Abstract
Most untreated cancer patients develop progressive tumors. We tested the capacity of T lymphocytes from patients with clinically progressive, multiple myeloma to develop killer function against fresh autologous tumor. In this malignancy, it is feasible to reproducibly evaluate freshly isolated tumor cells and T cells from the marrow tumor environment. When we did this with seven consecutive patients, with all clinical stages of disease, we did not detect reactivity to autologous cancer cells. However, both cytolytic and IFN-γ-producing responses to autologous myeloma were generated in six of seven patients after stimulation ex vivo with dendritic cells that had processed autologous tumor cells. The antitumor effectors recognized fresh autologous tumor but not nontumor cells in the bone marrow, myeloma cell lines, dendritic cells loaded with tumor-derived Ig, or allogeneic tumor. Importantly, these CD8+ effectors developed with similar efficiency by using T cells from both the blood and the bone marrow tumor environment. Therefore, even in the setting of clinical tumor progression, the tumor bed of myeloma patients contains T cells that can be activated readily by dendritic cells to kill primary autologous tumor.
Keywords: tumor immunity‖vaccine‖multiple myeloma‖tolerance‖gammopathy
Multiple myeloma is a common, incurable B cell malignancy characterized by the clonal proliferation of malignant plasma cells in the bone marrow (1). Most myeloma patients develop progressive disease and are treated with chemotherapy. Whether the host immune system is able to control tumor growth in myeloma patients is as yet unknown. Most studies to date have focused only on the function of immune T cells in the circulation, which may not fully reflect antitumor immunity in situ in the tumor bed [reviewed in Cook and Campbell (2)]. Myeloma is a malignancy in which it is possible to reliably access the tumor environment of the bone marrow, and, as a result, one can purify both the primary tumor and immune cells from tumor bed. In contrast to most solid tumors, this also does not require enzymatic treatment to isolate tumor cells, nor is it necessary to generate cell lines from tumor or tumor-infiltrating lymphocytes, which may alter tumor antigen/gene expression and other properties.
These features of multiple myeloma, when coupled to new developments in dendritic cell (DC) biology, allow one to more fully address the adaptive T cell response against autologous tumors. These specialized antigen-presenting cells (APCs) can present exogenous antigens from tumor cells and expand tumor-specific cytolytic T lymphocytes (CTLs) in vitro (3). Prior efforts to exploit this pathway have focused on DC phagocytosis of dying tumor cells, including, recently, those in myeloma (4–6). Recently, we observed that the targeting of tumor cells to human DCs via Fcγ receptors leads to significant enhancement of presentation of tumor antigens by DCs in culture (6). We now have used this strategy to show that T cells from the tumor bed even of patients with progressive myeloma can be activated readily ex vivo to yield tumor-specific CD8+ T cells that produce IFN-γ and lyse fresh autologous tumor.
Methods
Isolation of Tumor and Immune Cells.
Concurrent peripheral blood and bone marrow samples were obtained from patients with biopsy-proven Durie–Salmon clinical stage I–III multiple myeloma (stage I-U3, U6; stage II-U7; stage III-U1, U2, U4, U5, U8, U9, U10), after informed consent approved by the institutional review board. All patients had progressive tumor defined as >25% increase in paraprotein over baseline or newly diagnosed disease with progressive disease-related symptoms and were tested before initiation of any chemotherapy.
Mononuclear cells from blood or bone marrow were obtained by density gradient centrifugation with Ficoll/Hypaque. DCs were generated from monocytes by culture of adherent blood mononuclear cells in the presence of granulocyte/macrophage colony-stimulating factor (Immunex) and IL-4 (R&D Systems) as described (6). Nonadherent cells were used as a source of blood T cells. Bone marrow mononuclear cells were separated into tumor (CD138-positive) and nontumor (CD138-negative) fractions by using CD138 magnetic beads and following the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Tumor cells were cryopreserved in aliquots for use as sources of antigen as well as targets. Nontumor cell fractions also were cryopreserved for use as control targets as well as a source of T cells from the marrow. Bone marrow T cells were enriched from nontumor fractions by further depletion of B (CD20), monocyte (CD14), and natural killer cell (CD56) fractions after initial labeling with respective PE-labeled antibodies, followed by anti-PE microbeads (Miltenyi Biotec, Auburn, CA). For some experiments, we tested as APCs phytohemagglutinin (PHA) blasts, obtained by culturing bulk T and B cells with PHA (2 μg/ml) for 3–4 days, and autologous tumor cells cultured ex vivo for 2–3 days with IL-6 (1,000 units/ml) and granulocyte/macrophage colony-stimulating factor (10 ng/ml) (7). In some patients with IgG myeloma, circulating Igs were purified from plasma by affinity chromatography on a protein G-Sepharose column (Pharmacia). Ig concentrations were determined by ELISA and purity-assessed by SDS/PAGE.
