Abstract
Although a role for CD4+ helper cells in CD8+ cytotoxic T lymphocyte (CTL) induction by vaccines is widely recognized, much less is known about a counterbalancing role of CD4+ T cells in down-modulating this response, or about ways to optimize vaccine responses through abrogation of this negative regulatory mechanism. Here, we discovered a synergistic enhancement of vaccine-mediated CTL induction and protection by the relief of suppression through depletion of regulatory CD4+ cells, including CD4+ NKT cells, or blockade of IL-13 made by these cells, combined with the cytokine granulocyte/macrophage colony-stimulating factor and the costimulatory molecule CD40L. Indeed, in the absence of helper epitopes, granulocyte/macrophage colony-stimulating factor and the helper-mimetic molecule CD40L are not sufficient to replace help to induce CTL without abrogation of CD4+ T cell-mediated suppression, suggesting a role for T cell help in overcoming suppression. The increased CTL induction translated to striking protection against viral infection by a vaccine by using this synergistic combined approach. These results argue for a push–pull approach to maximize vaccine efficacy, especially for HIV and cancer.
The challenge in developing vaccines against persistent virus infections, parasitic infections, and cancer is to elicit cellular immune responses sufficient to control effectively infection and disease in the face of mechanisms by which the disease evades immune control. High levels of CD8+cytotoxic T lymphocyte (CTL) responses seen in HIV-infected nonprogressors suggest that this response may quantitatively correlate with protection from progression toward disease (1–3), and the protective role of CD8+ cells has been confirmed by depletion studies in macaques (4, 5). Although the progressive loss and dysfunction of the CD4+ Th cell population is the hallmark of AIDS, the sequence of events that ultimately leads to diminution of CTL responses and failure to control infection is unclear. Recent approaches to AIDS vaccines have emphasized CTL induction, and several complementary strategies have been developed recently to enhance CTL responses to vaccines (6).
Although studies have demonstrated that in virus infection specific CTL can be elicited in the absence of CD4+ cell help or even costimulation (7, 8), the role of Th1 CD4+ cells in eliciting CTL responses to vaccines and in maintaining functional CTL responses in persistent infections in mice, monkeys, and humans is compelling (2, 9–15). Thus, understanding ways to provide such help is critical in developing effective vaccines.
Here we show that abrogating CD4+ cell-mediated suppression plays a significant role in eliciting a CTL response both in the presence and absence of CD4+ cell help, and partially affects the need for help, and that optimizing this response requires potent cytokines and immunomodulating agents. A vaccine that elicits CTL responses in the absence of continued CD4+ cell help and suppression would be especially valuable in the immunotherapy of chronic diseases such as HIV infection and cancer.
Several types of CD4+ T cells that can suppress other T cell responses have been described, including the CD4+CD25+ suppressor cell (16), conventional Th2 cells (17), Th3 or Tr1 regulatory cells (18), and CD4+ natural killer (NK) T cells that we have found to inhibit natural tumor immunosurveillance through a mechanism involving IL-13 (19). Here, we find that the suppression of the vaccine response apparently is due at least in part to the NKT cell, because we can enhance vaccine efficacy by blockade of IL-13 or use of CD1-deficient mice that lack NKT cells.
Granulocyte/macrophage colony-stimulating factor (GM-CSF) alone has been used as an adjuvant in several vaccine studies (20–23). In previous work, we have shown that GM-CSF enhances the magnitude of both cellular and humoral immune responses to vaccine constructs by recruiting professional antigen-presenting cells (APCs) to inductive sites without skewing Th phenotype (24, 25). We have also found that CD4+ Th1 cell responses favor CTL induction and that GM-CSF acts synergistically with the Th1-inducing cytokine IL-12 to elicit CTL (24, 26). However, in a CD4+ T cell-mediated viral protection model a third cytokine, tumor necrosis factor α, was necessary to achieve complete protection (25). We have recently established that levels of CD40L expression on activated CD4+ Th cells correlated with functional ability to provide help for CTL induction (27), and we as well as others have shown that the mechanism of help involves signals communicated through CD40L–CD40 interaction to activate professional APCs to produce IL-12, IL-15, and enhanced costimulation (12, 27–31). An expression plasmid for CD40L has been used as an adjuvant in a DNA vaccine to increase cellular and humoral immunity (32, 33), and it has recently been shown that tumor cells transduced with the genes for GM-CSF and CD40L are effective at recruiting tumor-infiltrating dendritic cells capable of eliciting cellular immunity by a cross-priming mechanism (34). However, such a combination of GM-CSF and CD40L has not been tested for synergy in a vaccine. Therefore, we tested the combination of GM-CSF and CD40L for the ability to enhance synergistically vaccine induction of CTL responses. We reasoned that if GM-CSF recruits more professional APCs to the draining lymph nodes (25), and then CD40L matures and activates them, the combination might be synergistic. Further, if the primary role of T help is to activate APCs through GM-CSF and CD40L, we asked whether the combination of these might substitute for CD4+ T cell help. Here, we find that this combination is not sufficient to replace help unless CD4+-cell-mediated suppression is abrogated, suggesting that an additional unexpected function of CD4+ help is to overcome CD4+-cell-mediated suppression.
