Abstract
A significant number of exocytosis events recorded with amperometry demonstrate a prespike feature termed a “foot” and this foot has been correlated with messengers released via a transitory fusion pore before full exocytosis. We have compared amperometric spikes with a foot with spikes without a foot at chromaffin cells and found that the probability of detecting a distinct foot event is correlated to the amount of catecholamine released. The mean charge of the spikes with a foot was found to be twice that of the spikes without a foot, and the frequency of spikes displaying a foot was zero for small spikes increasing to ∼50% for large spikes. It is hypothesized that in chromaffin cells, where the dense core is believed to nearly fill the vesicle, the expanding core is a controlling factor in opening the fusion pore, that prefusion of two smaller vesicles leads to excess membrane, and that this slows pore expansion leading to an increased observation of events with a foot. Clearly, the physicochemical properties of vesicles are key factors in the control of the dynamics of release through the fusion pore and the high and variable frequency of this release makes it highly significant.
INTRODUCTION
Neurotransmitters are small molecules released by secretory cells through the exocytosis of vesicles in which they are stored at high concentrations (typically 0.5–1 M). Vesicular exocytosis in neuroendocrine cells, neurons or at the neuromuscular junction occurs through several steps. The docking of the vesicle to specific loci (Zenisek et al., 2000) of the cell membrane is promoted by the formation of SNAREs complexes between proteins of the vesicle (v-SNAREs) and proteins anchored or located at the plasma membrane (t-SNAREs). A supposedly second role of the SNAREs is to bring vesicle and cell membranes (in close contact) and help the formation of a transitory connection between the two membranes (Duman and Forte, 2003). Then, a nanometric-size channel, called the fusion pore, appears to be formed between vesicular and extracellular compartments, through which neurotransmitter molecules diffuse slowly out of the cell (Breckenridge and Almers, 1987; Chow et al., 1992; Clements et al., 1992). The fusion pore represents a pivotal intermediate before the complete fusion of the vesicle and plasma membranes, which may lead to the fast and total release of intravesicular compounds. It may also perform as a transient channel that closes itself after a partial release of neurotransmitters and allows the vesicle to be reused by the secretory machinery for another round of exocytosis (Stevens and Williams, 2000; Gandhi and Stevens, 2003). This “kiss and run” mode has been hypothesized to be the main mechanism of release at synapses (Klyachko and Jackson, 2002; Wightman and Haynes, 2004). In other secretory cells, the fusion pore appears to irreversibly expand and lead to the full-fusion of membranes and hence, to a massive release of neurotransmitters, as observed in chromaffin, mast, PC12 or pancreatic β-cells (Kennedy et al., 1993; Finnegan et al., 1996; Pihel et al., 1996). Although the mechanisms underlying the fusion pore formation and its expansion leading to full fusion are of great importance, they are only partially characterized experimentally and still not understood.
The dynamics of the fusion pore have been mainly investigated at the level of single cells by two techniques: patch-clamp measurements of the electrical capacitance of cell membrane (Neher and Marty, 1982; Haller et al., 1998; Dernick et al., 2003) and the amperometric detection of catecholamine neurotransmitters with carbon fibers (Leszczyszyn et al., 1991; Chow et al., 1992). Whereas patch-clamp detects changes of cell membrane area and conductance due to vesicular fusion, the electrochemical method analyzes quantitatively the release of catecholamines from each exocytotic event. When the fusion pore is sufficiently stable, i.e., when its time life is typically higher than a millisecond, the very low amounts of catecholamines emitted during this phase are detected at the microelectrode surface and lead to low amperometric currents, typically in the range of a few picomperes. The time course of release through the pore is then observed as a “foot” preceding the amperometric spike. The spike phase corresponds to the fast and massive release of catecholamines out of the vesicular matrix after the fusion of cell and vesicular membranes.
Based on amperometric studies of exocytosis from adrenal chromaffin cells, a physicochemical model accurately describing the dynamics of full-fusion events from dense core vesicles has been proposed (Amatore et al., 1999, 2000a,b, 2003). This model predicts that the vesicular matrix has a determining role in the opening of the vesicle during the final stage of exocytosis. As soon as the fusion pore opens, the concentration gradient of catecholamine cations (mostly adrenaline in chromaffin cells) provokes their outward diffusion. To maintain electroneutrality within the matrix, this is accompanied by the entry of extracellular hydrated monovalent cations such as Na+ and H+ into the intravesicular matrix. The physicochemical principle of ion exchange, which powers exocytosis of matrix-containing vesicles, was first experimentally demonstrated on the isolated matrix of beige mouse mastocyte granules (Curran and Brodwick, 1991; Marszalek et al., 1997). This was further supported by experiments on chromaffin cells reported by some of us and others (Jankowski et al., 1994; Amatore et al., 2003), where the external cell medium was transitory supplemented with bi- and trivalent cations to modify the exchange between extracellular cations and the ones in the matrix. The loss of exocytosis in these experiments demonstrated a contrario the necessity of dipole-dipole repulsive interactions, created when monovalent cations complex to the matrix anionic sites at the onset of local matrix swelling (Curran and Brodwick, 1991; Barrat and Joanny, 1996; Marszalek et al., 1997). The swelling may increase up to 30% the matrix volume (Fesce et al., 1994; Marszalek et al., 1997) and offers much more pathways of diffusion to the catecholamine molecules.
This overall process allows an outward catecholamine flux to be released through the fusion pore, which is observed as a low amperometric “foot” current (see above). It is usually considered that during this phase, the fusion pore has not yet expanded but rather fluctuates between open and closed phases (deToledo et al., 1993; Neher, 1993). Its geometry may be imposed by a specific architecture, which is still a matter of debate, possibly implicating SNARE protein complexes or phospholipids or a combination of both (Brunger, 2000; Almers, 2001; Zimmerberg, 2001). However, at this step, it may be considered that the pore energy is necessarily controlled by two opposite phenomena. The first one is positive and tends to close the pore. It reflects the edge energy of the pore rim. The second one is negative and tends to open the pore. It stems from Laplace tensions of the cell and vesicle membranes (Amatore et al., 1999, 2000a,b, 2003; Chizmadzhev et al., 2000). Initially, the edge energy is larger than the surface energy so that the pore remains stable. Owing to the continuous cation exchange, which occurs as soon as the pore is opened, the matrix swells. In dense core vesicles, the matrix expansion likely increases the pressure within the vesicle and provokes a continuous increase of the vesicle membrane tension. Unless the pore closes before, the surface energy of a toroidal pore thus ineluctably increases until reaching the point where it compensates the pore edge energy (Amatore et al., 2000a,b, 2003). When this point is reached, the pore becomes unstable and enlarges irreversibly (Taupin et al., 1975; Sandre et al., 1999; Amatore et al., 2000a,b, 2003).