Generation of Antigen-Loaded Dendritic Cells.
Autologous tumor cells were opsonized by using anti-syndecan-1 (1 μg/ml for 30 min at 4°C, clone B-B4; Serotec) or isotype (IgG1) control antibody, γ-irradiated (30 Gy), and then fed after 2–6 h to immature DCs (on day 5 of culture) at a tumor/DC ratio of 1:1 for 16 h in 96-well U-bottom microtest plates in 200 μl of final volume, as described (6). DCs then were induced to mature by overnight culture in the presence of a cytokine mixture consisting of TNF-α (10 ng/ml), IL-1β (10 ng/ml), IL-6 (1,000 units/ml; all from R&D Systems), and PGE2 (1 μg/ml; Sigma). For some experiments, autologous immature DCs (days 4–5 of culture) were pulsed overnight with purified Ig at 10 μg/ml and induced to mature on the following day with the cytokine mixture.
Stimulation of T Cell Responses in Culture.
Antigen-loaded (or unpulsed) mature DCs were cocultured with autologous T cells (2 × 105 cells per well) from either the marrow or blood at a DC/T cell ratio of 1:10–30 in 200 μl of 5% pooled human serum. Cultures were supplemented with IL-2 (Chiron) on days 4 and 7 (10 units/ml). In some experiments, IL-7 (R&D Systems; 5 ng/ml) was added to cultures on day 7. Exogenous IL-2 was not required for the expansion of influenza matrix peptide-specific T cells. After 12–16 days in most experiments, T cells from several microcultures were pooled and tumor-specific killer T cells were assayed by cytotoxicity and ELISPOT assays.
Quantitation of Cytokine-Producing T Cells.
The presence of tumor-reactive IFN-γ-producing T cells in both blood and marrow T cells was quantified both directly ex vivo and after in vitro stimulation (as described above) with antigen-pulsed DCs by using a 16-h ELISPOT assay as described (8). Briefly, autologous T cells from blood or marrow were cocultured with tumor or nontumor cells as APCs at a T/APC ratio of 30–50:1. For some experiments, autologous DCs loaded with autologous or allogeneic myeloma cells were used as APCs. After 16 h, the presence of antigen-specific spot-forming cells (SFCs) was quantified.
Cytolysis Assays.
The presence of tumor-specific killer T cells in bulk T cell cultures after in vitro stimulation was evaluated by using standard 5-h 51Cr-release assays, with autologous tumor cells (CD138+) as targets. Autologous nontumor fractions from the same patient, as well as K562 cells (to monitor natural killer cell-mediated lysis) and PHA blasts (cultured with 2 μg/ml PHA for 3–5 days), served as control targets. For some experiments, targets were preincubated with anti-MHC I (W6/32), anti-MHC II (anti-HLA DR; Immunotech, Westbrook, ME), or isotype control antibody before use in CTL assays.
Results
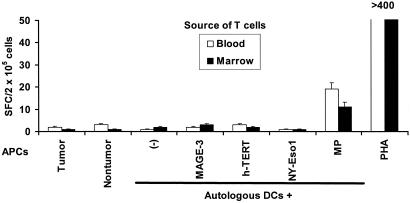
We isolated tumor and T cells from the blood and marrow of seven consecutive myeloma patients with clinically progressive disease before the initiation of any chemotherapy. The freshly isolated T cells from all seven patients failed to recognize and secrete IFN-γ in response to autologous tumor as well as HLA A2-restricted peptides (in HLA A2-positive patients) from defined tumor antigens when tested directly without ex vivo culture (Fig. 1). However, these cells were capable of producing IFN-γ in response to a polyclonal mitogen (PHA) or a defined influenza virus peptide (the latter in HLA A2-positive patients). These results indicate that the myeloma patients did not have global unresponsiveness but, rather, lacked tumor-reactive effectors in the circulation and the tumor environment, despite the abundance of tumor there.
Figure 1.