Materials and Methods
Mice.
BALB/c mice were obtained from the Charles River Breeding Laboratories. CD1(−/−) mice were obtained from the breeding colony of W. Paul, from breeders obtained from M. Grusby (35), after eight backcrosses to BALB/c. The CD1 knockout mice did not reject BALB/c skin, and their spleen cells did not proliferate in response to BALB/c spleen cells (M. Terabe and C. Khanna, unpublished observations). All animal work was performed in accordance with National Institutes of Health guidelines.
Vaccine Constructs and Immunizations.
Multideterminant cluster peptides (36) containing overlapping T helper cell epitopes that elicit help in mice and humans of multiple MHC types were colinearly synthesized with P18IIIB, the major neutralizing antibody epitope located at the tip of the V3 loop and an immunodominant CTL epitope in mice (37) to form a synthetic peptide vaccine construct (38). The constructs used for immunization were PCLUS 3–18IIIB, KQIINMWQEVGKAMYAPPISGQIRRIQRGPGRAFVTIGK, comprising Th determinants from HIV-1 IIIB envelope, amino acid residues 421–444 according to numbering in the Los Alamos sequence database (39) linked to P18IIIB shown in bold, amino acid residues 308–322), and PCLUS 6.1–18IIIB, DRVIEVVQGAYRAIRHIPRRIRQGLERRIQRGPGRAFVTIGK, consisting of the helper segment PCLUS6.1 (residues 827–853) followed by P18IIIB. We also used the minimum class I, Dd binding peptide epitope, I-10 (RGPGRAFVTI) (40). Peptides were synthesized and purified by Multiple Peptide Systems (San Diego), and were single peaks on HPLC in two solvent systems as well as by mass spectrometry. Cytokine GM-CSF (PeproTech, Rocky Hill, NJ) and dendritic cell-activating factor CD40L trimer (Immunex, Seattle) were given s.c. incorporated in the emulsion adjuvant ISA-51 (Seppic, Fairfield, NJ). In vivo depletion of CD4+ cells was achieved by four doses of anti-CD4 antibody (GK1.5, 0.5 mg per mouse per 0.1 ml) given i.p. 1 day before immunization and for 3 consecutive days. IL-13Rα2-Fc (Wyeth-Genetics Institute) (200 μg) was given i.p. on days 0, 1, 2, 4, and 6 after immunization.
CTL Assay.
Bulk spleen cell populations from immunized mice were restimulated in vitro with peptide-pulsed (0.5 μM P18MN for 2 h) syngeneic spleen cells in 12-well plates (Costar). Twenty hours later, Rat T Stim (Collaborative Biomedical Products, Bedford, MA) was added as a source of IL-2 to a final concentration of 7.5%. Six days later, nonadherent cells were spun over mouse Lympholyte M (Cedarlane Laboratories), and replated in fresh medium without IL-2. Cells were harvested on days 7 and 8 to test for CTL activity as described. Nonspecific background lysis was less than 10% except when the IL-13 inhibitor was used, where the background was 15–20%.
Virus Challenge.
Recombinant vaccinia virus vPE16 expressing HIV-IIIB gp160 envelope protein was a kind gift of B. Moss and P. Earl, National Institute of Allergy and Infectious Diseases, National Institutes of Health (41). Virus was injected i.p. into female mice in protection experiments, and ovaries were harvested 5 days after challenge. Ovaries were homogenized in 1 ml of medium, suspensions sonicated, and virus titered by plaque assay on BSC-1 cells (25).
Statistical Methods.