The pore expansion initiates a massive release of catecholamines reflected by a sharp increase of current detected at the electrode immediately after the foot current. The ensuing membrane fusion ends by fully exposing the vesicular matrix to the external medium. Afterwards, the progressive depletion of catecholamines by diffusion is observed by a decrease in current. This scheme is proposed for the exocytosis of dense core vesicles that constitute the main category of vesicles within chromaffin cells. Vesicles without a dense core as in nerve terminals (Li et al., 1996) or with a smaller dense core versus the total vesicle volume as observed in PC12 cells (Colliver et al., 2000) may proceed differently in the fusion pore expansion. The main difference should lie in the physicochemical origin of the building up of the internal pressure within the vesicle and consequently, of the surface tension of the fusion pore. It might undoubtedly affect its dynamics and provide differences between cell types in their release dynamics detected by amperometry. Nevertheless, although prespike feet were observed in the early experiments of amperometric detection of exocytosis, a few studies of the biological (Burgoyne et al., 2001; Wang et al., 2001; Burgoyne and Barclay, 2002; Robinson et al., 2002) or physicochemical parameters influencing their dynamics have been reported (Zhou et al., 1996; Amatore et al., 2003; Haque and Lentz, 2004; Sombers et al., 2004).
In this article, we wish to report the analysis of a very large population of amperometric spikes with a foot in comparison with spikes without a foot detected within the same set of experiments on chromaffin cells. The probability of detecting a distinct foot event is correlated to the catecholamine content of the vesicle as represented by the electrical charge of the amperometric spike. We infer that the amperometric events with a foot correspond in general to vesicles with a higher content in catecholamines. The mean charge of the spikes with a foot was found to be twice that of the spikes without a foot, which might be explained by a significant proportion of the large vesicles that demonstrate long duration feet in chromaffin cells being created from prefusion between two smaller vesicles.
EXPERIMENTAL SECTION
Cell culture and preparation
Bovine chromaffin cells were prepared by collagenase digestion of the medulla of adrenal glands obtained from a local slaughterhouse (Meaux, France). Cells were purified and cultured using previously described methods (Livett, 1984). They were then plated at a density of 4 × 104 cells/cm2 on collagen-poly-L-lysine coated glass coverslips placed in 24 wells plates and incubated in a 5% CO2 atmosphere at 37°C. Cells were used on days 3–10 after culture and 24 h maximum after plating.
Electrode preparation and single cell experiments
Carbon fiber microelectrodes (7-μm diameter, Thornel Carbon Fibers, Cytec Engineered Materials, Greenville, SC) were constructed as described previously (Arbault et al., 1995) and backfilled with mercury. Electrode tips were polished at a 45° angle on a diamond dust-embedded micropipette beveling wheel (Model EG-4, Narishige, London, UK) for 20–30 min before experiments. Only electrodes with a very stable amperometric baseline current were used for cell measurements.
Cells were prepared by placing each coverslip into a 35 mm plastic dish filled with 5 mL of isotonic physiological saline (154 mM NaCl, 4.2 mM KCl, 0.7 mM MgCl2, 11.2 mM glucose, 10 mM HEPES, pH 7.4). After positioning the dish onto the stage of an inverted microscope (Axiovert-135, Carl Zeiss, Germany), the carbon fiber microelectrode surface was positioned with a micromanipulator (Model MHW-103, Narishige, Japan) in contact with the membrane of an isolated chromaffin cell. The close proximity of the electrode surface to the cell surface was confirmed by a slight deformation in the outline of the cell. Then, a glass microcapillary (20–30 μm diameter) was positioned with a second micromanipulator at a distance of 20–30 μm from the cell and used to inject for 10 s (Femtojet injector, Eppendorf, Hamburg, Germany) a stimulating solution (BaCl2 2mM in Locke buffer supplemented with 0.7 mM MgCl2, without carbonates) toward the cell surface. The microelectrode was kept in place during the stimulation and all along the secretion process, the mean time length of which was ∼3 min. Each cell was only stimulated once. All experiments were performed at room temperature.
Data acquisition and data analysis
Electrodes were held at + 0.65 V versus a silver/silver chloride reference electrode using a commercially available picoamperometer (model AMU-130, Radiometer Analytical Instruments, Copenhagen, Denmark), for which the adjustable time response was set at 0.05–0.5 ms, depending on the experiment. The output was digitized at 10–40 kHz, displayed in real time and stored on a computer (Powerlab-4SP A/D converter and software Chart, ADinstruments, Colorado Springs, CO) with no subsequent digital filtering.
Each amperometric trace obtained during cell secretion was visually inspected and signals were designated as exocytotic spikes if their maximum current values were three times higher than the RMS noise (0.4–0.7 pA) of the baseline current (30 ms minimum time length) recorded before each signal. Special attention was applied to verify the baseline stability before and after each spike to avoid spike superimposition. Generally, 50–200 spikes could be isolated from each trace following these criteria. Spikes were designated as having a foot by the existence of a current increase and an inflection point or a slope discontinuity distinguishing the end of the foot portion of the trace from the onset of the main event. Each spike characteristic, i.e., the maximum oxidation current Imax (pA), the total electrical charge Q (fC), the half-width t1/2 (ms), and the rise time t20–90 (the delay between I = 20% of Imax and I = 90% of Imax in ms), was determined using locally written software. All values are reported as the mean ± SE considering Gaussian-type distributions of the data (Imax, Q, t1/2, t20–90) or of its logarithm (Q) and all plots were created using Kaleidagraph (Synergy Software, Essex Junction, VM). The statistical analysis of histograms was done using Origin 7 software (OriginLab, Northampton, MA).
RESULTS
Quantitative and kinetic parameters of the amperometric spikes with and without a foot on chromaffin cells
These experiments were conducted under control conditions, i.e., following a commonly used protocol, where a single chromaffin cell is stimulated for 10 s by a 2-mM barium ion solution, without any previous or further modification of the cells or of the medium used for measurements. The exocytotic response of 32 cells in six different experiments corresponding to different animal origins was monitored by amperometry on carbon fiber microelectrodes. These experiments provided a large sample of data, representative of the mean response of chromaffin cells (a typical response is shown in Fig. 1 a). 1390 exocytotic events could be isolated from the amperometric recordings following the criteria for selection and analysis described above. The quantitative and kinetic parameters analyzed for each spike were in this case the maximum oxidation current Imax, the total electrical charge Q, the half-width t1/2, and the rise time t20–90 (Amatore et al., 2003). Mean values of these parameters, reported in Table 1, are in good agreement with those previously reported, being within the usual variability, presumably due to variations of the cell origin, the experimental conditions for the detection of the spikes and then, the criteria for their selection (Borges et al., 1997, 2001; Haller et al., 1998; Elhamdani et al., 2001).