Lack of detectable, myeloma-specific effector cells in freshly isolated T cells from the blood or marrow of myeloma patients. Freshly isolated T cells (105 cells per well) from the blood or bone marrow of an HLA A2-positive myeloma patient were cultured overnight (APC/responder ratio, 1:50) with fresh autologous tumor (CD138-positive) or nontumor (CD138-negative) fractions from the bone marrow (isolated by using magnetic beads) or autologous mature DCs pulsed for 2 h with several known HLA A2-restricted tumor antigen peptides (MAGE3-271–279 FLWGPRALV, NYESO-1-157–165 SLLMWITQC, and h-TERT1540 ILAKFLHWL, at 1 μg/ml), or control influenza peptide (MP58–66, GILGFLVFTL), or PHA (10 μg/ml) as a positive control. Antigen-specific IFN-γ-producing cells were quantified by using an ELISPOT assay. Data are representative of three HLA A2-positive patients tested.
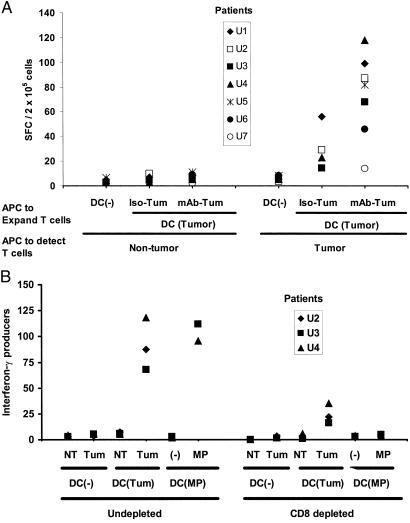
We then used autologous DCs to examine whether the circulating T cell repertoire contained cells that could be activated/expanded to recognize and kill autologous tumor. Blood T cells were stimulated for 12–16 days with monocyte-derived DCs loaded with γ-irradiated autologous tumor cells, with or without prior coating of tumor cells with anti-syndecan-1 antibody to enhance presentation on MHC class I products as described (6). T cells from these cultures, in contrast to freshly isolated T cells from blood and marrow (Fig. 1), now secreted IFN-γ in response to autologous tumor (CD138-positive) but not the nontumor fraction (CD138-negative) from the same marrow (Fig. 2A). Opsonization of tumor cells with anti-syndecan-1 mAb before irradiation and DC loading led to greater activation of tumor-reactive T cells, as compared with DCs loaded with isotype control-treated dying tumor cells (Fig. 2A). This autologous tumor-reactive T cell response was detectable in six of seven previously untreated patients. The antitumor T cell response consisted predominantly of CD8+ T cells but may include CD4+ T cells as well, because the depletion of CD8+ T cells led to a partial decline (70%) in tumor-reactive cells but a complete loss of IFN-γ producers specific for the MHC I-restricted influenza viral matrix peptide (Fig. 2B). In contrast to virus-specific T cells, the expansion of tumor-specific T cells required the addition of exogenous IL-2 and could not be detected in shorter, 7-day cultures (not shown). We focused our subsequent studies on the CD8+ T cell response, because these could directly lyse fresh autologous tumor as long as the latter had not developed immune escape mechanisms.
Figure 2.
Reliable expansion of tumor-specific effectors after stimulation with antigen-loaded DCs. (A) Antibody coating leads to enhanced generation of tumor-specific T cell responses. Monocyte-derived immature DCs from seven untreated patients with progressive myeloma were loaded with irradiated tumor cells, with or without prior opsonization with anti-syndecan-1 antibody (vs. isotype control) as described (7), or were left unpulsed. DCs then were matured by using cytokine mixture, and mature DCs were used to stimulate autologous blood T cells for 12–16 days, whereupon tumor-specific effectors were quantified against tumor (CD138-positive) or nontumor (CD138-negative) fractions from the marrow (APC/responder ratio, 1:50) by using an IFN-γ ELISPOT assay. (B) Effect of depletion of CD8+ T cells on reactivity of the tumor-specific, IFN-γ-producing cells. Blood T cells from an HLA A2-positive myeloma patient were stimulated for 12 days by using unpulsed DCs or DCs loaded with antibody-coated tumor cells or pulsed with an HLA A2-restricted influenza matrix peptide (MP), as described in Methods. After expansion, T cells were depleted of CD8+ fraction (or left undepleted) and tested for the presence of tumor-reactive or influenza peptide-specific T cells by using an ELISPOT assay, as described earlier (7). Data for influenza MP-specific effectors are per 5 × 104 cells, and others are per 2 × 105 cells. Tum, tumor; NT, nontumor fraction from autologous marrow.