A two-way analysis of variance was applied to CTL data, thereby averaging the respective values for each curve over all of the E:T ratios above background. Means for each treatment were compared by using Tukey's multiple comparison test or Dunnett's method and significance determined at α = .05. Departures from additivity (synergism or antagonism) were assessed by using the construct AB + control − A − B, where A and B denote individual treatment factors, and AB denotes the combination. Protection from viral challenge data were analyzed by using both an unequal-variance t test (Welch's ANOVA) and the nonparametric Wilcoxon Rank Sum. In some pairwise comparisons variance of log10 viral titers were equal resulting in analysis by the Student's t test. Statistically significant differences between groups were determined at P values <0.05, <0.01, and <0.001. Analysis was performed by using JMP (SAS Institute, Cary, NC) statistical software for the Macintosh.
Results
Synergy of Cytokine GM-CSF with Costimulatory Molecule CD40L.
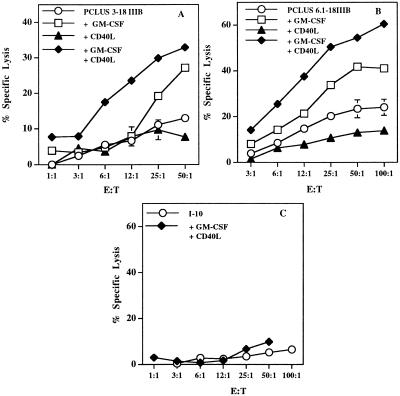
We tested the hypotheses above by using two different Th-CTL HIV-1 peptide vaccine constructs (42), PCLUS 3–18IIIB and PCLUS 6.1–18IIIB, with distinct helper epitopes. With both vaccines, the combination of GM-CSF and CD40L incorporated in the emulsion adjuvant was synergistic in enhancing CTL induction (Fig. 1 A and B). As previously found, GM-CSF alone enhanced the vaccine-induced CTL response, whereas CD40L alone provided some enhancement after boosting (not shown) but did not significantly enhance the CTL response after a single immunization (Fig. 1 A and B). Thus, the combination of GM-CSF and CD40L is synergistic. We conclude that recruitment of professional APCs to the induction site by GM-CSF is a prerequisite for the effect of CD40L in activating sufficient APCs to optimize induction of naïve CD8+ CTL precursors.
Figure 1.
GM-CSF and CD40L trimer synergize for induction of CD8+CTL. (A) Specific lysis of P18IIIB (0.5 μM) coated target cells by splenic effectors from BALB/c mice immunized s.c. with 20 nmol (17 μg CTL epitope P18IIIB) HIV-1 Th-CTL vaccine construct, PCLUS 3–18IIIB, with or without individual or a combination of factors GM-CSF (5 μg/mouse), and CD40L (15 μg/mouse), incorporated in adjuvant montanide-ISA 51. Spleen cells are pooled from two mice. Error bars < the symbols are not shown. (B) CTL response is significantly (P < 0.05) enhanced by GM-CSF and CD40L after boosting with HIV Th-CTL vaccine construct, PCLUS 6.1–18IIIB. Similar results were obtained in four additional experiments. (C) Mice immunized with the minimum CTL epitope, I-10, plus GM-CSF and CD40L failed to elicit a CTL response, even after boosting and with a 4-fold higher peptide dose (80 nmol/mouse) (≈80 μg).
To determine whether this combination of agents could bypass CD4+ T cell help elicited by the vaccine constructs for induction of CTL, in view of the role of CD40L in mediating the mechanism of help (27–31), we immunized mice with the free CTL epitope peptide I-10 in adjuvant, without a helper epitope. The combination of GM-CSF and CD40L was unable to substitute for CD4+-mediated help in eliciting CTL (Fig. 1C), even when animals were immunized with a 4-fold higher molar dose (80 nmol) of the CTL epitope, I-10, than given in the Th-CTL vaccine construct. Thus, we hypothesized that help served to do more than recruit and activate APCs, despite the findings cited above that much of CD4 help for CTL is mediated by CD40L, and that anti-CD40 could bypass CD4+ cell help in a tumor model (43).
Conflicting Roles of CD4+ Cells.