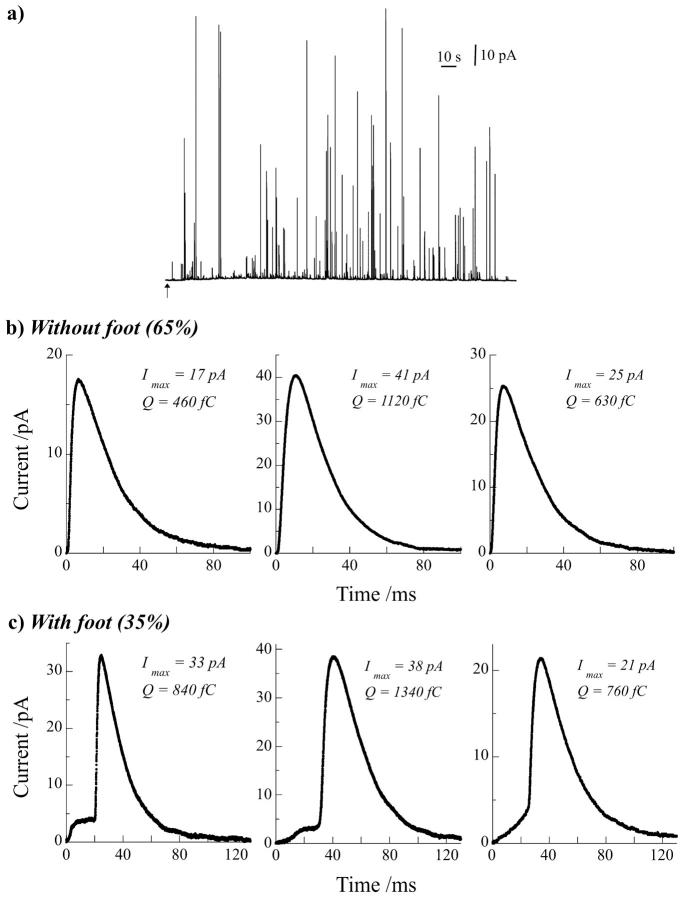
FIGURE 1.
(a) Representative exocytotic response of a bovine chromaffin cell detected by amperometry at a carbon fiber microelectrode. The arrow under the trace represents the start of injection (10 s) on the cell of the stimulus solution containing Ba2+ (2 mM). From these traces three representative amperometric events were extracted without (b) and with a discernable foot (c) preceding the spike. Three different shapes of foot were usually observed (c) that differ on how the foot current onsets (fast versus progressive rise, see left and middle spikes, respectively) and if the foot current linearly increases with time until the spike onset (right spike) or stabilizes as a plateau current after its initial rising phase until the current burst (middle spike). Quantitative parameters of each spike are given.
TABLE 1.
Quantitative and kinetic parameters of the amperometric spikes detected during exocytosis of bovine chromaffin cells
| All spikes n = 1390 | Spikes W/O foot n = 905 | Spikes W foot n = 485 | |
|---|---|---|---|
| Imax (pA) | 25 ± 2 | 23 ± 2 | 30 ± 3 |
| Q (fC) | 950 ± 70 | 850 ± 90 | 1140 ± 110 |
| t1/2 (ms) | 32.5 ± 1.0 | 33.0 ± 1.0 | 32.0 ± 1.0 |
| t20–90 (ms) | 8.5 ± 0.5 | 8.5 ± 0.5 | 8.5 ± 0.5 |
| Log (Q) | 2.75 ± 0.01 | 2.65 ± 0.02 | 2.91 ± 0.02 |
(n = 32 cells from six different experiments), as a function of the presence (noted W for “with”) or absence (noted W/O for “without”) of a foot preceding the spike. Imax is the maximum oxidation current of the spike, Q is its total electrical charge, t1/2 is its half-width, and t20–90 is a measurement of its rise time, i.e., the delay between I = 20% of Imax and I = 90% of Imax. Mean values of log (Q) reported here are means of the Gaussian curves fitting the statistical distribution of all log (Q) values in each condition (Fig. 4) and not the direct average calculation of these values (see text for details). Catecholamines release from chromaffin cells was elicited by a 10-s injection of a Ba2+ 2 mM solution. Data are presented as mean ± SE.
Overall, in these experiments, 35% of the amperometric spikes displayed an analyzable foot, i.e., with a maximum current Ifoot larger than 2 pA (signal/noise = 3) and a time duration tfoot larger than 1 ms. We reproducibly observed three distinct types of foot shape illustrated by the representative set of spikes shown in Fig. 1 c, in comparison with spikes without detectable foot in Fig. 1 b. These types differed on how the foot current onsets (fast versus progressive rise) and if the foot current linearly increased with time until the spike onset or stabilized as a plateau current after its initial rising phase until the current burst. The foot frequency and mean values of Ifoot (3.5 ± 0.5 pA) and tfoot (28 ± 3 ms) are in good agreement with the same measurements generally reported for chromaffin cells (Chow et al., 1992; Zhou et al., 1996; Albillos et al., 1997).
More interestingly, when spikes with a foot were separately analyzed from the ones without a foot (see Table 1), the mean values of the maximum current Imax and the electrical charge Q were significantly higher for spikes with a foot. Mean values of the kinetic parameters t1/2 and t20–90 were not significantly different between the two categories of spikes. We also observed that the percentage of spikes with a foot and the difference of amplitude, according to charge measurement, versus spikes without a foot were both relatively constant along the secretory response (Fig. 2, a and b). Oscillations of these parameters with time merely resulted from the large variability between spikes when the analysis was necessarily focused on small time windows, such as 10 s. Finally, these different analyses indicated that exocytotic vesicles leading to amperometric events with a foot generally contained larger quanta of catecholamines than the ones leading to events without a foot.
FIGURE 2.
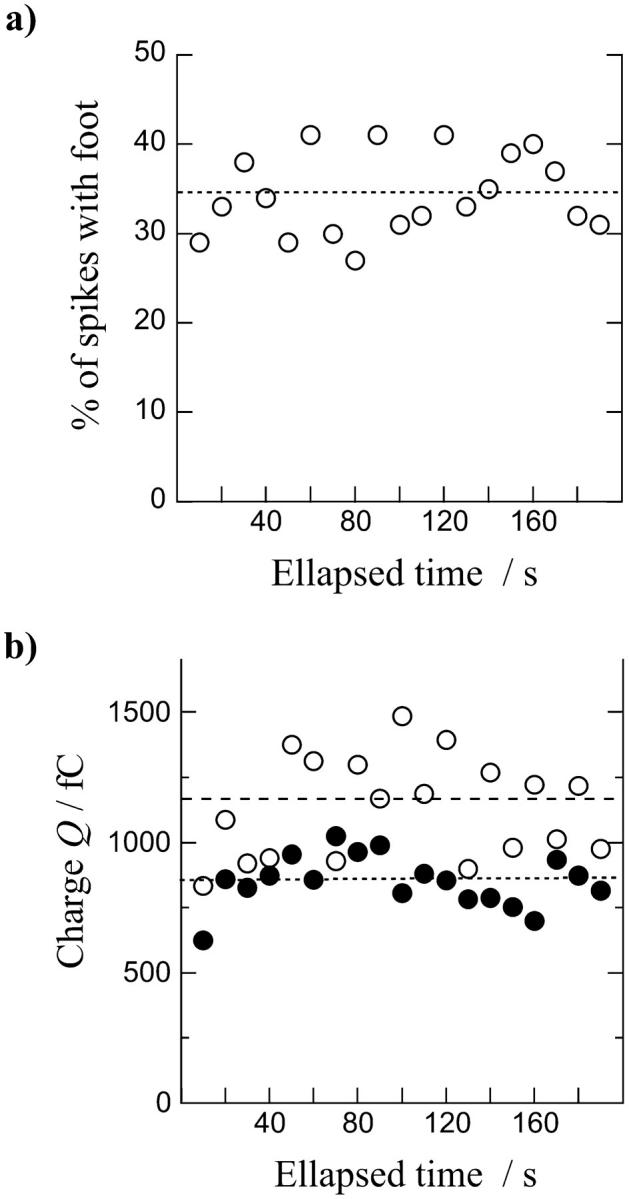
(a) Evolution of the percentage of spikes with a foot observed on amperometric traces as a function of time after the chromaffin cell response to stimulation (10-s injection of a 2 mM Ba2+ solution). (b) Compared evolution of the electrical charge Q of spikes with (○) or without (•) a foot along the experiment. Foot percentage and means of Q values were calculated every 10-s period. The mean of each parameter for all pooled values as given in Table 1 is represented on each graph (small dotted line for spikes without foot and large dotted line for spikes with foot), showing the variations of the values sampled over a small time window (10 s) across the global average.