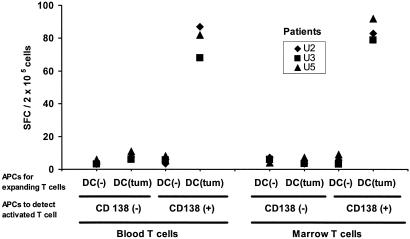
T cells from the tumor microenvironment conceivably could be tolerized by the high burden of tumor antigen there (9). However, stimulation of these T cells with tumor cell-loaded DCs again led to the activation of antitumor effector T cells from three of three untreated patients and, to a similar extent, to that seen with blood T cells (Fig. 3). As with the blood T cells, expansion of tumor-specific T cells from the tumor bed also required the addition of exogenous IL-2 and could not be detected in shorter, 7 day cultures (not shown). Therefore, T cells in the tumor bed, even from patients with clinically progressive disease, remain capable of responding to fresh, autologous tumor cells if activated by DCs.
Figure 3.
Comparable expansion of tumor-specific effectors from blood and marrow T cells. Blood or marrow T cells were cultured with unpulsed or tumor cell-loaded DCs for 12–16 days. After expansion, the presence of tumor-reactive T cells was quantified with an ELISPOT assay by using autologous tumor (CD138+) or nontumor (CD138-ve) bone marrow cells as APCs, as described (7).
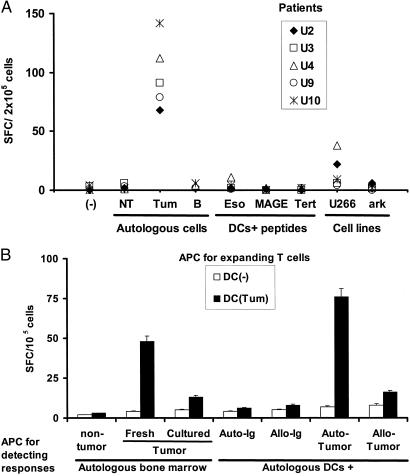
The elicited antitumor T cells were tumor-specific, because they did not react to either nontumor cells from the same bone marrow or autologous-purified B cells (Fig. 4A). To test whether this response was against known MHC class I-binding peptides for previously defined tumor antigens, we also looked for T cells specific for defined epitopes from cancer testis (C-T; for example, MAGE-3 and NY ESO-1) and universal (hTERT) antigens. Prior studies have documented the expression of C-T antigens in myeloma by RT-PCR (6, 10), but expression of these antigens in myeloma also can be demonstrated at the protein level by immunohistochemistry (A. Jungbluth and M.V.D., unpublished data). Before ex vivo expansion, no reactivity to these antigens was noted (Fig. 1). After stimulation with tumor cell-loaded DCs, low levels of NY-Eso-1-specific T cells were detected in only one (patient U3) of the three HLA A2-positive patients, which represented <10% of net tumor reactivity (Fig. 4A). In addition, only minor reactivity against HLA-A2-positive myeloma cell lines was observed (Fig. 4A), indicating that only few antigenic targets may be shared between these cell lines and autologous tumors tested.
Figure 4.
Nature of antigens by tumor-specific killer T cells. (A) T cells from HLA A2-positive patients expanded by using DCs loaded with antibody-coated tumor cells were tested for the recognition of autologous tumor or nontumor fractions from the marrow, purified autologous B cells, HLA A2.1-positive (U266) or negative (ark) myeloma cell lines, or HLA A2.1-restricted peptides derived from MAGE-3, h-TERT, or NY-ESO-1, using peptide-pulsed DCs as APCs (effector/APC ratio, 50:1). IFN-γ-producing cells (SFCs) were quantified in an ELISPOT assay. Data are shown as SFC/2 × 105 cells per well. (B) T cells (105 cells per well) from a patient with IgG myeloma expanded by using unpulsed DCs or DCs loaded with antibody-coated tumor cells were tested for the recognition of autologous CD138+ tumor [either fresh or cultured ex vivo in the presence of IL-6 (1,000 units/ml) and granulocyte/macrophage colony-stimulating factor (10 ng/ml) for 2 days] or nontumor cells (CD138-ve) from the bone marrow or autologous DCs pulsed with purified tumor-derived circulating Ig, allogeneic Ig, or DCs fed with autologous or allogeneic tumor cells (tumor/DC ratio, 1:1) as APCs. Effector cells were cultured overnight with APCs (effector/APC ratio, 50:1) and IFN-γ-producing cells (SFCs) quantified by an ELISPOT assay. Data are representative of experiments on two separate patients.