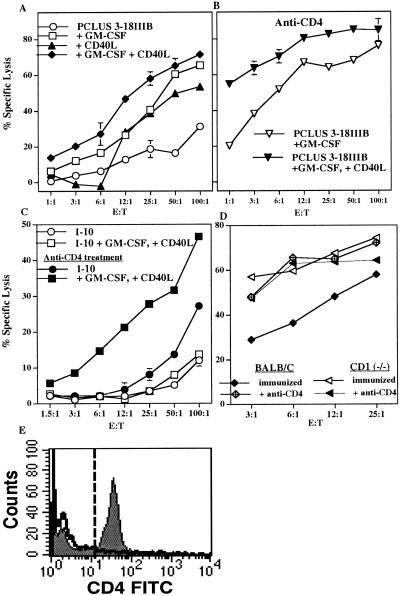
To examine the mechanism, we used another method to bypass CD4+ cell help. We depleted animals of CD4+ T cells with anti-CD4 treatment 1 day before and 3 consecutive days after immunization. Depletion was verified by the absence of double-positive CD3+ CD4+ cells in spleens of treated mice 14 days after depletion (Fig. 2E) and the absence of a proliferative response in treated animals to in vitro stimulation with T helper peptides. Because BALB/c mice lack the NK1.1 marker, we cannot distinguish depletion of NKT vs. conventional helper T cells except by function; but in the tumor model, similar anti-CD4 treatment depleted suppression more than it depleted help (19). However, the CD4+ cell depletion in vivo resulted in less than a 10% enrichment in the fraction of spleen cells that were CD8+, so the effects seen were not simply due to cell enrichment (data not shown). Surprisingly, anti-CD4 treatment enhanced the CTL response in animals immunized with the Th-CTL vaccine construct, PCLUS 3–18IIIB (compare Fig. 2 A and B) and was essential for induction of CTL to the free CTL epitope I-10 (Fig. 2C). Paradoxically, in the absence of a helper T cell epitope, removal of CD4+ cells actually revealed a CTL response. Thus, it seemed that a regulatory CD4+ cell was blocking induction of CTL to immunization with the free CTL epitope and down-modulating the response to the Th-CTL vaccine construct. Furthermore, the combination of dendritic cell-activating factors GM-CSF and CD40L was necessary for eliciting optimum CTL responses in CD4+-depleted animals. Thus, we conclude that GM-CSF and CD40L ligand could replace CD4 cell help if the negative regulatory influence of another CD4+ cell was eliminated.
Figure 2.
CD4+ cell depletion enhances CD8+ CTL response to HIV-1 Th-CTL vaccine and minimum CTL epitope. (A) Specific lysis of P18IIIB (0.5 μM) coated targets by splenic effectors after 7 days in vitro restimulation with P18IIIB and IL-2 after two s.c. immunizations with PCLUS 3–18IIIB alone, or plus individual or a combination of factors GM-CSF (5 μg/dose) and CD40L (10 μg/dose) 2 weeks apart. (B) Two additional groups of animals from the experiment in A were treated with anti-CD4 (GK1.5, 0.5 mg/day per mouse) 1 day before immunization and for 2 additional days and spleen cells harvested 15 days later, restimulated in vitro, and assayed for CTL. (C) Specific lysis by splenic effectors from mice immunized with minimum CTL epitope I-10 (60 nmol/mouse), alone, or I-10 plus GM-CSF and CD40L, restimulated in vitro with P18IIIB (0.5 μM) plus IL-2 and assayed for CTL 7 days later. Filled symbols represent mice treated with anti-CD4. Error bars < the symbols are not shown. This experiment was performed four times with comparable results. (D) CTL response of BALB/c and CD1-deficient mice after two immunizations with PCLUS 6.1–18IIIB plus GM-CSF (5 μg/dose) and CD40L (10 μg/dose). One group from each strain of mice also received anti-CD4 (GK1.5, 0.5 mg/day i.p.) 1 day before and 3 days after immunization. BALB/c and CD1(−/−) mice were significantly different (P < 0.05) without but not with CD4 depletion. Error bars < symbols are not shown. This experiment was performed two additional times with comparable results. (E) Animals treated or not with anti-CD4 were immunized with PCLUS 6.1–18IIIB, plus GM-CSF and CD40L and 15 days later spleen cells pooled from two mice each were examined for CD4+ cells, gated on CD3+ cells. Total CD4+ cells in untreated mice (gray) or mice treated 4 days with anti-CD4, 0.5 mg/day (solid line) are shown.