Correlation between the frequency of a spike's foot and the spike's charge
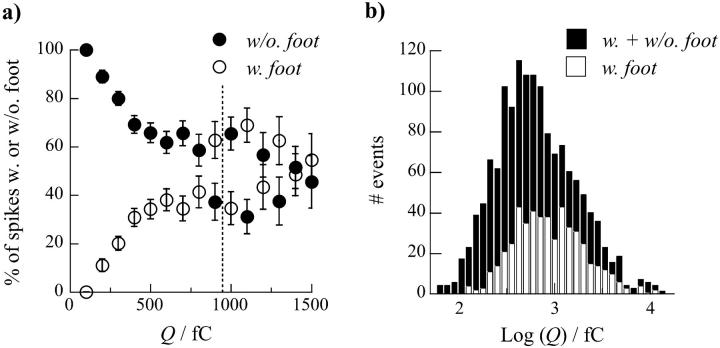
To investigate if a correlation exists between the probability to observe a spike's foot and the catecholamine content of each vesicle, we analyzed the evolution of the percentage of spikes with or without a foot as a function of the spikes' charge Q (see Fig. 3 a). The frequency of spikes displaying a foot was close to zero for small values of Q and regularly increased with Q until reaching a limiting value of ∼50% for Q > 800 fC. This result confirmed the above observations (Table 1 and Fig. 2) on the differences of mean charge values between the two populations and shows that occurrence of a prespike foot feature is clearly favored for vesicles containing larger quanta of catecholamines.
FIGURE 3.
(a) Evolution of the percentage of spikes with (○) or without (•) a foot as a function of their total electrical charge Q (bin = 100 fC). Error bars have been calculated as mean ± SE based on the probability p in a binomial law to observe a foot or not: σ2 = p(1 − p); SE = σ/(nvalues)1/2. Values above 1500 fC are not shown because of too large fluctuations in the percentages of spikes due to a small number of events occurring in this range of Q. The vertical dotted line is the mean charge considering all spikes (see Table 1). (b) Histograms of the Q value distribution for all spikes (black bar) and only for spikes with a foot (open bar). Log transformed values were used since histograms of raw values are heavily skewed to the right. The two histograms are superimposed so that one can visually see the contribution for spikes without a foot from the difference between the envelope of the distribution for all spikes and the one for spikes with a foot.
However, these conclusions have been inferred on the basis of charge Q analysis, for which values span a large range, so that a precise quantification of differences between the populations of vesicles leading to spikes with and without foot is currently difficult. Consequently, the statistical distribution of Q values for both types of spikes is largely skewed. These values can be satisfactorily fitted by log-normal laws (data not shown) (Amatore et al., 1999, 2000b) providing evidence that the range of values is >2 orders of magnitude. An analysis of the statistical distributions based on log (Q) values instead of Q provides a better statistical weight to all events. The normal-type distribution obtained for log (Q) is basically justified through its correlation with the normal-type distribution of vesicles' size in chromaffin cells (Coupland, 1968). Thus, the differences between log (Q) values for spikes with and without foot are more evident (see Table 1 and Figs. 3 b and 4). When these two distributions are both superimposed on a cumulative histogram of each population (overall 35% of spikes with a foot versus 65% of spikes without a foot) in the distribution for all spikes (see Fig. 3 b), it becomes apparent that spikes with a foot are predominant at higher values of log (Q). These differences can be quantified by fitting each log (Q) distribution with Gaussian laws (noted f (Q)). As can be observed on Fig. 4, the Gaussian fit matches extremely well the distributions relative to all spikes (noted fall(Q), Fig. 4 a) and to spikes with foot (noted fW(Q), Fig. 4 c), whereas the Gaussian fit is not as good for the distribution of spikes without a foot (noted fW/O(Q), Fig. 4 b). This latter distribution displays a shoulder after its maximum, possibly showing the overlap of two different populations (see below). The distributions for spikes with and without a foot are clearly different, respectively centered on 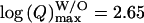 and
and 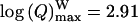 in chromaffin cells, leading to the conclusion that they correspond to different populations of vesicles. From that result, we estimate (compare in Table 1:
in chromaffin cells, leading to the conclusion that they correspond to different populations of vesicles. From that result, we estimate (compare in Table 1: 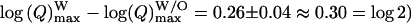 that the population of vesicles giving rise to a foot contains approximately twice the amount of catecholamines compared to the population giving no foot feature. Finally, it appears from these analyses that the overall distribution of charge values for all spikes (Fig. 4 a) corresponds to the overlap of at least two distributions with different means and standard deviation, corresponding to spikes with or without foot and reflecting two different categories or size of vesicles.
that the population of vesicles giving rise to a foot contains approximately twice the amount of catecholamines compared to the population giving no foot feature. Finally, it appears from these analyses that the overall distribution of charge values for all spikes (Fig. 4 a) corresponds to the overlap of at least two distributions with different means and standard deviation, corresponding to spikes with or without foot and reflecting two different categories or size of vesicles.
FIGURE 4.
