Abstract
Sucrose is a natural osmolyte accumulated in cells of organisms as they adapt to environmental stresses. In vitro, sucrose increases protein stability and forces partially unfolded structures to refold. Its effects on the native fold structure and dynamics are not fully established. This study, utilizing Trp phosphorescence spectroscopy, examined the influence of molar concentrations of sucrose on the flexibility of metal-free azurin from Pseudomonas aeruginosa. In addition, by means of specific mutants of the test protein, namely I7S, F110S, and C3A/C26A, that altered its thermodynamic stability, its intrinsic flexibility, and the extent of internal hydration, this investigation sought to identify possible correlations between these features of protein structure and the influence of the osmolyte on protein dynamics. Alterations of structural fluctuations were assessed by both the intrinsic phosphorescence lifetime (τ), which reports on local structure about the triplet probe, and the acrylamide bimolecular quenching rate constant (kq) that is a measure of the average acrylamide diffusion coefficient through the macromolecule. From the modulation of τ and kq across a wide temperature range and up to a concentration of 2M sucrose, it is concluded that sucrose attenuates structural fluctuations principally when macromolecules are internally hydrated and thermally expanded. Preliminary tests with trehalose and xylitol suggest that the effects of sucrose are general of the polyol class of osmolytes.
INTRODUCTION
Sucrose is a compatible osmolyte, belonging to a class of low molecular weight compounds produced by both prokaryotic and eukaryotic cells to protect proteins against the deleterious effects of harsh environmental conditions of water, salts, cold, and heat stress (Yancey et al., 1982; Borowitzka, 1985). Lately, the study of sucrose-protein interactions has attracted considerable attention for the importance of sucrose in biochemical science and in the pharmaceutical industry, where it is commonly used as an additive to protein formulations, to protect labile proteins from the harmful effects of high temperature, freezing, and drying (Vrkljan et al., 1994). It is now generally accepted that it is the preferential exclusion of the osmolyte from the biopolymer surface that provides the driving force for processes like protein refolding and subunit association, which reduce the water accessible surface area (ASA). It has also been suggested that the increase in chemical potential resulting from its preferential exclusion is proportional to the protein surface area, irrespective of the amino acid composition. Whence, the sucrose-mediated increase in stability of folded states over denatured states would simply derive from the much larger ASA of the latter (Lee and Timasheff, 1981; Arakawa and Timasheff, 1982; Timasheff, 1993; Kendrick et al., 1997; Webb et al., 2001; Qu and Bolen, 2003).
Thermodynamics points out the increase in free energy difference between folded and unfolded states induced by sucrose but gives no indication as to the extent the individual states are perturbed by the osmolyte. Of particular relevance are possible effects on the structure of the native fold. The osmolyte pressure to reduce the protein surface area is expected to translate into a decrease in the size of internal cavities, in the withdrawal of internal water molecules, and, where plasticity of the native fold permits it, in the adoption of more compact spherical shape. As a result, the native state should assume a more compact rigid form, and higher activation barriers should slow down structural fluctuations involving surface expansion. Because stability, structural flexibility, and biological function are closely interrelated, subtle alterations of conformation and (or) internal dynamics by sucrose are bound to bear directly on the catalytic efficiency of enzymatic proteins and, in turn, have major implications for the cell metabolism under osmotic stress.
To date, the impact of osmolytes on protein native structure is still poorly characterized. Evidence of structural alterations has been inferred indirectly from modulation of enzymatic activity (Burg et al., 1996, 1999; Fields et al., 2001). In the case of lactate dehydrogenase, the increase in substrate affinity by sucrose was explained in terms of a shift in the native (N)-state ensemble to more compact substates whereas the inhibition of the turnover rate provided evidence for the slowing down of functionally relevant structural fluctuations (Fields et al., 2001). As assessed by CD and IR spectroscopy, changes in the native fold structure by 1–2 M sucrose are generally modest or negligible (Lee and Timasheff, 1975; Borowitzka, 1985; Kim et al., 2003), unless a protein is marginally stable (Kendrick et al., 1997). Effects of sucrose are likely to be more manifest on conformational dynamics rather than on the overall fold of these macromolecules. Flexibility information has been obtained predominantly from amide H-D exchange (HX) rates and all data available concur that sucrose attenuates structural fluctuations (Butler and Falke, 1996; Kendrick et al., 1997; Kim et al., 2003; Qu and Bolen, 2003). HX rates suggest that the mobility of deeply buried, rigid segments of the polypeptide is more affected than superficial domains, although interpretation of HX data has sometime been controversial. For example, studies employing site-specific NMR-detected HX rates (Wang et al., 1995; Foord and Leatherbarrow, 1998; Qu and Bolen, 2003) have concluded that the osmolyte inhibits slow, large scale unfolding like transitions but has no detectable effects on small-scale fluctuations. On the contrary, studies employing FTIR to follow the overall fraction of exchanged protons have indicated that both slow and fast exchange rates are affected by the osmolyte (Kendrick et al., 1997; DePaz et al., 2000; Kim et al., 2000, 2003). Recently, Kim et al. (2003) suggested that the adoption of different exchange conditions might account for the discrepancy. The analysis of exchange rates in terms of structural fluctuations also presumes knowledge of the prevailing exchange regime (EX1 or EX2). Alternative exchange routes, such as solvent penetration, which are difficult to distinguish from EX2, as it would exhibit a similar pH dependence, are generally dismissed as unimportant, more from the impossibility to determine their relative magnitude rather than on experimental grounds. Last, questions have been raised on the sensitivity of HX rates for reporting on the flexibility of the protein native state. Recently, Qu and Bolen (2003) and Wooll et al. (2000) emphasized that HX rates are sensitive to large amplitude unfolding like transitions but are intrinsically insensitive with respect to fluctuations of the native fold. It is argued that even if the osmolyte did inhibit the latter fluctuations they would be hard to detect in EX2 rates as open and closed states involved in the exchange are similar in surface area and there will be a negligible shift in the closed to open equilibrium. The need of alternative probes of protein dynamics, selective of motions within of the native state ensemble, has been emphasized.
In recent years, Trp phosphorescence spectroscopy has proven to be a remarkably sensitive technique for probing the flexibility of globular proteins (Saviotti and Galley, 1974; Strambini, 1989; Vanderkooi, 1991; Cioni and Strambini, 2002a; Gonnelli and Strambini, 2005). Further, this method monitors selectively the flexibility of proteins in their native structure as the room-temperature phosphorescence of less than fully folded proteins is effectively quenched, and partial unfolding transitions generally occur in a timescale longer than the millisecond–second of phosphorescence. In this work, we pursue the subject of sucrose effects on protein dynamics by means of two sensitive parameters related to the phosphorescence emission of internal Trp residues. One is the intrinsic room-temperature phosphorescence lifetime (τ), which is a direct probe of the local flexibility of the protein matrix around the chromophore (Strambini and Gonnelli, 1995; Gonnelli and Strambini, 1995, 2005). The other is the bimolecular rate constant (kq) for the quenching of phosphorescence by acrylamide in solution. kq is determined by the diffusion of the solute through the protein fold to the chromophore's site and is therefore correlated to the structural flexibility of the macromolecule (Cioni and Strambini, 1998). Recently, these parameters have proved instrumental in detecting variations of protein dynamics induced by a variety of perturbations, among which are those brought about by chemical denaturants (Strambini and Gonnelli, 1986; Cioni and Strambini, 1998), hydrostatic pressure (Cioni and Strambini, 1996, 2002b), and heavy water (Cioni and Strambini, 2002a).