Myeloma cells secrete a monoclonal Ig that may serve as an immune target. However, in contrast to lymphoma, the Ig is secreted in large amounts in myeloma, conditions that may promote the deletion of high-affinity T cells recognizing Ig-derived epitopes (11, 12). In fact, the antitumor effectors elicited in our cultures did not react to autologous DCs fed with autologous or allogeneic Ig, indicating that the effectors are not directed against Ig-derived determinants (Fig. 4B). Interestingly, in three of three patients tested, the T cell reactivity was directed against autologous tumors or DCs fed with autologous tumors, but not allogeneic tumors, suggesting that the dominant antigens in myeloma are specific to each individual patient's tumor. In addition, the recognition of cultured autologous tumor cells was significantly lower than those of fresh cells in two of two patients tested (Fig. 4B), suggesting that even short-term in vitro cultures may alter the antigenic properties of tumor cells.
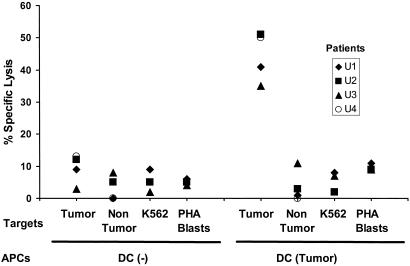
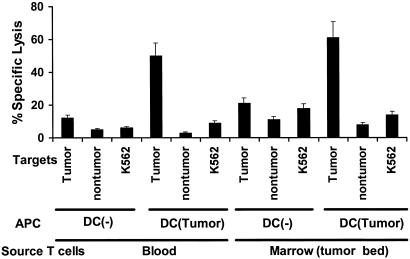
The capacity of T cells to secrete IFN-γ is likely to be critical for tumor resistance (13), but we also tested whether the myeloma-reactive T cells could specifically kill the tumor in short-term lytic assays. After 2 weeks of in vitro expansion with autologous tumor cell-loaded DCs, we found that the cultures from all four patients tested developed strong lytic activity against autologous tumor but not the control nontumor fraction or K562 cells, the latter being targets for natural killer cells (Fig. 5). The elicited T cells also did not recognize autologous PHA blasts expressing self-lymphoid antigens. The lysis of tumor targets was inhibited by pretreatment of tumors with anti-MHC I, but not anti-MHC II or isotype control antibody, indicating the MHC I-restricted nature of the lytic cells (not shown). Again, stimulation of marrow T cells with tumor-loaded DCs led to a similar activation of antitumor killers, as seen with blood T cells (Fig. 6). Thus, T cells from both the circulation and tumor microenvironment of myeloma patients can be activated ex vivo to yield tumor-reactive killer T cells, even in the setting of clinically progressive disease.
Figure 5.
Generation of tumor-specific killer T cells in vitro. Blood T cells were cocultured with unpulsed or tumor cell-loaded DCs as in Fig. 2. T cells were tested for the ability to kill autologous tumor cells, nontumor fraction from the marrow, or K562 cells and autologous PHA blasts as controls at an effector-to-target ratio of 20:1.
Figure 6.
Expansion of tumor-specific killers from blood or marrow T cells. T cells from blood and marrow T cells were expanded under conditions identical to those in Fig. 3 and tested for the ability to kill tumor or nontumor targets or K562 cells as controls. Data are representative of similar experiments on three separate patients.
Discussion
These data provide direct evidence that the tumor bed of patients with clinically progressive myeloma contains T cells that can be activated ex vivo to kill freshly isolated autologous tumor. The generation of tumor-specific killers is feasible in all clinical stages of disease. The inability of the immune system to naturally control tumor progression in myeloma patients is therefore unlikely to be due to immune escape from loss of expression or presentation of antigens by tumor cells, generalized CD8+ T cell dysfunction, or deletion of tumor-specific CD8+ T cells. These data also suggest that either there is no tolerance to myeloma tumor antigens or that it can be easily broken by DCs loaded with autologous fresh tumor cells, particularly if delivered in an opsonized fashion. However, there is tolerance to self-antigens of normal lymphocytes, which cannot be overcome under these conditions. Reactivity against the tumor was not detected in freshly isolated T cells from the tumor bed, despite the abundance of tumor there. This could have several explanations. There could be a low frequency or a lack of activation of tumor-specific T cells in vivo—e.g., because of lack of access of tumors to DCs or defective DC activation—as shown recently for blood-derived DCs in myeloma (14, 15). Also, the immune responses could be silenced in vivo—e.g., by regulatory T cells or suppressive factors in the tumor environment. The former possibilities are supported by our observation that tumor-reactive effectors remain undetectable in shorter (7-day) cultures, whereas virus-specific T cells are already expanded severalfold in 1 week.