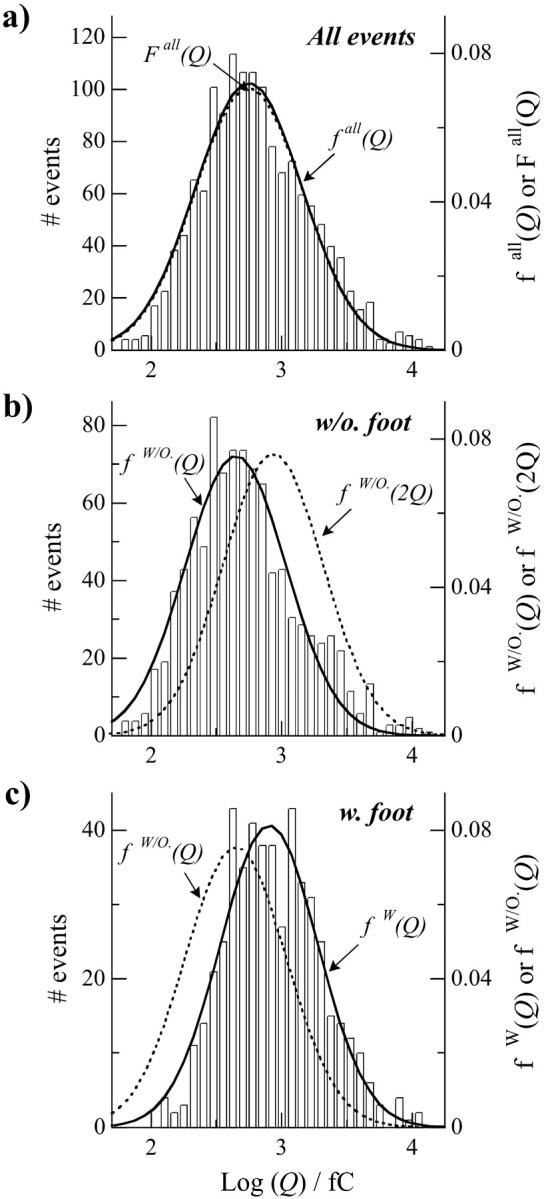
Histograms of the log (Q) values for all spikes (a), and separately for the ones without a foot (b) and the ones with a foot (c). Each distribution is presented as a function of events number (left y axis) or the statistical probability density (right y axis) and is fitted by a single Gaussian distribution, named fall(Q) for all spikes, fW/O(Q) for spikes without a foot, and fW/O(Q) for spikes with a foot. The parameters for the Gaussian distributions are 2.75 ± 0.02 and 0.82 ± 0.02 for all spikes, 2.65 ± 0.02 and 0.77 ± 0.02 for spikes without a foot, and 2.91 ± 0.02 and 0.75 ± 0.02 for spikes with a foot for their center position (log (Q)max) and their half-width, σ, respectively. Graphs are presented in a column so that one can easily observe the shift between centers of the different distributions. The comparison between the Gaussian fitting fW/O(Q) and the distribution for spikes with a foot in c shows the differences of vesicular quantal size between spikes with and without a foot. In b, a mathematically constructed Gaussian distribution fW/O(2Q) was created using the same conditions but applied to a mean charge of 2Q instead of Q (parameters: log (Q)max = 2.95; σ = 0.77). fW/O(2Q) and fW(Q) coincide well within the accuracy of their determination, showing that vesicles leading to the events with a foot contain on average twice the quantity of catecholamines of the vesicles that lead to events without foot. Finally, we constructed a Gaussian distribution Fall(Q) corresponding to all spikes by the combination of the two individual distributions fW/O(Q) and fW/O(2Q), weighted each as a function in the overall of the percentage of spikes without and with foot, respectively, 65% and 35%. Fall(Q) is compared in a with the distribution of log (Q) values.
To test more precisely the above hypothesis that vesicles leading to events with a foot contained on average twice the quantity of catecholamines of the vesicles that lead to events without a foot, we have constructed mathematically a would-be Gaussian distribution fW/O(2Q), viz. using the same law as fW/O(Q) but applied to a mean charge 2Q instead of Q. As noted in Fig. 4 b, fW/O(2Q) could not be definitively match with the experimental distribution for events without a foot even when considering the accuracy of Gaussian distribution determination. In contrast, when fW/O(2Q) was compared with the experimental distribution describing events with a foot (Fig. 4 c), the two distributions fW/O(2Q) and fW(Q) coincided within the accuracy of their determination. This provides further evidence that the distribution of quantal sizes of the two populations of vesicles are almost identical when considering their distribution around their mean charge, but that the mean charge is twice as large for vesicles giving rise to a foot, compared to those not displaying a foot. This led us to propose ex nihilo an analytical distribution for all spikes by combining the two individual distributions fW/O(Q) and fW/O(2Q) ≈ fW(Q) where each is weighted as a function of the overall percentage of spikes with foot (viz. 35% for fW/O(2Q), see above). This constructed mathematical distribution F(Q) almost coincides with the experimental one for fall(Q) obtained for all spikes shown in Fig. 4 a.
DISCUSSION
Amperometric measurements of exocytotic events on chromaffin cells provide information and quantification of the dynamics of the initial fusion pore when a prespike foot can be detected. Owing to an increased accuracy in the measurements of amperometric events and to the use of a large and representative sample of data from several experiments, it has been possible to investigate with precision several properties of spikes with a foot. In these experiments and in agreement with previous reports, a foot was observed for 35% of the exocytotic events from chromaffin cells. This means that for the other 65% of spikes, the fusion pore stage is too transient to lead to a detectable flux of neurotransmitters with current techniques, so that it cannot be detected as a distinct foot feature before the full-fusion stage of exocytosis. It seems plausible that this dichotomy may reflect differences in biological mechanisms controlling the pore structure and regulating the different phases of exocytosis, but it also might reflect differences in physicochemical parameters that contribute at different levels to the control of the fusion pore stability and command the kinetics of its expansion (Marszalek et al., 1997; Amatore et al., 1999, 2000a,b, 2003). Nevertheless, since a foot was clearly observed on 35% of the exocytotic events, the slow and low release of catecholamines through the fusion pore represents an unambiguous mode for release of neurotransmitters by chromaffin cells, and potentially for other cells. This is even more evident in the case of synapses where the pore-release mechanism appears to be a basic feature of neurotransmission (Bruns et al., 2000; Klyachko and Jackson, 2002). Thus, the determination of all parameters governing this type of release and the reasons that provoke the shift toward the second mode of release (viz. full fusion of the vesicle with the membrane) leading to massive exocytosis of compounds is obviously of great importance (Neher, 1993).
In this context, we have recently reported several issues on the relations between vesicles characteristics and the fusion pore dynamics in PC12 cells (Sombers et al., 2004). Under control conditions, although the relative proportion of spikes with a foot is clearly not associated with higher values of spikes charge than spikes without a foot for PC12 cells, the parameters of each foot (i.e., their charge and duration) did increase for events involving larger amounts of release of catecholamine. In addition to these parameters, the frequency of observation of feet was modified when PC12 cells were pharmacologically treated by reserpine or L-DOPA. Reserpine favored the observation of a foot but decreased its duration and charge in parallel with a decrease in the average vesicle volume observed in TEM. L-DOPA induced the opposite trend since it increased the vesicle volume and feet parameters (i.e., charge and duration) while decreasing their frequency. These results on PC12 cells led us to hypothesize that the physicochemical characteristics of the vesicles are key factors in the control of the fusion pore dynamics. This was reasoned to result from dense core vesicles with a core that is significantly smaller than the vesicular volume. Thus, in this scenario the expanding core increases the tension of the vesicle membrane and stabilizes the fusion pore in a nanotube versus a toroidal transition state.
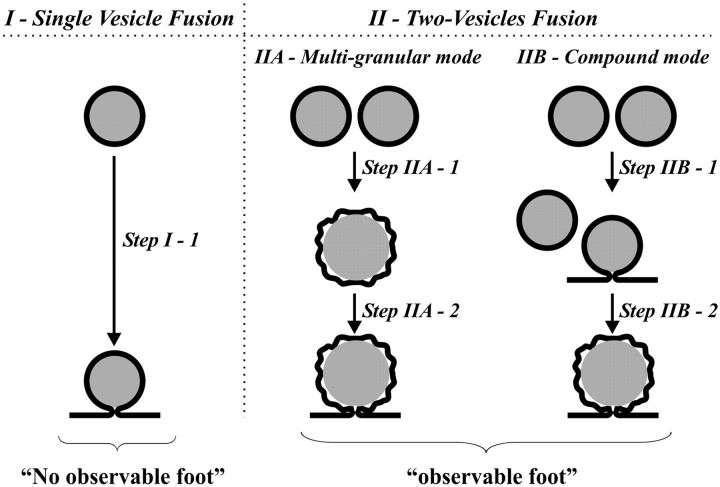
In contrast, these experiments have been performed on normal chromaffin cells where the dense core is thought to fill the vesicle for normal vesicles and thus an expanding core will destabilize a toroidal transition state and push open the fusion pore. Thus, smaller vesicles, having a larger surface/volume ratio, should have a lower probability for significant release via the fusion pore (Amatore et al., 1999, 2000a, 2000b). In this case, the spikes displaying an analyzable foot correspond clearly to the exocytosis of vesicles containing larger quanta of catecholamines. On average, these larger vesicles contain twice the amount of adrenaline (and/or nor-adrenaline) than the vesicles that lead to the events without a detectable foot. Such a factor too may readily be explained by the occurrence of compound or multigranular exocytosis (deToledo and Fernandez, 1990; Bokvist et al., 2000; Lollike et al., 2002) of two individual vesicles from the population that leads to events without foot (Fig. 5). In the compound mode, a vesicle fuses with another one, already fusing with the cell membrane and undergoing exocytosis. In the multi-granular mode, the two vesicles may fuse together to form a larger vesicle, which then fuses with the plasma membrane. These processes are well documented in synapses and mast cells, but have not been reported in chromaffin cells to the best of our knowledge.