Azurin from Pseudomonas aeruginosa was chosen as the model protein system for the wealth of structural (crystallographic, spectroscopic, and theoretical), thermodynamic, and kinetic data available on both native and mutated forms. Azurin is a 14-kDa copper-binding protein with an eight-stranded β-sandwich structure forming a highly hydrophobic core about the unique Trp residue (W48) of the polypeptide (Nar et al., 1991). Because of a rigid structure about the indole ring the phosphorescence emission (of the copper free protein, apo-azurin) is long-lived up to temperatures approaching thermal denaturation. Mutated forms are available varying in thermodynamic stability, overall flexibility, extent of internal hydration, and number of covalent links between β-strands that permit to inquire on potential correlations between the effectiveness of sucrose on protein dynamics and specific features of the macromolecule. With this aim we have examined the dynamics of azurin wild-type (WT), of the two single point mutants, I7S and F110S, and of the double mutant C3A/C26A in which the disulfide bridge linking strands β1and β2 has been severed. From the modulation of τ and kq over a wide temperature range and up to 2 M concentration of sucrose, we find that the attenuation of structural fluctuations by the sugar is significant only with loose, internally hydrated macromolecules and thermally expanded conformations. Preliminary tests with trehalose and xylitol suggest that the effects of sucrose are probably general for the polyol class of osmolytes.
MATERIALS AND METHODS
All chemicals were of the highest purity grade available from commercial sources. Acrylamide (> 99.9% electrophoretic purity) was from Bio-Rad Laboratories (Richmond, CA). Ultrapure guanidine hydrochloride (GdnHCl) was purchased from United States Biochemical (Cleveland, OH). Sucrose, trehalose, and xylitol were purchased from Pfanstiehl (Cleveland, OH). Water, doubly distilled over quartz, was purified by Milli-Q Plus system (Millipore, Bedford, MA). All glassware used for sample preparation was conditioned in advance by standing for 24 h in 10% HCl suprapur (Merck, Darmstadt, Germany).
Azurin WT and mutants I7S, F110S, and C3A/C26A, were prepared following published protocols. The plasmid carrying the WT sequence was a generous gift from Prof. A. Desideri (Università di Roma, “Tor Vergata”). The procedure of isolation and purification of WT has been described by van de Kamp et al. (1990). Details about site-directed mutagenesis, protein expression, isolation, and purification of I7S, F110S, and C3A/C26A mutants have been described elsewhere (Gilardi et al., 1994; Bonander et al., 2000). Copper free azurins (apo-azurins) were prepared from holo-azurins by adding potassium cyanide and EDTA to final concentrations of 0.1 M potassium cyanide and 1 mM EDTA in 0.15 M Tris-HCl, pH 8, followed by column chromatography (van de Kamp et al., 1990). The proteins were dialyzed and stored in Tris HCl, 10 mM pH 7.5.
The viscosity of polyol/water mixtures was determined at 0, 20, and 40°C with Ostwald viscosimeter. Each viscosity value is the average of three independent determinations. The standard deviation of these measurements was better than 5%.
Fluorescence and phosphorescence measurements
All luminescence measurements were conducted on homemade instrumentation. Briefly, for emission spectra, continuous excitation is provided by a Cermax xenon lamp (LX150UV, ILC Technology, Sunnyvale, CA) whose output is selected (6 nm bandpass) by a 0.25 m double grating monochromator optimized for maximum stray light rejection (SPEX, Model 1680, Spex Industries, Edison, NJ). The emission collected at 90° from the excitation is dispersed by a 0.25 m grating monochromator (Jobin-Yvon, H-25, Lille, France) set to a bandpass of 1 nm. For phosphorescence decays, pulsed excitation is provided by a frequency-doubled, Nd/Yag-pumped dye laser (Quanta Systems, Milan, Italy) (λex = 290 nm) with a pulse duration of 5 ns and a typical energy per pulse of 0.5–1 mJ. An interference filter (DTblau, Balzer, Milan, Italy) with a transmission window between 410 and 450 nm selects the phosphorescence emitted at 90° from the excitation. A gating circuit that inverts the polarity of dynodes 1 and 3, for up to 1.5 ms after the laser pulse, protects the photomultiplier (Hamamatsu R928, Hamamatsu, Japan) from the intense fluorescence light pulse. As for spectral measurements, the photocurrent signal is amplified, digitized, and multiple sweeps averaged by the same computer-scope system. All phosphorescence decays were analyzed in terms of a sum of exponential components by a nonlinear least-squares fitting algorithm (Global Unlimited, University of Illinois, Champaign, IL).
For phosphorescence measurements in fluid solutions, it is paramount to rid the solution of all O2 traces. The samples were placed in 5 × 5 square quartz cuvettes especially designed to allow thorough removal of O2 by the alternative application of moderate vacuum and inlet of ultrapure N2 (Strambini and Gonnelli, 1995). All experiments were conducted in 2 mM Tris-HCl, pH 7.5 at a protein concentration of 2–5 μM. Acrylamide quenching experiments were carried out as described before (Cioni and Strambini, 1998).
Denaturation of azurin by guanidine hydrochloride (GdnHCl) was performed incubating the samples at 20°C for 3 days in the presence of suitable amounts of denaturant. The process was reversible as renaturation could be achieved upon diluting the samples in buffer. Equilibrium unfolding was analyzed according to the simple two-state (N↔U) model. The fraction of unfolded protein (fU) was determined at each GdnHCl concentration from the displacement of the center of mass of the Trp fluorescence spectrum (Mei et al., 1999). Briefly, the center of spectral mass (ν) is defined as ν = (ΣνiFi)/(ΣFi), where Fi is the fluorescence intensity at the wavenumber νi. fU is related to ν by the expression
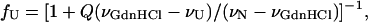 |
where Q is the ratio of the quantum yields of unfolded and folded protein, (ν)GdnHCl is the center of spectral mass at each GdnHCl concentration, and νU and νN are the corresponding values for the unfolded and folded protein, respectively.
The free energy change, ΔG°, in buffer and in 2 M sucrose was estimated from the linear plot (Pace, 1986)
 |
where ΔG = −RTlnK = −RTln(fU/fN).