Several possible CD4+ regulatory cells might play a role in inhibition of induction of CTL by immunization with vaccine constructs. These include the CD4+CD25+ suppressor cell (16), conventional Th2 cells (17), Th3 or Tr1 regulatory cells (18), and CD4+ NKT cells that we have found to inhibit tumor immunosurveillance (19) and that others have found to play a role in immune privilege in the eye (44) and in allograft and xenograft tolerance (45, 46). Because the suppressive effect of CD4+ cells was induced when the immunogen was the pure class I-restricted CTL epitope peptide I10, which does not bind to class II molecules in BALB/c mice (40) or elicit a conventional CD4+ cell response or antigen-specific T cell proliferation, we believe the suppressor cell is less likely to be an antigen-specific CD4+ cell such as a Th2 cell, a Th3 cell, or a CD4+CD25+ suppressor cell. To distinguish among some of these possibilities, we immunized CD1(−/−) mice, that lack NKT cells, with the Th-CTL vaccine construct PCLUS 6.1–18IIIB. A higher CTL response was seen in CD1(−/−) compared with intact BALB/c mice when GM-CSF and CD40L were used in the vaccine (Fig. 2D). Furthermore, depleting CD4+ cells by anti-CD4 treatment during immunization did not further enhance the CTL response in CD1(−/−) mice although the response dramatically increased in normal BALB/c (Fig. 2D). This result suggests that the CD4+ suppressive cell is already absent in the CD1(−/−) mice and so cannot be further deleted, implicating the CD4+ NKT cell as the primary suppressive cell in this response.
Synergy of GM-CSF and CD40L in Protection Against Viral Infection.
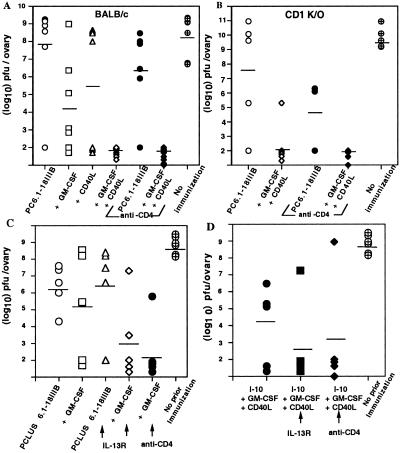
The ultimate measure of viral vaccine efficacy is protection against virus, not just induction of CTL. To determine whether immunization with the synergistic combination GM-CSF and CD40L would enhance protection from viral challenge, we immunized animals twice with the vaccine construct PCLUS 6.1–18IIIB and challenged i.p. with 1 × 107 plaque-forming units (pfu) of recombinant vaccinia virus expressing HIV-1 IIIB gp160. Only animals that received both GM-CSF and CD40L were completely protected in this CD8+ CTL-dependent viral challenge model (Fig. 3A). Indeed, the synergistic combination of GM-CSF and CD40L was required for complete protection in both intact and anti-CD4-treated animals (P < 0.01). In the mice receiving only peptide vaccine PCLUS6.1–18IIIB without cytokines, the variance was too great to see a statistically significant effect of CD4 depletion. The high level of CD8+ CTL elicited in CD1(−/−) mice receiving GM-CSF and CD40L also correlated with complete protection in untreated and anti-CD4-treated animals (P < 0.01) (Fig. 3B). However, in accord with the CTL response (Fig. 2D), anti-CD4 treatment did not significantly further improve the protective response in CD1(−/−) mice, consistent with the major CD4+-suppressive cell in this system being CD1-dependent (Fig. 3B). In a pilot experiment, we also found GM-CSF and CD40L to be essential for protection in the group of anti-CD4-treated animals immunized with the free CTL epitope I-10, thereby bypassing the need for CD4 helper cell function in this vaccine model (data not shown). Thus, although suppression of CTL induction against the free CTL epitope I-10 can be relieved by anti-CD4 treatment, enhancement of this response by dendritic cell-activating factors GM-CSF and CD40L is required to elicit a response of sufficient magnitude to afford protection from viral challenge.
Figure 3.