FIGURE 5.
Cartoon depicting the different possibilities considered here for vesicle fusion and exocytosis in relevance with the presence of an observable foot. See text for the definition of each mode.
In these two modes of exocytosis, the combination of two dense core vesicles of radius r0 and volume Vsingle entirely filled by the matrix will create a vesicle of membrane area:
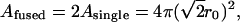 |
(1) |
assuming the addition of phospholipids from the two membranes as observed during full fusion events (Zenisek et al., 2000). Assuming that the fused vesicle membrane is not crippled, its surface area would correspond to a spherical vesicle of radius being 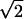 times larger than those of the original vesicles. The inner volume of such a new vesicle of radius
times larger than those of the original vesicles. The inner volume of such a new vesicle of radius 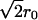 would be:
would be:
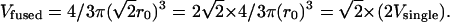 |
(2) |
Consequently, Vfused would be 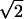 times larger than the direct sum of the volumes of the original vesicles. Thus, immediately after fusion, the resulting new vesicle must compensate this discrepancy between its real granule volume and its membrane surface area. This may occur either by the formation of a halo between its matrix and its membrane as clearly observed in PC12 cells or by having a crippled membrane surrounding closely its matrix (wavy shape of the resulting vesicle in Fig. 5). In this context, it was recently reported (Pothos et al., 2002) that after stimulation of a cell to release the volume of many chromaffin vesicles increased and that so called active vesicles displayed a clear halo around their dense core. Vesicles formed that have a significant halo or a crippled membrane should develop a slower increase and/or a lower maximum value of surface tension than the membranes of the original noncompound vesicles when they undergo exocytosis. Since the equalization of membrane tension energy is likely the condition for pore expansion in the toroidal model (Chanturiya et al., 1997; Sandre et al., 1999; Amatore et al., 2000a,b; Chizmadzhev et al., 2000), the lifetime of the pore of fused vesicles should increase for the less tense vesicles, therefore leading to the observation of a foot on the amperometric spikes. Moreover, the presence of a liquid film in the halo between the vesicle membrane and the matrix would increase the local diffusion coefficient and the exposed matrix area. Both factors increase the rate of release through the pore. The foot current duration may then increase and again facilitate its observation. Such variations in the structure of vesicles after either multigranular fusion or either compound exocytosis can thus explain the increase of percentage of events that display a foot as the overall amount released increases. Nevertheless, at this stage our data cannot distinguish these different versions of mutivesicle fusion and exocytosis. Indeed, amperometric measurements give access to kinetics of vesicle membrane fusion step but do not contain information about the kinetic stages arising before exocytosis starts.
times larger than the direct sum of the volumes of the original vesicles. Thus, immediately after fusion, the resulting new vesicle must compensate this discrepancy between its real granule volume and its membrane surface area. This may occur either by the formation of a halo between its matrix and its membrane as clearly observed in PC12 cells or by having a crippled membrane surrounding closely its matrix (wavy shape of the resulting vesicle in Fig. 5). In this context, it was recently reported (Pothos et al., 2002) that after stimulation of a cell to release the volume of many chromaffin vesicles increased and that so called active vesicles displayed a clear halo around their dense core. Vesicles formed that have a significant halo or a crippled membrane should develop a slower increase and/or a lower maximum value of surface tension than the membranes of the original noncompound vesicles when they undergo exocytosis. Since the equalization of membrane tension energy is likely the condition for pore expansion in the toroidal model (Chanturiya et al., 1997; Sandre et al., 1999; Amatore et al., 2000a,b; Chizmadzhev et al., 2000), the lifetime of the pore of fused vesicles should increase for the less tense vesicles, therefore leading to the observation of a foot on the amperometric spikes. Moreover, the presence of a liquid film in the halo between the vesicle membrane and the matrix would increase the local diffusion coefficient and the exposed matrix area. Both factors increase the rate of release through the pore. The foot current duration may then increase and again facilitate its observation. Such variations in the structure of vesicles after either multigranular fusion or either compound exocytosis can thus explain the increase of percentage of events that display a foot as the overall amount released increases. Nevertheless, at this stage our data cannot distinguish these different versions of mutivesicle fusion and exocytosis. Indeed, amperometric measurements give access to kinetics of vesicle membrane fusion step but do not contain information about the kinetic stages arising before exocytosis starts.
Such interpretations are also based on the assumption that large vesicles resulting from prefusion of smaller vesicles do not undergo any membrane readjustments, such as phospholipid retrieval during a maturation process before fusion. One may envision that a fraction of these fused vesicles may be formed far from the docking loci (deToledo and Fernandez, 1990; Zenisek et al., 2000), so that they may be transformed during trafficking and may thus lose their excess of membrane area (phospholipid content) and consequently, adjust their internal volume to the size of the matrix. This hypothesis is supported by the results recently obtained by Gong et al. (2003) with patch amperometry analysis on chromaffin cells treated by reserpine and L-DOPA. Modification of vesicle charge observed from catecholamine release was followed by changes in vesicle volume so that the internal concentration remained quite constant under control, reserpine, or L-DOPA incubation. This was also reported for PC12 cell vesicles (Colliver et al., 2000). In both cases, it is not clear where the phospholipids are transported, but vesicles appear to loose or incorporate phospholipids in the membrane to adapt to the vesicle content. Such a tailoring process may produce large dense core vesicles with membrane properties similar to the ones of smaller original dense core vesicles that had not fused, though their mean charge would be twice as large. This may readily explain the observation reported above that a significant population (∼50% in Fig. 3 a) of vesicles containing high quantities of catecholamines (Q > 800 fC) lead to spikes without a foot.
The existence of such fused and matured vesicles could also be responsible of the presence of the shoulder in the distribution of large-size release events (Figs. 3 b and 4 b). Within the framework of this hypothesis one need then to consider that the process should be iterative. Such fused vesicles that may have been “tailored” may further fuse together or fuse with other unfused vesicles, so that a population of vesicles should exist, for which the charge observed upon release is equivalent to four times or three times, respectively, the mean value of Q for single unfused vesicles, and so on. This should give rise to an overall population consisting of a series of distributions of vesicles with a released charge, centered at n × Q, with n = 1, 2, 3, etc., and with a statistical probability that decreases with n. This could be the reason for the existence of the wavy tail at large Q values, which is apparent on each distribution shown in Fig. 4, a–c. Note that similar shoulders have also been frequently observed and reported previously (Finnegan et al., 1996; Elhamdani et al., 2001), though to the best of our knowledge such information has never been rationalized nor used. Nevertheless, a sound statistical treatment with this iterative fusion framework will clearly require the treatment of a much larger set of exocytotic events and was therefore beyond the scope of this work.