RESULTS
Influence of sucrose on the stability of metal-free WT and mutant azurins
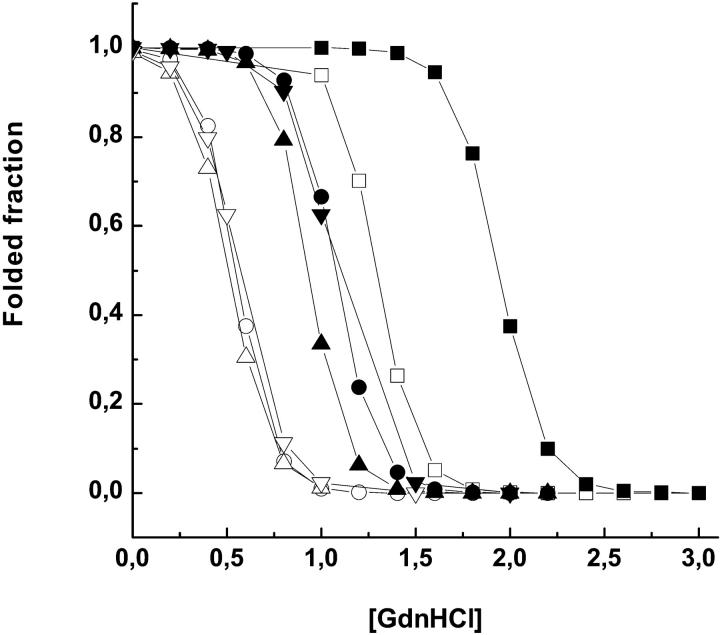
The stability of azurin at 20°C was assessed from its denaturation in GdnHCl, as described under material and methods. The effect of 2 M sucrose on the denaturation profile of the various azurin species is shown in Fig. 1 whereas the unfolding free energy (ΔG°) is given in Table 1. The values of ΔG° in buffer are similar to those reported in the literature (Mei et al., 1999). All proteins exhibited increased thermodynamic stability in the presence of sucrose; the magnitude of ΔΔGD is ∼2.5 kcal/mole, very similar among WT and mutant proteins irrespective of the much lower stability of the latter. Likewise, the m value is practically invariant among the azurins suggesting that the extent of surface exposure to the solvent on unfolding is not significantly different among them (Myers et al., 1995). Constant m values (i.e., ΔASA) and ΔΔG are consistent with the notion that the degree of stabilization by sucrose is simply proportional to the change in solvent accessible surface area (ΔASA).
FIGURE 1.
Denaturation profile of azurin WT and mutant forms in buffer open symbols) and in 2 M sucrose (solid symbols) at 20°C: WT (□), F110S (▵), I7S (○), and C3A/C26A (▿)). The buffer is 2 mM TrisHCl pH 7.5.
TABLE 1.
Unfolding free energies and m values relative to the denaturation of azurin species by GdnHCl, in buffer and in 2 M sucrose at 20°C
| Protein | Solvent | ΔGD (kcal/mole) | m (kcal/mole2) | ΔΔGD (kcal/mole) |
|---|---|---|---|---|
| WT | Buffer | 7.1 ± 0.8 | 5.5 ± 0.4 | 2.4 |
| Buffer + sucrose | 9.5 ± 1.0 | 4.9 ± 0.4 | ||
| C3A/C26A | Buffer | 2.8 ± 0.3 | 5.0 ± 0.5 | 2.5 |
| Buffer + sucrose | 5.3 ± 0.7 | 5.0 ± 0.4 | ||
| F110S | Buffer | 2.7 ± 0.2 | 5.3 ± 0.4 | 2.9 |
| Buffer + sucrose | 5.5 ± 0.7 | 5.9 ± 0.5 | ||
| I7S | Buffer | 3.3 ± 0.3 | 6.0 ± 0.7 | 2.5 |
| Buffer + sucrose | 5.8 ± 0.5 | 5.4 ± 0.5 |
Effect of sucrose on the intrinsic lifetime of W48
For WT azurin, the phosphorescence decay of W48, in buffer (2 mM Tris, pH 7.5) at 20°C, is uniform with a lifetime of 0.63 s. For the cavity-forming mutants F110S and I7S the decay is heterogeneous reflecting the presence of more than one stable conformation of the macromolecule in the millisecond–second timescale, each with its own τ (Cioni et al., 2004). Throughout, heterogeneous decays were adequately fitted in terms of two lifetime components (see as an example F110S in Fig. 2). This analysis yielded an averaged lifetime (τav = α1τ1 + α2τ2) of 1.1 s for F110S and 0.16 s for I7S (Table 2). The phosphorescence emission of the C3A/C26A double mutant, which had not been characterized before, is similar to that of the WT protein in that the decay is uniform and the lifetime is 0.47 s.
FIGURE 2.

Trp phosphorescence decay of F110S azurin (1.4 μM) in 2 mM Tris-HCl pH 7.5 before and after the addition of 2 M sucrose at 20°C. The plots of residuals refer to mono- and biexponential fitting of the decay in buffer. The parameters for a biexponential fit are α1 = 0.53, α2 = 0.47, τ1 = 1.40 s, and τ2 = 0.8 s for the decay in buffer, and α1 = 0.97, α2 = 0.03, τ1 = 1.93 s, and τ2 = 0.40 s in 2 M sucrose.
TABLE 2.
Typical lifetimes and relative amplitudes derived from a biexponential fitting of the phosphorescence decay of the cavity mutants in buffer and in 2 M sucrose
|
T = 20°C
|
T = 40°C
|
|||||
|---|---|---|---|---|---|---|
| Sample/solvent | τ1 (s) | τ2 (s) | α1 | τ1 (s) | τ2 (s) | α1 |
| F110S/buffer | 1.4 | 0.8 | 0.53 | 0.26 | 0.07 | 0.05 |
| F110S/sucrose | 1.93 | 0.40 | 0.97 | 0.27 | 0.08 | 0.95 |
| I7S/buffer | 0.19 | 0.08 | 0.33 | 0.031 | 0.02 | 0.26 |
| I7S/sucrose | 0.21 | 0.09 | 0.95 | 0.037 | 0.023 | 0.85 |
Sucrose increased the phosphorescence lifetime and, in the case of the cavity mutants, rendered the decay more uniform (Table 2). The increase of τ signals a tightening of the structure about W48 (Gonnelli and Strambini, 1995, 2005). Thus, with the addition of sucrose the internal structure of azurin becomes more rigid and the native state ensemble of the cavity mutants more homogeneous. As an example of raw data, the effect of 2 M sucrose on the decay of F110S is shown in Fig. 2. Fig. 3 a indicates that although τ increases smoothly with sucrose concentration, the extent of the change varies markedly among the four azurins. At the highest concentration examined (2 M), the change of τ is modest for WT (5%) and C3A/C26A (8%) but rises to 23% for I7S and to 70% for F110S. From the empirical relationship between the increase of τ and solvent viscosity in model systems (Strambini and Gonnelli, 1995) one estimates a reduction in local flexibility (effective “viscosity”, ητ) of ∼10% for WT, 14% for C3A/C26A, 40% for I7S, and 255% for F110S.
FIGURE 3.

Influence of sucrose concentration on the phosphorescence lifetime of the four azurin species at 20°C and 40°C: WT (▴), F110S (•), I7S (▪), and C3A/C26A (▾). Each point is the average of at least three independent experiments. The error bars (reported only for F110S mutant azurin) indicate the range of τ variations, which is typically <5%.
