyelins. Biophys. J. 77:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt, B., and J. P. Slotte. 1999b. Interaction of cholesterol with sphingomyelins and acyl-chain-matched phosphatidylcholines: a comparative study of the effect of the chain length. Biophys. J. 76:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld, A., and K. Simons. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta. 1376:467–479. [DOI] [PubMed] [Google Scholar]
- Ruocco, M. J., D. Atkinson, D. M. Small, R. P. Skarjune, E. Oldfield, and G. G. Shipley. 1981. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 20:5957–5966. [DOI] [PubMed] [Google Scholar]
- Ruocco, M. J., G. G. Shipley, and E. Oldfield. 1983. Galactocerebroside-phospholipid interactions in bilayer membranes. Biophys. J. 43:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt, H. A., P. Muller, A. Herrmann, and D. Huster. 2003. The potential of fluorescent and spin-labeled steroid analogs to mimic natural cholesterol. J. Biol. Chem. 278:45563–45569. [DOI] [PubMed] [Google Scholar]
- Schneider, P. B., and E. P. Kennedy. 1967. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid Res. 8:202–209. [PubMed] [Google Scholar]
- Schroeder, F., G. Nemecz, E. Gratton, Y. Barenholz, and T. E. Thompson. 1988. Fluorescence properties of cholestatrienol in phosphatidylcholine bilayer vesicles. Biophys. Chem. 32:57–72. [DOI] [PubMed] [Google Scholar]
- Schroeder, R., E. London, and D. Brown. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA. 91:12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, J., J. M. Atienza, R. I. Duclos Jr., A. V. Rawlings, Z. Dong, and G. G. Shipley. 1995. Structural and thermotropic properties of synthetic C16:0 (palmitoyl) ceramide: effect of hydration. J. Lipid Res. 36:1936–1944. [PubMed] [Google Scholar]
- Silvius, J. R., D. del Giudice, and M. Lafleur. 1996. Cholesterol at different bilayer concentrations can promote or antagonize lateral segregation of phospholipids of differing acyl chain length. Biochemistry. 35:15198–15208. [DOI] [PubMed] [Google Scholar]
- Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- Sot, J., F. J. Aranda, M. I. Collado, F. M. Goni, and A. Alonso. 2005. Different effects of long- and short-chain ceramides on the gel-fluid and lamellar-hexagonal transitions of phospholipids. A calorimetric, NMR, and x-ray diffraction study. Biophys. J. 88:3368–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott, C. M., I. Vorobyov, D. Borchman, K. G. Taylor, D. B. DuPre, and M. C. Yappert. 2000. Conformational studies of sphingolipids by NMR spectroscopy. II. Sphingomyelin. Biochim. Biophys. Acta. 1467:326–337. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk, W. J., A. H. Van Der Luit, R. J. Veldman, M. Verheij, and J. Borst. 2003. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duyl, B. Y., D. Ganchev, V. Chupin, B. de Kruijff, and J. A. Killian. 2003. Sphingomyelin is much more effective than saturated phosphatidylcholine in excluding unsaturated phosphatidylcholine from domains formed with cholesterol. FEBS Lett. 547:101–106. [DOI] [PubMed] [Google Scholar]
- Wang, T. Y., and J. R. Silvius. 2003. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys. J. 84:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, C., K. Koumanov, B. Tenchov, and P. J. Quinn. 2001. Cholesterol favors phase separation of sphingomyelin. Biophys. Chem. 89:163–172. [DOI] [PubMed] [Google Scholar]
- Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- Xu, X., and E. London. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 39:843–849. [DOI] [PubMed] [Google Scholar]
- Yeagle, P. L., A. D. Albert, K. Boesze-Battaglia, J. Young, and J. Frye. 1990. Cholesterol dynamics in membranes. Biophys. J. 57:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]