Pioneering studies in melanoma have shown that tumor-infiltrating lymphocytes contain T cells specific for defined antigens expressed by human melanomas and normal melanocytes (16, 17). However, functional studies of autologous tumor cell recognition in this tumor by intratumoral immune cells are confounded by difficulties in reliably isolating tumor and immune cells from the tumor. Usually, this requires enzymatic treatment followed by in vitro culture (18–20). Such cultures, however, may alter gene-expression profiles and properties of tumors as well as T cells. Because of these difficulties with accessing the tumor bed, prior studies testing the recognition of fresh autologous tumor mostly have used blood T cells, generally after prior in vitro culture (5, 21–23). In myeloma, one can directly assess the reactivity of freshly isolated T cells from both blood as well as the tumor bed with authentic autologous tumor, and when this is done, no T cell reactivity is detected. Nonetheless, strong killing can be generated against the tumor but not the nontumor cells in the tumor bed. Interestingly, even short-term culture led to significant reduction in tumor cell recognition as compared with fresh autologous tumor cells in two patients who were tested, suggesting that in vitro culture may alter the antigenic properties of tumors.
The tumor-reactive T cells that were generated with tumor-loaded DCs in vitro were tumor-specific and not detectably directed against autologous lymphoid cells, even though there must be extensive sharing of self-antigens between myeloma and other lymphoid cells. Similar findings also were reported recently by Wen et al. (5), who tested T cell lines generated from blood of myeloma patients after repetitive stimulation with tumor lysate-pulsed DCs.
However, the identity of antigens recognized by the antitumor T cells in these cultures is not known and likely includes reactivity against multiple tumor antigens, several of which may be unique to each patient's tumor (24). The experimental system reported here might be useful in identifying novel antigenic targets in myeloma. The monoclonal Ig secreted by tumor cells has been proposed as an immune target in B cell tumors (11). However, in contrast to lymphoma, the Ig is secreted in large amounts in myeloma, conditions that may promote the deletion of high-affinity T cells recognizing Ig-derived epitopes (12). In our cultures, the elicited T cells did not recognize or kill autologous B cells or DCs pulsed with autologous tumor-derived Ig. This suggests that the dominant antitumor reactivity in these cultures is not derived from Ig determinants. Recently, T cell lines generated after repetitive stimulation with Ig protein-pulsed DCs were shown to recognize tumor cells (23, 25), but this required multiple cycles of stimulation and selection. The finding that most of these T cells did not recognize DCs loaded with allogeneic tumor or myeloma cell lines suggests that the dominant reactivity might have been against antigens specific for each patient's tumor. Although myeloma cells were purified by using anti-CD138 antibody, the immune reactivity was not against mouse Ig, because the T cells do not recognize antigens from anti-CD138-purified allogeneic myeloma cells or antibody-purified autologous B cells (Fig. 4), whereas they kill unfractionated bone marrow cells with a high tumor burden (not shown). Although our studies, to date, have focused only on CD8+ killer T cells, further studies are needed to characterize the antitumor CD4+ T cell response in myeloma.
One possibility, that we favor, is that the antitumor immune repertoire is directed predominantly to antigens overexpressed on patient-specific fresh tumor cells. More work needs to be done to identify the relevant antigens/epitopes and the frequency of these precursors. Nonetheless, these data support the use of fresh autologous tumor, as opposed to cell lines, or even cultured autologous tumor cells as sources of antigen or targets for DC-based active immunotherapy (reviewed in refs. 26–28). Alternatively, the T cells might be activated by such DCs ex vivo and then transferred adoptively into patients (26, 29).
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA81138, CA84512, and MO-1-RR00102, an investigator award from the Cancer Research Institute, and funds from the Irma T. Hirschl Foundation and New York Community Trust.