On raising the temperature from 20 to 40°C the structure tightening effect of sucrose is significantly enhanced becoming quite distinct also with WT and C3A/C26A azurins (Table 3 and Fig. 3 b). The reduction in flexibility at 40°C is again most pronounced for the cavity mutants, ητ increasing by 80% for I7S and 387% for F110S. The opposite trend is observed on cooling the samples to 0°C where, except for the small increase with F110S, τ was hardly affected by sucrose (data not shown).
TABLE 3.
Effect of 2 M sucrose on the phosphorescence lifetime (τS) and on the acrylamide quenching rate constant ( ) of azurin species, at 20 and 40°C
) of azurin species, at 20 and 40°C
| Protein | T (°C) | τB (s) | τS (s) | τS/τB† |  |
 (M−1s−1) (M−1s−1) |
 (M−1s−1) (M−1s−1) |
|---|---|---|---|---|---|---|---|
| WT | 20 | 0.63 | 0.66 | 1.05 | 1.10 | 32 | 1.0 |
| C3AC26A | 20 | 0.47 | 0.52 | 1.06 | 1.13 | 180 | 1.0 |
| F110S | 20 | 1.10* | 1.87* | 1.70 | 3.53 | 8.9 × 104 | 1.6 |
| I7S | 20 | 0.16* | 0.20* | 1.25 | 1.40 | 5.0 × 105 | 1.8 |
| WT | 40 | 0.117 | 0.114 | 1.17 | 1.26 | 3.5 × 102 | 1.5 |
| C3AC26A | 40 | 0.101 | 0.120 | 1.20 | 1.30 | 1.0 × 103 | 1.4 |
| F110S | 40 | 0.080* | 0.261* | 3.20 | 5.20 | 2.8 × 105 | 3.0 |
| I7S | 40 | 0.023* | 0.035* | 1.52 | 1.83 | 1.1 × 106 | 2.5 |
Average lifetimes from a biexponential fit of the decay.
Effective viscosity derived from the empirical relationship between τ and bulk solvent viscosity in model compounds (Strambini and Gonnelli, 1995).
Effect of sucrose on quenching of W48 phosphorescence by acrylamide
An independent assessment of the flexibility of the macromolecule was obtained from the ease with which neutral quenching molecules like acrylamide diffuse through the globular fold to the site of W48 and quench its phosphorescence by a relatively short-range interaction (Cioni and Strambini, 1998). Quenching experiments involved measuring the phosphorescence lifetime at increasing acrylamide concentrations and derivation of the bimolecular rate constant (kq) of the reaction from the gradient of the lifetime Stern-Volmer plot (1/τ = 1/τ0 + kq [acryl], where τ0 is the unperturbed lifetime). Where the phosphorescence emission is intrinsically heterogeneous, as for F110S and I7S, lifetime Stern-Volmer plots where constructed from the average lifetime, (τav = Σαiτi), so the rate constant derived from them is an average quantity.
For every protein, acrylamide quenching experiments were conducted in buffer and in the presence of 2 M sucrose, at both 20 and 40°C. In each case 1/τ increased linearly with acrylamide concentration (the [acryl] was increased until τ decreased ∼5-fold) as should be expected for a dynamic quenching reaction. A representative Stern-Volmer plot is shown in Fig. 4 for quenching of F110S phosphorescence, at 40°C. The magnitude of kq in buffer (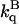 ) and in 2 M sucrose (
) and in 2 M sucrose (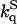 ) is reported in Table 3. In most cases the ratio
) is reported in Table 3. In most cases the ratio 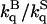 is significantly >1 implying that in sucrose the accessibility of acrylamide to the core of azurin is reduced relative to buffer. As for the triplet lifetime, the slowing down of acrylamide diffusion by sucrose is more pronounced with the two cavity mutants and at high temperature. kq is proportional to the diffusion coefficient for acrylamide migration to the site of W48, (kq α Dp α T/ηp, ηp is an effective frictional drag to acrylamide diffusion inside the protein) and the ratio
is significantly >1 implying that in sucrose the accessibility of acrylamide to the core of azurin is reduced relative to buffer. As for the triplet lifetime, the slowing down of acrylamide diffusion by sucrose is more pronounced with the two cavity mutants and at high temperature. kq is proportional to the diffusion coefficient for acrylamide migration to the site of W48, (kq α Dp α T/ηp, ηp is an effective frictional drag to acrylamide diffusion inside the protein) and the ratio  represents the extent by which sucrose slows down structural fluctuations underlying the diffusion process. Assuming for simplicity that a single slow rate-limiting step governs the diffusion process the results would indicate that the frequency of the putative structural fluctuation, at 20°C, is hardly affected in WT and C3A/C26A but is reduced by 60% in F110S and by 80% in I7S. Increasing the temperature to 40°C, the inhibitory effect of sucrose is sensibly larger, the diffusion rate decreasing by 50% for WT, 40% for C3A/C26A, 250% for F110S and 150% for I7S. Throughout, we note that there is a good correlation between the effect of sucrose on the frictional coefficient to acrylamide diffusion, ηp, and the change in local “viscosity” inferred from τ, ητ,. Both parameters point out that sucrose dampens structural fluctuations in the native fold but the effect is large only with the more flexible cavity forming mutant proteins and is amplified as the temperature is raised above room temperature.
represents the extent by which sucrose slows down structural fluctuations underlying the diffusion process. Assuming for simplicity that a single slow rate-limiting step governs the diffusion process the results would indicate that the frequency of the putative structural fluctuation, at 20°C, is hardly affected in WT and C3A/C26A but is reduced by 60% in F110S and by 80% in I7S. Increasing the temperature to 40°C, the inhibitory effect of sucrose is sensibly larger, the diffusion rate decreasing by 50% for WT, 40% for C3A/C26A, 250% for F110S and 150% for I7S. Throughout, we note that there is a good correlation between the effect of sucrose on the frictional coefficient to acrylamide diffusion, ηp, and the change in local “viscosity” inferred from τ, ητ,. Both parameters point out that sucrose dampens structural fluctuations in the native fold but the effect is large only with the more flexible cavity forming mutant proteins and is amplified as the temperature is raised above room temperature.
FIGURE 4.
Lifetime Stern-Volmer pots for the quenching of F110S azurin mutant phosphorescence by acrylamide at 40°C, before (▪) and after (•) the addition of 2 M sucrose. Each point is the average of at least three independent experiments and the error bars indicate the range of τ variations. Other experimental conditions are as in Fig. 2.
Effect of trehalose and xylitol of the phosphorescence lifetime of F110S
Like sucrose, other polyols such as trehalose and xylitol are known to be preferentially excluded from the protein surface and to stabilize the globular structure against chemical and thermal denaturation (Xie and Timasheff, 1997a,b). To verify whether the structure tightening effect observed for sucrose is a general property of this class of compounds we have examined the influence of trehalose and of xylitol on τ of F110s, the azurin mutant most sensitive to compaction by sucrose. The results, at 20 and 40°C, indicate that trehalose increases the lifetime by roughly the same extent as sucrose whereas the influence of xylitol is significantly less (data at 40°C is shown in Fig. 5 a). If the lifetime data, however, is plotted versus a weight fraction scale instead of the molar scale (Fig. 5 b) then we note that xylitol approaches the effectiveness of the disaccharides. The latter finding suggests that the molecular size of the solute is an important factor in determining the stabilizing interaction and lends support to the proposal that the osmolyte-excluded volume plays a predominant role in the interaction free energy (Schellman, 2003). From this limited set of data, it would appear that attenuation of conformational dynamics of the native state is a general property of preferentially excluded polyols.