Finally, in this work a detailed analysis of the amperometric events recorded during the exocytosis of chromaffin cells under controlled conditions has shown that the physicochemical properties of vesicles are key factors in the control of the dynamics of release through the fusion pore. In chromaffin cells, the vesicles leading to events where a foot is observed contain approximately twice as much catecholamine as the vesicles leading to releasing events without an observable foot. These double vesicles may originate to some extent from fusion between vesicles that have been created by fusion of two vesicles before or during exocytosis (Fig. 5, panel IIA and IIB). The ensuing increase of volume may modify the conditions of matrix expansion, yield a decrease in initial vesicle membrane tension, change the pattern and properties of catecholamines diffusion and, consequently, the dynamics of the fusion pore expansion.
These results correlate with the ones we obtained on PC12 cells since in both models, the dynamics of the fusion pore is modulated by the properties of the vesicles. Though these cell types display opposing trends in behavior with vesicle size, overall our results strongly suggest that in addition to biological factors, some physicochemical mechanisms can modulate the release events and it appears that membrane tension and its cause are critically important factors in determining the probability of observing significant release via the fusion pore. Consistent with our data, the effect of membrane tension on vesicle opening appears to be related to the vesicle type and pore transition state and this in fact, can be used to explain the differences between cell types. In chromaffin cells, where the dense core is believed to nearly fill the vesicle, the expanding core appears to be a controlling factor in opening a toroidal fusion pore. Occurrence of prefusion of two smaller vesicles leads to excess membrane, which slows pore expansion leading to an increased observation of events with a foot. In PC12 cell vesicles, the core does not fill the interior volume and pressure on the membrane from an expanding dense core is hypothesized to stabilize a nanotube transition state in the fusion pore versus a toroidal transition state in the normal chromaffin cell vesicles. Thus, the mechanism of vesicle opening and release via the fusion pore appears to differ depending on the characteristics and size of dense core vesicles. Exocytosis is obviously more complex and variable than what has long been described leading to the hypothesis of discrete quantal size.
Acknowledgments
This work has been supported in part by CNRS (UMR 8640; Program “Dynamique et réactivité des assemblages biologiques”), Ecole Normale Supérieure, and by the French Ministry of Research (MDRNT). I.B. acknowledges a PhD fellowship from CONACYT-SFERE. We are also greatly indebted to the slaughterhouse of Meaux (France) for the supply of adrenal glands and to UPR CNRS 1929 (team of Dr. F. Darchen, IBPC, Paris) and to its director, Dr. J.P. Henry, for help during the chromaffin cells preparation. A.G.E. acknowledges support from the National Institutes of Health.
References
- Albillos, A., G. Dernick, H. Horstmann, W. Almers, G. A. deToledo, and M. Lindau. 1997. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 389:509–512. [DOI] [PubMed] [Google Scholar]
- Almers, W. 2001. Fusion needs more than SNAREs. Nature. 409:567–568. [DOI] [PubMed] [Google Scholar]
- Amatore, C., Y. Bouret, and L. Midrier. 1999. Time-resolved dynamics of the vesicle membrane during individual exocytotic secretion events, as extracted from amperometric monitoring of adrenaline exocytosis from chromaffin cells. Biochimie. 5:2151–2162. [Google Scholar]
- Amatore, C., Y. Bouret, E. R. Travis, and R. M. Wightman. 2000a. Adrenaline release by chromaffin cells: Constrained swelling of the vesicle matrix leads to full fusion. Angew. Chem. Int. Ed. 39:1952–1955. [DOI] [PubMed] [Google Scholar]
- Amatore, C., Y. Bouret, E. R. Travis, and R. M. Wightman. 2000b. Interplay between membrane dynamics, diffusion and swelling pressure governs individual vesicular exocytotic events during release of adrenaline by chromaffin cells. Biochimie. 82:481–496. [DOI] [PubMed] [Google Scholar]
- Amatore, C., S. Arbault, I. Bonifas, Y. Bouret, M. Erard, and M. Guille. 2003. Dynamics of full fusion during vesicular exocytotic events: release of adrenaline by chromaffin cells. ChemPhysChem. 4:147–154. [DOI] [PubMed] [Google Scholar]
- Arbault, S., P. Pantano, J. A. Jankowski, M. Vuillaume, and C. Amatore. 1995. Monitoring an oxidative stress mechanism at a single human fibroblast. Anal. Chem. 67:3382–3390. [DOI] [PubMed] [Google Scholar]
- Barrat, J. L., and J. F. Joanny. 1996. Theory of polyelectrolyte solutions. In Advances in Chemical Physics, Vol. XCIV. I. Prigogine and S. A. Rice, editors. J. Wiley and Sons, UK. 1–66.
- Bokvist, K., M. Holmqvist, J. Gromada, and P. Rorsman. 2000. Compound exocytosis in voltage-clamped mouse pancreatic beta-cells revealed by carbon fibre amperometry. Pflueg Archiv Eur J Physiol. 439:634–645. [DOI] [PubMed] [Google Scholar]
- Borges, R., E. R. Travis, S. E. Hochstetler, and R. M. Wightman. 1997. Effects of external osmotic pressure on vesicular secretion from bovine adrenal medullary cells. J. Biol. Chem. 272:8325–8331. [DOI] [PubMed] [Google Scholar]
- Borges, R., R. Jaen, F. Freire, J. F. Gomez, C. Villafruela, and E. Yanes. 2001. Morphological and functional characterization of beige mouse adrenomedullary secretory vesicles. Cell Tissue Res. 304:159–164. [DOI] [PubMed] [Google Scholar]
- Breckenridge, L. J., and W. Almers. 1987. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature. 328:814–817. [DOI] [PubMed] [Google Scholar]
- Brunger, A. T. 2000. Structural insights into the molecular mechanism of Ca2+-dependent exocytosis. Curr. Opin. Neurobiol. 10:293–302. [DOI] [PubMed] [Google Scholar]
- Bruns, D., D. Riedel, J. Klingauf, and R. Jahn. 2000. Quantal release of serotonin. Neuron. 28:205–220. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., R. J. Fisher, M. E. Graham, L. P. Haynes, and A. Morgan. 2001. Control of membrane fusion dynamics during regulated exocytosis. Biochem. Soc. Trans. 29:467–472. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., and J. W. Barclay. 2002. Splitting the quantum: regulation of quantal release during vesicle fusion. Trends Neurosci. 25:176–178. [DOI] [PubMed] [Google Scholar]
- Chanturiya, A., L. V. Chernomordik, and J. Zimmerberg. 1997. Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 94:14423–14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizmadzhev, Y. A., P. I. Kuzmin, D. A. Kumenko, J. Zimmerberg, and F. S. Cohen. 2000. Dynamics of fusion pores connecting membranes of different tensions. Biophys. J. 78:2241–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, R. H., L. Vonruden, and E. Neher. 1992. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 356:60–63. [DOI] [PubMed] [Google Scholar]