Abbreviations
- DC
dendritic cell
- CTL
cytolytic T lymphocyte
- APC
antigen-presenting cell
- SFC
spot-forming cell
- PHA
phytohemagglutinin
References
- 1.Tricot G. In: Hematology: Principles and Practice. Hoffman R, editor. New York: Churchill Livingstone; 2000. pp. 1398–1415. [Google Scholar]
- 2.Cook G, Campbell J D. Blood Rev. 1999;13:151–162. doi: 10.1054/blre.1999.0111. [DOI] [PubMed] [Google Scholar]
- 3.Heath W R, Carbone F R. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Berard F, Blanco P, Davoust J, Neidhart-Berard E M, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini J C, Banchereau J, Palucka A K. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen Y J, Min R, Tricot G, Barlogie B, Yi Q. Blood. 2002;99:3280–3285. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- 6.Dhodapkar K, Krasovsky J, Williamson B, Dhodapkar M. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X G, Bataille R, Jourdan M, Saeland S, Banchereau J, Mannoni P, Klein B. Blood. 1990;76:2599–2605. [PubMed] [Google Scholar]
- 8.Dhodapkar M V, Krasovsky J, Steinman R, Bhardwaj N. J Clin Invest. 2000;107:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotomayor E M, Borrello I, Rattis F M, Cuenca A G, Abrams J, Staveley-O'Carroll K, Levitsky H I. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 10.van Baren N, Brasseur F, Godelaine D, Hames G, Ferrant A, Lehmann F, Andre M, Ravoet C, Doyen C, Spagnoli G C, et al. Blood. 1999;94:1156–1164. [PubMed] [Google Scholar]
- 11.Tao M H, Levy R. Nature (London) 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 12.Bogen B. Eur J Immunol. 1996;26:2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran V, Ikeda H, Bruce A T, White J M, Swanson P E, Old L J, Schreiber R D. Nature (London) 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 14.Brown R D, Pope B, Murray A, Esdale W, Sze D M, Gibson J, Ho P J, Hart D, Joshua D. Blood. 2001;98:2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 15.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna G R, Tura S, et al. Blood. 2002;100:230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 16.Aebersold P, Hyatt C, Johnson S, Hines K, Korcak L, Sanders M, Lotze M, Topalian S, Yang J, Rosenberg S A. J Natl Cancer Inst. 1991;83:932–937. doi: 10.1093/jnci/83.13.932. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg S A, Packard B S, Aebersold P M, Solomon D, Topalian S L, Toy S T, Simon P, Lotze M T, Yang J C, Seipp C A, et al. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 18.Lotze M T, Grimm E A, Mazumdar A, Strausser J L, Rosenberg S A. Cancer Res. 1981;41:4420–4425. [PubMed] [Google Scholar]
- 19.Romero P, Rod Dunbar P, Valmori D, Pittet M, Ogg G S, Rimoldi D, Chen J L, Lienard D, Cerottini J C, Cerundolo V. J Exp Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valmori D, Dutoit V, Lienard D, Lejeune F, Speiser D, Rimoldi D, Cerundolo V, Dietrich P Y, Cerottini J C, Romero P. J Immunol. 2000;165:533–538. doi: 10.4049/jimmunol.165.1.533. [DOI] [PubMed] [Google Scholar]
- 21.Molldrem J J, Lee P P, Wang C, Felio K, Kantarjian H M, Champlin R E, Davis M M. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom I, Ledbetter J A, Scholler N, Yang Y, Ye Z, Goodman G, Pullman J, Hayden-Ledbetter M, Hellstrom K E. Proc Natl Acad Sci USA. 2001;98:6783–6788. doi: 10.1073/pnas.021557498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen Y J, Barlogie B, Yi Q. Blood. 2001;97:1750–1755. doi: 10.1182/blood.v97.6.1750. [DOI] [PubMed] [Google Scholar]
- 24.Gilboa E. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Bendandi M, Deng Y, Dunbar C, Munshi N, Jagannath S, Kwak L W, Lyerly H K. Blood. 2000;96:2828–2833. [PubMed] [Google Scholar]
- 26.Steinman R, Dhodapkar M. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Schuler-Thurner B, Palucka A K, Schuler G. Cell. 2001;106:271–274. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 28.Fong L, Engleman E G. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 29.Yee C, Thompson J A, Roche P, Byrd D R, Lee P P, Piepkorn M, Kenyon K, Davis M M, Riddell S R, Greenberg P D. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]