FIGURE 5.
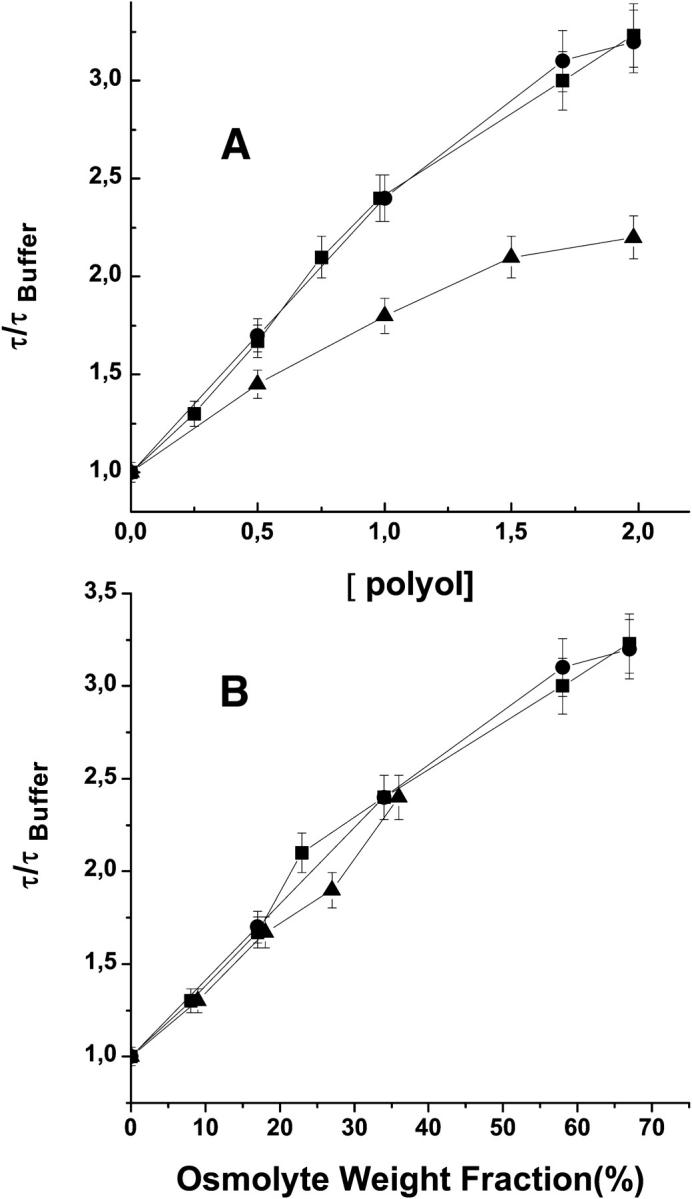
Comparative effects of sucrose (•), trehalose (▪), and xylitol (▴) on the phosphorescence lifetime of azurin F110S at 40°C on a molar concentration scale (A) and on a weight fraction scale (B).
DISCUSSION
Our investigation inquired on the influence that sugars and polyols, at physiological concentrations, have on the internal flexibility of globular proteins. By means of specific mutants of the test protein azurin that altered its thermodynamic stability, its intrinsic flexibility and the extent of internal hydration we also sought to identify possible correlations between these features of the macromolecule and the influence of the osmolytes on protein dynamics. We shall start by first recalling specific structural and dynamical features of WT and mutant azurin forms and then discuss how the variable response of these proteins to sucrose correlates to them.
Azurin dynamics in buffer as probed by τ and kq
Frauenfelder and co-workers have argued that a single monomeric protein can possess many nearly isoenergetic conformations Ni separated by barriers of different height and that the dynamics of its internal motions, spanning several decades in time, is a direct consequence of the complexity of the underlying energy landscape (Frauenfelder et al., 1988). Schematically, for any given temperature and observation timescale t0, the native state may be pictured as an ensemble of distinct Ni substates interconverting at rates kij < 1/t0 (in this scheme faster structural fluctuations, kij > 1/t0, that occur in substate Ni characterize its intrinsic ground state flexibility):
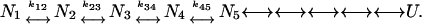 |
(1) |
Moving to the right of scheme 1 conformations become increasingly expanded and intrinsically more flexible until eventually the polypeptide becomes fully unfolded (U). In the above scheme, the flexibility of the native structure increases as both the equilibrium distribution of the N-ensemble shifts to more expanded substates and the interconversion rates kij < 1/t0 become more rapid. This representation emphasizes that the assessment of structural fluctuations characterizing the native fold will differ between probes that integrate over all frequencies from those that are merely sensitive to the equilibrium distribution of Ni states (e.g., EX2 type hydrogen-exchange rates) or to a subset of fluctuations (kij < 1/t0) (e.g., slow, large amplitude, EX1 type, hydrogen-exchange rates).
Both τ and kq are flexibility monitors specific of the native state ensemble. In azurin, τ reflects the fluidity of the protein matrix surrounding the indole ring of W48 (Gonnelli and Strambini, 1995, 2005). It represents an ensemble-averaged parameter weighted by the relative effectiveness of individual frequencies/amplitudes in deactivating the triplet state. τ is determined essentially by the equilibrium distribution of Ni states. By comparison, acrylamide migration to the site of W48 requires transient opening of the compact inner core. Acrylamide being a relatively large solute, diffusion jumps will rely on structural fluctuations of sufficiently large amplitude (kij) between Ni states each of which may be regarded essentially impermeable to diffusion. Hence, kq must be considered a kinetic parameter governed by the free energy barriers separating putative Ni states. A confirm that a wide range of smaller amplitude motions are not seen by acrylamide diffusion, the internal flexibility of proteins estimated by kq (ηp) is typically 4 orders of magnitude smaller than that estimated by τ (ητ), which is sensitive to a very broad range of frequencies and amplitudes (Cioni and Strambini, 1998).
W48 of azurin is located roughly at the center of the β-barrel, the indole ring surrounded by nonpolar side chains that form a compact hydrophobic core (Adman, 1991; Nar et al., 1991). By all criteria, crystallographic B-factors (Nar et al., 1991), T1 NMR relaxation times (Mei et al., 1996), and molecular dynamic simulations (Arcangeli et al., 1999, 2001), the aromatic ring is immobilized in an unusually rigid domain. In accord with a rigid environment, W48 exhibits a long phosphorescence lifetime (0.63 s) at ambient temperature that translates into a local “viscosity” ητ = 5 × 104 cPoise. Acrylamide migration to the site of W48 is highly hindered. A value of kq of 32 M−1s−1, relative to 109 M−1s−1 for Trp on the surface (Kerwin et al., 2002), implies a protein frictional coefficient ηp = 3 × 108 cPoise.