The combination of GM-CSF and CD40L is essential for vaccine-mediated protection from viral challenge. Female BALB/c (A) and CD1(−/−) (B) mice were immunized s.c. with 20 nmol HIV-1 vaccine PCLUS 6.1–18IIIB in ISA-51 plus indicated cytokines and costimulatory molecules and boosted i.p. Two groups immunized with PCLUS 6.1–18IIIB alone or with the combination GM-CSF and CD40L were depleted of CD4+ cells by treating with GK1.5 antibody (0.5 μg/dose i.p.) 1 day before and 3 consecutive days after immunization. Mice were challenged i.p. 5 days later with 1 × 107 pfu recombinant vaccinia expressing the HIV-1 IIIB gp160. Ovaries from individual mice were harvested 5 days after challenge, and titered on BSC-1 cells as described (25). (C) Female BALB/c mice were immunized twice with PCLUS 6.1–18IIIB with or without GM-CSF and challenged with recombinant vaccinia as in A. Mice immunized with PCLUS 6.1–18IIIB alone (open triangles), or plus GM-CSF (open diamonds), received five treatments (250 μg/mouse i.p. on day 0, 1, 2, 4, and 6 after immunization) with IL-13Rα2-Fc, an inhibitor of IL-13. Another group of animals receiving the vaccine plus GM-CSF were depleted of CD4+ cells (filled circles). (D) Female BALB/c mice were immunized twice with the minimum CTL epitope I-10 (80 nmol) with GM-CSF (5 μg/mouse) and CD40L (10 μg/mouse) and challenged with recombinant vaccinia (filled circles) as in C. Animals also received either the IL-13 inhibitor IL-13Rα2-Fc (filled squares), or anti-CD4 treatment (filled diamonds). Each symbol represents an individual animal and horizontal bars represent the geometric mean titers for each group.
Enhancement of Vaccine-Induced CTL Response by Inhibition of IL-13.
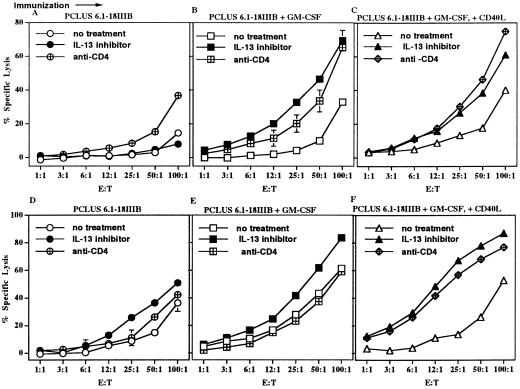
We had shown the cytokine IL-13 made by NKT cells was responsible for suppression of CD8+ CTL-mediated tumor immunosurveillance and allowing tumor recurrence (19). We therefore wanted to see whether IL-13 played a role in inhibiting vaccine induction of CTL. When BALB/c mice were treated with IL-13Rα2Fc, an in vivo inhibitor interfering with IL-13 binding to its receptor, a significant enhancement in CTL occurred in those immunized with the vaccine plus GM-CSF or GM-CSF and CD40L (Fig. 4 B and C). Enhancement of the CTL response to the peptide vaccine construct by inhibition of IL-13 during immunization was at least as great as that induced by depletion of CD4+ cells during immunization. Thus, an inhibitory activity of a CD4+-positive cell population down-modulates CTL induction and could be abolished by anti-CD4 treatment or by giving animals IL-13Rα2-Fc as an inhibitor of IL-13. This effect of treating animals with IL-13 inhibitor at the time of immunization also had a significant effect on maintaining memory CD8+ CTL. When spleen cells from another group of mice were tested 7 weeks after a single immunization with the vaccine construct, animals treated with the IL-13 inhibitor showed a significantly higher level of CTL response than animals without the inhibitor (Fig. 4 D–F, P < 0.05). This effect was most pronounced in the group given GM-CSF and CD40L. The responses in all of the IL-13Rα2Fc-treated groups were better than those in the vaccinated groups receiving anti-CD4 treatment, consistent with a positive role for CD4+ cells in maintaining memory lost with anti-CD4 treatment but not disrupted by the more selective treatment with the IL-13 inhibitor. In fact, treatment with the IL-13 inhibitor led to a slight enhancement in CD4+ proliferation to the vaccine construct PCLUS 6.1–18IIIB compared with immunized groups that did not receive the IL-13 inhibitor or those groups receiving anti-CD4 treatment, in which no significant proliferation above background was seen (data not shown). In addition, the IL-13 inhibitor significantly increased the levels of CD8+ IFN-γ production in animals immunized with PCLUS6.1–18IIIB and GM-CSF and CD40L, and these IFN-γ levels correlated with the CTL response (data not shown).
Figure 4.