- Clements, J. D., R. A. J. Lester, G. Tong, C. E. Jahr, and G. L. Westbrook. 1992. The time course of glutamate in the synaptic cleft. Science. 258:1498–1501. [DOI] [PubMed] [Google Scholar]
- Colliver, T. L., S. J. Pyott, M. Achalabun, and A. G. Ewing. 2000. VMAT-mediated changes in quantal size and vesicular volume. J. Neurosci. 20:5276–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland, R. E. 1968. Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature. 217:384–389. [DOI] [PubMed] [Google Scholar]
- Curran, M. J., and M. S. Brodwick. 1991. Ionic control of the size of the vesicle matrix of beige mouse mast-cells. J. Gen. Physiol. 98:771–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernick, G., G. A. de Toledo, and M. Lindau. 2003. Exocytosis of single chromaffin granules in cell-free inside-out membrane patches. Nat. Cell Biol. 5:358–362. [DOI] [PubMed] [Google Scholar]
- Detoledo, G. A., and J. M. Fernandez. 1990. Compound versus multigranular exocytosis in peritoneal mast-cells. J. Gen. Physiol. 95:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detoledo, G. A., R. Fernandezchacon, and J. M. Fernandez. 1993. Release of secretory products during transient vesicle fusion. Nature. 363:554–558. [DOI] [PubMed] [Google Scholar]
- Duman, J. G., and J. G. Forte. 2003. What is the role of SNARE proteins in membrane fusion? Am. J. Physiol. Cell Physiol. 285:C237–C249. [DOI] [PubMed] [Google Scholar]
- Elhamdani, A., H. C. Palfrey, and C. R. Artalejo. 2001. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 31:819–830. [DOI] [PubMed] [Google Scholar]
- Fesce, R., F. Valtorta, and J. Meldolesi. 1994. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 4:1–4. [DOI] [PubMed] [Google Scholar]
- Finnegan, J. M., K. Pihel, P. S. Cahill, L. Huang, S. E. Zerby, A. G. Ewing, R. T. Kennedy, and R. M. Wightman. 1996. Vesicular quantal size measured by amperometry at chromaffin, mast, pheochromocytoma, and pancreatic beta-cells. J. Neurochem. 66:1914–1923. [DOI] [PubMed] [Google Scholar]
- Gandhi, S. P., and C. F. Stevens. 2003. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 423:607–613. [DOI] [PubMed] [Google Scholar]
- Gong, L. W., I. Hafez, G. A. de Toledo, and M. Lindau. 2003. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J. Neurosci. 23:7917–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller, M., C. Heinemann, R. H. Chow, R. Heidelberger, and E. Neher. 1998. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophys. J. 74:2100–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, M. E., and B. R. Lentz. 2004. Roles of curvature and hydrophobic interstice energy in fusion: Studies of lipid perturbant effects. Biochemistry. 43:3507–3517. [DOI] [PubMed] [Google Scholar]
- Jankowski, J. A., J. M. Finnegan, and R. M. Wightman. 1994. Extracellular ionic composition alters kinetics of vesicular release of catecholamines and quantal size during exocytosis at adrenal-medullary cells. J. Neurochem. 63:1739–1747. [DOI] [PubMed] [Google Scholar]
- Kennedy, R. T., L. Huang, M. A. Atkinson, and P. Dush. 1993. Amperometric monitoring of chemical secretions from individual pancreatic beta-cells. Anal. Chem. 65:1882–1887. [DOI] [PubMed] [Google Scholar]
- Klyachko, V. A., and M. B. Jackson. 2002. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 418:89–92. [DOI] [PubMed] [Google Scholar]
- Leszczyszyn, D. J., J. A. Jankowski, O. H. Viveros, E. J. Diliberto, J. A. Near, and R. M. Wightman. 1991. Secretion of catecholamines from individual adrenal-medullary chromaffin cells. J. Neurochem. 56:1855–1863. [DOI] [PubMed] [Google Scholar]
- Li, J. Y., L. Edelmann, R. Jahn, and A. Dahlstrom. 1996. Axonal transport and distribution of synaptobrevin I and II in the rat peripheral nervous system. J. Neurosci. 16:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livett, B. G. 1984. Adrenal-medullary chromaffin cells-invitro. Physiol. Rev. 64:1103–1161. [DOI] [PubMed] [Google Scholar]
- Lollike, K., M. Lindau, J. Calafat, and N. Borregaard. 2002. Compound exocytosis of granules in human neutrophils. J. Leukoc. Biol. 71:973–980. [PubMed] [Google Scholar]
- Marszalek, P. E., B. Farrell, P. Verdugo, and J. M. Fernandez. 1997. Kinetics of release of serotonin from isolated secretory granules.2. Ion exchange determines the diffusivity of serotonin. Biophys. J. 73:1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, E., and A. Marty. 1982. Discrete changes of cell-membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 79:6712–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, E. 1993. Cell physiology—secretion without full fusion. Nature. 363:497–498. [DOI] [PubMed] [Google Scholar]
- Pihel, K., E. R. Travis, R. Borges, and R. M. Wightman. 1996. Exocytotic release from individual granules exhibits similar properties at mast and chromaffin cells. Biophys. J. 71:1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos, E. N., E. Mosharov, K. P. Liu, W. Setlik, M. Haburcak, G. Baldini, M. D. Gershon, H. Tamir, and D. Sulzer. 2002. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. J Physiol (Lond) 542:453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, I. M., R. Ranjan, and T. L. Schwarz. 2002. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C(2)A domain. Nature. 418:336–340. [DOI] [PubMed] [Google Scholar]
- Sandre, O., L. Moreaux, and F. Brochard-Wyart. 1999. Dynamics of transient pores in stretched vesicles. Proc. Natl. Acad. Sci. USA. 96:10591–10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers, L. A., H. J. Hanchar, T. L. Colliver, N. Wittenberg, A. Cans, S. Arbault, C. Amatore, and A. G. Ewing. 2004. The effects of vesicular volume on secretion through the fusion pore in exocytotic release from PC12 cells. J. Neurosci. 24:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, C. F., and J. H. Williams. 2000. “Kiss and run” exocytosis at hippocampal synapses. Proc. Natl. Acad. Sci. USA. 97:12828–12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin, C., M. Dvolaitzky, and C. Sauterey. 1975. Osmotic-pressure induced pores in phospholipid vesicles. Biochemistry. 14:4771–4775. [DOI] [PubMed] [Google Scholar]
- Wang, C. T., R. Grishanin, C. A. Earles, P. Y. Chang, T. F. J. Martin, E. R. Chapman, and M. B. Jackson. 2001. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 294:1111–1115. [DOI] [PubMed] [Google Scholar]
- Wightman, R. M., and C. L. Haynes. 2004. Synaptic vesicles really do kiss and run. Nat. Neurosci. 7:321–322. [DOI] [PubMed] [Google Scholar]
- Zenisek, D., J. A. Steyer, and W. Almers. 2000. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 406:849–854. [DOI] [PubMed] [Google Scholar]
- Zhou, Z., S. Misler, and R. H. Chow. 1996. Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys. J. 70:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg, J. 2001. How can proteolipids be central players in membrane fusion? Trends Cell Biol. 11:233–235. [DOI] [PubMed] [Google Scholar]