Severing the superficial C3–C26 disulfide bridge linking the ends of strands β1and β2 does not alter the WT fold or the compactness of the protein core. The main effect on its internal dynamics is sixfold increase of kq, due to lowering the activation barrier from 22 to 15.7 kcal/mole (P. Cioni, unpublished data). On the contrary, by substituting internal I7 or F110 with smaller Ser a cavity (of 40 and 100 Å3 for I7S and F110S, respectively) is engineered in proximity of W48, which is presumably filled with water (Hammann et al., 1996). The consequences on τ are diverse between the two mutants but both proteins have become highly permeable to acrylamide. Relative to WT, kq increased 3 and 4 orders of magnitude for F110S and I7S, respectively, primarily as a result of lowering the activation barrier (Cioni et al., 2004). Another consequence of creating an internal cavity is a greater plasticity of the globular fold and the appearance of structural heterogeneity as evidenced by the multiplicity of phosphorescence lifetimes. Heterogeneity is confirmed by a wider distribution of fluorescence lifetimes and ground state energies (Gilardi et al., 1994; Mei et al., 1996; Kroes, et al., 1998), the loss of the near-UV CD signal (Mei et al., 1996) and the large B-factors of Ser-7 or of Ser-110, in addition to a variable number of water molecules in the latter's cavity (Hammann et al., 1996). Viewed in terms of the energy landscape, the mutants have comparable free energy minima but whereas the N-state ensemble of the C3A/C26A double mutant (and WT) is confined within a relatively narrow well in configurational space multiple near isoenergetic wells are accessible to the cavity forming mutants.
Effect of sucrose on the flexibility of azurin
Both τ and kq attest to a slowing down of azurin dynamics by sucrose. The change is progressive with concentration and as it requires molar quantities, the effect is clearly one of bulk rather than of potential binding of the sugar to the protein. Attenuation of protein dynamics finds an adequate explanation from the increase in chemical potential associated with more expanded structures, in that sucrose will shift the equilibrium of Ni states to more compact rigid forms and raise the barriers of structural fluctuations between Ni states involving surface expansion. This rationalization is advocated by the observed effects of sucrose on azurin stability, where roughly constant values of m and ΔΔGD among WT and mutant forms entail that the increase in free energy of the protein in sucrose solutions is simply proportional to its solvent accessible surface area (ASA). Before adopting this interpretation key, we recall that motions in the protein can also be slowed down by the increase in solvent viscosity. There is evidence to suggest, however, that under these conditions, coupling of internal motions to solvent viscosity is weak and that consequently the latter plays a minor role on the attenuation of conformational dynamics by the sugar. First, we note that up to 20°C, the ∼15-fold increase in viscosity by 2 molar sucrose has small or negligible effects on both τ and kq of WT and C3A/C26A proteins. Even with I7S, the most flexible of the four azurin species examined, the influence of sucrose is very modest on approaching freezing temperature. Further, although the disaccharides, sucrose and trehalose, are more viscogenic than xylitol, their effectiveness on the lifetime of F110S, the protein most affected by these osmolytes, is comparable (Fig. 5). Last, Fig. 6 emphasizes that: 1), for each osmolyte most of the lifetime increase occurs at relatively small increments of solvent viscosity; 2), the change of τ is sharper with xylitol than with the sugars; and 3), that it is much more marked at 40 rather than at 20°C. A weak influence of solvent viscosity was also inferred for the HX rates of RNase A (Qu and Bolen, 2003).
FIGURE 6.
Variations of the phosphorescence lifetime of F110S azurin, induced by polyols, plotted as a fraction of solvent: sucrose (•), trehalose (▴), and xylitol (▪) at 20°C; sucrose (○), trehalose (▵), and xylitol (□) at 40°C.
At ambient temperature, the effectiveness of sucrose on slowing down structural fluctuations in the native state ensemble seems to depend critically on the compactness of the globular fold. With internally well-packed WT and C3A/C26A azurins species τ reports a modest, barely detectable (<10%) reduction of local motions about W48 whereas an essentially constant kq points to no significant changes in acrylamide diffusion rate. The same behavior was found with the corresponding Cd2+-complexed azurins (data not shown) which are as compact and even more stable relative to the apo-proteins. We interpret this relative inertness of sucrose with respect to the natural breathing motions of well-packed polypeptides to mean that with already well-defined rigid structures there is little room for osmolytes to shift the Ni population toward more compact forms. Further, fluctuations about this lowest energy configuration, permitting slow diffusion of acrylamide, can occur without exhibiting large expansion of the protein surface.
Significant dampening of conformational dynamics by sucrose occurs upon the creation of an internal cavity that can host one or more water molecules. Relative to buffer, in 2 M sucrose ητ was 40% higher with I7S and as much as 250% with F110S, the latter having a cavity at least twice as large as the former. Within the framework of scheme 1 the lengthening of τ entails a narrowing down of the distribution of Ni states toward the compact end. A more uniform compact native state ensemble is also inferred from the concomitant decrease in lifetime heterogeneity (Table 2). If for simplicity we assume that in buffer only two lifetimes describe the emission of the N-state ensemble, i.e., that the latter is essentially represented by two main substates, we find that at increasing sucrose concentrations the decay is progressively dominated by the amplitude of the long-lived component (αL). In the case of F110S, αL goes from 0.53 in buffer to 0.97 in 2 M sucrose (Fig. 2, Table 2). With these cavity mutants sucrose also decreased kq by 80% with I7S and 60% with F110S implying that the osmolyte is equally effective in slowing down the transitions between Ni states governing acrylamide migration in these permeable proteins. Conversely, from their sensitivity to sucrose, we deduce that these fluctuations between Ni states, which drastically enhance the diffusion of acrylamide relative to WT, involve expanded transition states.
Another key factor that modulates the influence of sucrose on protein dynamics is temperature. The attenuation of proteins motions was considerably enhanced on raising the temperature from 20 to 40°C. At the higher temperature the effects have become evident also with compact WT and C3A/C26A azurin species (Table 3). From 20 to 40°C, there is a sizable decrease of τ of all azurins suggesting that the N-state ensemble becomes populated with more expanded states, namely, that the Ni distribution in scheme 1 has shifted to the right. Thus, a larger sucrose-induced increase in τ at higher temperature can again be explained by the contraction of thermally expanded states, a response analogous to that observed with the application of hydrostatic pressure (Cioni and Strambini, 2002b). The onset of new structural fluctuations (kij) between these thermally expanded states, characterized by transition states with significant ΔASA, would instead account for the sizable sucrose-induced decrease of kq also for WT and C3A/C26A species. The opposite trend, a decreasing influence of sucrose on protein dynamics, was observed on cooling the solution to near freezing temperatures. Lifetime measurements conducted at near 0°C (data not shown) do indicate that in three out of four azurins τ is not affected by 2 M sucrose and increases by merely 30% (compared to 70% at 20°C) only with the largest cavity mutant, F110S. Likewise, changes in kq are negligibly small with all azurins. The implication is that, although sucrose remains an effective promoter of folded structures over a wide temperature range, its influence on protein dynamics tapers off on cooling, until it probably becomes negligible at temperatures where expanded states of the native fold ensemble and large amplitude fluctuations are thermally inaccessible. A smaller inhibition of conformational dynamics by sucrose at low temperature was also inferred from the enzymatic activity of lactic dehydrogenase (Fields et al., 2001). The preservation of structural flexibility at low temperature may represent a selective advantage of these osmolytes, fundamental for maintaining biological activity in cells protected from freeze damage by large concentrations of sucrose or other saccharides.