Enhancement of vaccine elicited CD8+ CTL response and memory by relief of CD4+ cell mediated suppression by treatment with an in vivo inhibitor of IL-13. (A and D) CTL response of female BALB/c mice given a single s.c. immunization with 20 nmol vaccine construct PCLUS 6.1–18IIIB in Montanide ISA51. Animals received five doses (175 μg/dose at day 0, 1, 2, 4, 6) i.p. of either IL-13Rα2Fc (filled circles) or control human IgG (Panglobulin) (open circles). Anti-CD4 (crossed circle) (0.5 mg/day) was given 1 day before immunization and 3 consecutive days thereafter. In all six exhibits, spleen cells were restimulated in vitro with P18IIIB peptide and IL-2 for 7 days before testing in a 51Cr release assay. Error bars < the symbols are not shown. Shown is specific lysis of P18IIIB (0.5 μM)-coated target cells by spleen cell effectors from the immunized mice harvested 14 days (A) or 7 weeks (D) after a single immunization. (B and E) CTL response of female BALB/c mice given a single s.c. immunization with 20 nmol of PCLUS 6.1–18IIIB plus GM-CSF, and either IL-13Rα2Fc (filled squares), control human IgG (Panglobulin) (open squares), or anti-CD4 (crossed square) (0.5 mg/day), and spleen cells tested for lysis as in A and D harvested 14 days (B) or 7 weeks (E) after immunization. (C and F) CTL response of female BALB/c mice given a single s.c. immunization with 20 nmol of PCLUS 6.1–18IIIB and the combination of GM-CSF (5 μg/mouse) and CD40L (15 μg/mouse), incorporated in adjuvant montanide-ISA 51. Mice were treated with control Ig (open triangle) or the IL-13 inhibitor (filled triangle) or anti CD4 antibody (crossed diamond) as in A and D and spleen cells harvested 14 days (C) or 7 weeks (F) after immunization tested as above. Except in A, CTL responses are significantly different in all vaccine groups given IL-13Rα2Fc treatment (filled symbols) compared with control IgG (open symbols) (P < 0.05–0.01). Nonspecific lysis was <15% in immunized groups and completely absent in anti-CD4-treated mice.
Enhancement of Protection Against Viral Infection by Inhibition of IL-13, and Synergy with Cytokines.
To see whether the enhancement of CTL activity by the IL-13 inhibitor would translate to increased protection, we immunized mice that had been treated with IL-13 inhibitor or depleted of CD4+ cells and challenged them with a recombinant vaccinia virus expressing HIV-1 gp160 (Fig. 3 C and D). In this experiment we used GM-CSF without CD40L to distinguish better protection with and without IL-13Rα2-Fc or anti-CD4, because with CD40L as well included in the immunization, maximal protection was achieved without these treatments (Fig. 3 A and B). IL-13Rα2Fc improved protection of mice immunized with peptide and GM-CSF (Fig. 3C), although not in the absence of GM-CSF, exactly parallel to the enhancement of CTL activity primarily in the presence of GM-CSF (Fig. 4). By the Welch's ANOVA test, the groups treated with GM-CSF plus IL-13 inhibitor or anti-CD4 were both statistically different from the unimmunized control group (P < 0.01), whereas the group with GM-CSF alone was not quite significant (P = 0.06), indicating that blocking the suppressive pathway led to more significant protection. Further, in mice immunized with the free CTL epitope I10 alone, with GM-CSF and CD40L, protection was increased by use of IL-13Rα2-Fc as an inhibitor of IL-13 just as effectively as depletion of CD4+ cells (P < 0.01 for both) (Fig. 3D). Thus, the more selective inhibition of IL-13 was as effective as CD4+ cell depletion in overcoming negative regulation and enhancing protection against viral infection, presumably without the potential negative side effects of CD4+ cell depletion, and thus maybe a valuable new approach to enhance vaccines.
Discussion
The current results show that CD4+ cells both positively and negatively regulate CD8+ CTL responses, and that whereas the combination of dendritic cell-activating agents GM-CSF and CD40L can substitute for some of the positive effects of helper CD4+ cells, this combination is not adequate to overcome the negative regulatory influences, which are somewhat surmounted by actual CD4+ Th1 helper cells. Thus, CD4+ T cell help seems to contribute to overcoming suppression, perhaps by adjusting the cytokine balance, as well as to activate APCs. CD40L and GM-CSF together, although capable of activating APCs, are not sufficient to replace CD4+ T cell help as long as suppression is present. Rather, a third component, relief of suppression, is needed to replace T cell help fully.