Preliminary tests with trehalose and xylitol, limited to τ of the largest cavity mutant F110S, suggest that the dampening of protein motions observed with sucrose is likely to be a general property of stabilizing polyols. The efficacy of xylitol is smaller than that of the disaccharides, on a molar concentration scale, but becomes similar when compared on a weight fraction scale. In the still open debate as to the relative contribution of specific interactions leading to the preferential exclusion of the osmolyte (a repulsive interaction with the peptide backbone (Wang et al., 1995), the increase in surface tension (Kaushik and Bhat, 2003) and the supremacy of the excluded volume (Schellman, 2003)), the differences between xylitol, and the disaccharides could be largely accounted by their difference in excluded volume.
In summary, molar concentrations of sucrose, stabilized azurin against unfolding and in some mutants altered significantly the conformational flexibility of the native state. A constant increase in ΔG° for the four azurin species, irrespective of differences in their intrinsic stability and internal flexibility, confirms that the increase in thermodynamic stability is due primarily to the large surface area of the denatured state. Attenuation of structural fluctuation in the native state, on the other hand, is not the general response to the osmolyte. The sharp distinction between proteins with a compact globular fold and internally hydrated proteins, together with the positive role of temperature seem to indicate that important effects of sucrose on protein dynamics are linked to the possibility of dehydrating-compacting the native state. The findings reported here largely confirm the inhibition of protein dynamics inferred from hydrogen exchange studies. However, they caution that with well-packed polypeptides the effects of sucrose on the internal dynamics are expected to be moderate or insignificant below ambient temperature. Hence, the large decrease in hydrogen-exchange rate, generally observed for deeply buried amides protons in compact stable proteins, probably refers to the slowing down of extended, unfolding like transitions occurring in a timescale longer than the natural breathing modes of the native state.
References
- Adman, E. T. 1991. Copper protein structures. Adv. Protein Chem. 42:144–197. [DOI] [PubMed] [Google Scholar]
- Arakawa, T., and S. N. Timasheff. 1982. Stabilization of protein structure by sugars. Biochemistry. 21:6536–6544. [DOI] [PubMed] [Google Scholar]
- Arcangeli, C., A. R. Bizzarri, and S. Cannistraro. 1999. Long-term molecular dynamics simulation of copper azurin: structure, dynamics and functionality. Biophys. Chem. 78:247–257. [DOI] [PubMed] [Google Scholar]
- Arcangeli, C., A. R. Bizzarri, and S. Cannistraro. 2001. Concerted motions in copper plastocyanin and azurin: an essential dynamics study. Biophys. Chem. 90:45–56. [DOI] [PubMed] [Google Scholar]
- Bonander, N., J. Leckner, H. W. Guo, B. G. Karlsson, and L. Sjolin. 2000. Crystal structure of the disulfide bond-deficient azurin mutant C3A/C26A - How important is the S-S bond for folding and stability? Eur. J. Biochem. 267:4511–4519. [DOI] [PubMed] [Google Scholar]
- Borowitzka, L. J. 1985. Glycerol and other carbohydrate osmotic effectors. In Transport Processes, Iono- and Osmoregulation. R. Gilles and M. Gilles-Baillien, editors. Springer and Verlag, Berlin. 437–453.
- Burg, M. B., E. D. Kwon, and E. M. Peters. 1996. Glycerophosphocholine and betaine counteract the effect of urea on pyruvate kinase. Kidney Int. Suppl. 57:S100–S104. [PubMed] [Google Scholar]
- Burg, M. B., E. M. Peters, K. M. Bohren, and K. H. Gabbay. 1999. Factors affecting counteraction by methylamines of urea effects on aldolase reductase. Proc. Natl. Acad. Sci. USA. 96:6517–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, S. L., and J. J. Falke. 1996. Effects of protein stabilizing agents on thermal backbone motions: a disulfide trapping study. Biochemistry. 35:10595–10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni, P., E. de Waal, G. W. Canters, and G. B. Strambini. 2004. Effects of cavity-forming mutations on the internal dynamics of azurin. Biophys. J. 86:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni, P., and G. B. Strambini. 1996. Pressure effects on the structure of oligomeric proteins prior to subunit dissociation. J. Mol. Biol. 263:789–799. [DOI] [PubMed] [Google Scholar]
- Cioni, P., and G. B. Strambini. 1998. Acrylamide quenching of protein phosphorescence as a monitor of structural fluctuations in the globular fold. J. Am. Chem. Soc. 120:11749–11757. [Google Scholar]
- Cioni, P., and G. B. Strambini. 2002a. Effect of heavy water on protein flexibility. Biophys. J. 82:3246–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni, P., and G. B. Strambini. 2002b. Tryptophan phosphorescence and pressure effects on protein structure. Biochim. Biophys. Acta. 1595:116–130. [DOI] [PubMed] [Google Scholar]
- DePaz, R. A., C. C. Dale, J. F. Carpenter, A. L. Gaertner, and T. W. Randolph. 2000. The excluding effects of sucrose on a protein chemical degradation pathway: methionine oxidation in subtilisin. Arch. Biochem. Biophys. 384:123–132. [DOI] [PubMed] [Google Scholar]
- Fields, P. A., B. D. Wahlstrand, and G. N. Somero. 2001. Intrinsic versus extrinsic stabilization of enzymes. The interaction of solute and temperature on A4-lactate dehydrogenase orthologs from warm-adapted and cold-adapted marine fishes. Eur. J. Biochem. 268:4497–4505. [DOI] [PubMed] [Google Scholar]
- Foord, R. L., and R. J. Leatherbarrow. 1998. Effects of osmolytes on the exchange rates of backbone amide hydrogens in proteins. Biochemistry. 37:2969–2978. [DOI] [PubMed] [Google Scholar]
- Frauenfelder, H., F. Parak, and R. D. Young. 1988. Conformational substates in proteins. Ann. Rev. Biophys. Chem. 17:451–479. [DOI] [PubMed] [Google Scholar]
- Gilardi, G., G. Mei, N. Rosato, G. W. Canters, and A. Finazzi-Agrò. 1994. Unique environment of Trp48 in Pseudomonas aeruginosa azurin as probed by site-directed mutagenesis and dynamic fluorescence spectroscopy. Biochemistry. 33:1425–1432. [DOI] [PubMed] [Google Scholar]
- Gonnelli, M., and G. B. Strambini. 2005. Intramolecular quenching of Trp phosphorescence in short peptides and proteins. Photochem. Photobiol. In press. [DOI] [PubMed]
- Gonnelli, M., and G. B. Strambini. 1995. Phosphorescence lifetime of tryptophan in proteins. Biochemistry. 34:13847–13857. [DOI] [PubMed] [Google Scholar]
- Hammann, C., A. Messerschmidt, R. Huber, H. Nar, G. Gilardi, and G. W. Canters. 1996. X-ray crystal structure of the two site-specific mutants Ile7Ser and Phe110Ser of azurin from Pseudomonas aeruginosa. J. Mol. Biol. 255:362–366. [DOI] [PubMed] [Google Scholar]
- Kaushik, J. K., and R. Bhat. 2003. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 278:26458–26465. [DOI] [PubMed] [Google Scholar]
- Kendrick, B. S., B. S. Chang, T. Arakawa, B. Peterson, T. W. Randolph, M. C. Manning, and J. F. Carpenter. 1997. Preferential exclusion of sucrose from recombinant interleukin-1 receptor antagonist: role in restricted conformational mobility and compaction of native state. Proc. Natl. Acad. Sci. USA. 94:11917–11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin, B. A., B. S. Chang, C. V. Gegg, M. Gonnelli, T. Li, and G. B. Strambini. 2002. Interactions between PEG and type I soluble tumor necrosis factor receptor: modulation by pH and by PEGylation at the N terminus. Protein Sci. 11:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. S., L. S. Jones, A. Dong, B. S. Kendrick, B. S. Chang, M. C. Maniing, T. W. Randolph, and J. F. Carpenter. 2003. Effects of sucrose on conformational equilibria and fluctuations within the native-state ensemble of proteins. Protein Sci. 12:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.-S., J. S. Meyer, C. Murphy, T. W. Randolph, M. C. Manning, A. Solomon, and J. F. Carpenter. 2000. Thermodynamic modulation of light chain amyloid fibril formation. J. Biol. Chem. 275:1570–1574. [DOI] [PubMed] [Google Scholar]
- Kroes, S. J., G. W. Canters, G. Gilardi, A. van Hoek, and J. W. G. Visser. 1998. Time-resolved fluorescence study of azurin variants: conformational heterogeneity and tryptophan mobility. Biophys. J. 75:2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. C., and S. N. Timasheff. 1975. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 14:5183–5187. [DOI] [PubMed] [Google Scholar]
- Lee, J. C., and S. N. Timasheff. 1981. The stabilization of proteins by sucrose. J. Biol. Chem. 256:7193–7201. [PubMed] [Google Scholar]
- Mei, G., A. Di Venere, F. Malvezzi Campeggi, G. Gilardi, N. Rosato, F. De Matteis, and A. Finazzi-Agrò. 1999. The effect of pressure and guanidine hydrochloride on azurins mutated in the hydrophobic core. Eur. J. Biochem. 265:619–625. [DOI] [PubMed] [Google Scholar]
- Mei, G., G. Gilardi, M. Venanzi, N. Rosato, G. W. Canters, and A. Finazzi-Agrò. 1996. Probing the structure and mobility of Pseudomonas aeruginosa azurin by circular dichroism and dynamic fluorescence anisotropy. Protein Sci. 5:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, J. K., C. N. Pace, and J. M. Scholtz. 1995. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 4:2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nar, H., A. Messerschimdt, R. Huber, M. van de Kamp, and G. W. Canters. 1991. Crystal structure analysis of oxidized Pseudomonas aeruginosa azurin at pH 5.5 and pH 9.0. J. Mol. Biol. 221:765–772. [DOI] [PubMed] [Google Scholar]
- Pace, C. N. 1986. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 131:266–280. [DOI] [PubMed] [Google Scholar]
- Qu, Y., and D. W. Bolen. 2003. Hydrogen exchange kinetics of RNase A and the urea: TMAO paradigm. Biochemistry. 42:5837–5849. [DOI] [PubMed] [Google Scholar]
- Saviotti, M. L., and W. C. Galley. 1974. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc. Natl. Acad. Sci. USA. 71:4154–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellman, J. A. 2003. Protein stability in mixed solvents: a balance of contact interaction and excluded volume. Biophys. J. 85:108–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strambini, G. B. 1989. Tryptophan phosphorescence as monitor of protein flexibility. J. Mol. Liq. 42:155–165. [Google Scholar]
- Strambini, G. B., and M. Gonnelli. 1986. Effects of urea and guanidine hydrochloride on the activity and dynamical structure of equine liver alcohol dehydrogenase. Biochemistry. 25:2471–2476. [DOI] [PubMed] [Google Scholar]
- Strambini, G. B., and M. Gonnelli. 1995. Tryptophan phosphorescence in fluid solution. J. Am. Chem. Soc. 117:7646–7651. [Google Scholar]
- Timasheff, S. N. 1993. The control of protein stability and association by weak interactions with water. Annu. Rev. Biophys. Biomol. Struct. 22:67–97. [DOI] [PubMed] [Google Scholar]
- van de Kamp, M., M. C. Silvestrini, M. Brunori, J. van Beeumen, F. C. Hali, and W. C. Canters. 1990. Involvement of the hydrophobic patch of azurin in the electron-transfer reactions with cytochrome-C551 and nitrite reductase. Eur. J. Biochem. 194:109–118. [DOI] [PubMed] [Google Scholar]
- Vanderkooi, J. M. 1991. Tryptophan phosphorescence from proteins at room temperature. In Topics in Fluorescence Spectroscopy, Vol. 3, Biochemical Applications. J. R. Lakowicz, editor. Plenum Publishing, New York. 113–136.
- Vrkljan, M., T. M. Foster, M. E. Powers, J. Henkin, W. R. Porter, H. Staack, J. F. Carpenter, and M. C. Manning. 1994. Pharm. Res. 11:1004–1008. [DOI] [PubMed] [Google Scholar]
- Wang, A., A. D. Robertson, and D. W. Bolen. 1995. Effects of a naturally occurring compatible osmolyte on the internal dynamics of ribonuclease A. Biochemistry. 34:15096–15104. [DOI] [PubMed] [Google Scholar]
- Webb, J. N., S. D. Webb, J. L. Cleland, J. F. Carpenter, and T. W. Randolph. 2001. Partial molar volume, surface area, and hydration changes for equilibrium unfolding and formation of aggregation transition state: high pressure and cosolute studies on recombinant human IFN-γ. Proc. Natl. Acad. Sci. USA. 98:7259–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooll, J. O., J. O. Wrabl, and V. J. Hilser. 2000. Ensemble modulation as an origin of denaturant-independent hydrogen exchange in proteins. J. Mol. Biol. 301:247–256. [DOI] [PubMed] [Google Scholar]
- Xie, G. F., and S. N. Timasheff. 1997a. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 64:25–43. [DOI] [PubMed] [Google Scholar]
- Xie, G. F., and S. N. Timasheff. 1997b. Temperature dependence of the preferential interactions of ribonuclease A in aqueous co-solvent systems: Thermodynamic analysis. Prot. Sci. 6:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolytes system. Science. 217:1214–1222. [DOI] [PubMed] [Google Scholar]