The suppressive effects of CD4+ cells on CTL induction are at least in part mediated by IL-13. Thus, inhibition of IL-13 or depletion of the CD4+-negative regulatory cells allows the GM-CSF/CD40L combination to have its maximal effect. Both CD1-restricted NKT cells and conventional CD4+ Th2 cells [or the CD4+CD25+ suppressor cells (16)] may contribute to this suppressive effect, and both NKT and Th2 cells can make IL-13 (19, 47), but on the basis of the use of CD1 knockout mice, a major mechanism may involve NKT cells. Indeed, the fact that no further enhancement is achieved by anti-CD4 treatment in CD1 knockout mice suggests that the major suppressive cell being deleted by anti-CD4 in this system is the NKT cell, which is already absent in the CD1 knockout mice (Fig. 2D). Moreover, because inhibition of IL-13 was as effective as CD4 depletion in increasing CTL activity (Fig. 4), it is likely that the major mechanism of CD4+ T cell-mediated suppression in this system involves IL-13. Combining these last two points would lead logically to the conclusion that the major suppressive mechanism here requires IL-13 production by NKT cells. However, we cannot exclude a contribution by IL-13 made by other cells. The current findings are important not only in understanding the conflicting role of CD4+ T cells in CTL responses, but also in designing a vaccine to activate CTL maximally. One combination that achieves this effect in optimizing vaccines is the synergistic combination of GM-CSF and CD40L to push the response (especially when help is weak) used in concert with an inhibitor of IL-13 to relieve the negative regulatory effects that dampen the CTL response.
Our results represent an important direction in optimizing vaccine induction of CD8+ CTL responses. Relief of suppressive cell interactions or cytokine-mediated suppression may prove to be essential for eliciting cellular immune response in persistent viral infections and cancer. Although such immunosuppressive mechanisms have been of interest in transplantation, autoimmunity, and cancer, approaches to inhibit these have not been applied to the optimization of vaccines for infectious diseases. Because these ongoing disease states involve manifold mechanisms of immune deviation and suppression, identification and development of molecules that block suppressive cells and their cytokines may also have a therapeutic potential in enhancing preexisting immunity.
We have recently demonstrated both a quantitative and qualitative role for CD40L–CD40 interaction in polarizing dendritic cells toward driving Th1 responses and CTL induction (27). Although dendritic cells may be activated and CTL elicited in both CD4-dependent and independent pathways (48, 49), a critical role for CD4+ help mediated through CD40L–CD40 interaction in eliciting protective antitumor immunity (32, 50) and maintaining memory CTL responses, particularly in resolving chronic viral infection (8, 12), emphasizes the need for eliciting optimum CD4+ effector function in vaccine immunotherapy. Here, we have shown that CD4+ T cell activity may have opposing effects on vaccine induction of CTL, so that CD4+ cell depletion paradoxically enhances vaccine effectiveness when exogenous cytokines and costimulatory molecules are included to compensate for loss of T cell help.
Cytokines that recruit and activate antigen-presenting cells, such as GM-CSF, and Th1-promoting cytokines, such as IL-12, synergistically enhance vaccine-induced CTL responses (24, 26). Here we show that enhancing help is a major factor in eliciting optimal CTL responses; however, when help is absent or limiting, relieving suppressive cytokine influences such as the one we have demonstrated here for IL-13 may be essential for eliciting CTL responses. The push–pull combination of enhancing cytokines such as GM-CSF and IL-12, and molecules that enhance antigen-presenting function and costimulation, such as CD40L, with inhibitors of negative regulatory mechanisms, such as IL-13-mediated suppression, may optimize vaccine efficacy and overcome some of the mechanisms by which viruses and cancer evade protective immunity. In HIV-infected patients, this approach may synergize with antiretroviral therapy.
Acknowledgments
We thank Drs. William E. Paul and Alan Sher for critical reading of the manuscript and helpful suggestions. We thank Jean-Pierre Planchot, Seppic, Inc., for a kind gift of Montanide ISA 51 adjuvant, and Dr. William E. Paul and Cynthia Watson (National Institute of Allergy and Infectious Diseases) for a gift of the CD1 knockout mice.
Abbreviations
- APC
antigen-presenting cell
- CTL
cytotoxic T lymphocyte(s)
- NK
natural killer
- GM-CSF
granulocyte/macrophage colony-stimulating factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rowland-Jones S, Tan R, McMichael A. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 2.Goulder P J R, Rowland-Jones S L, McMichael A J, Walker B D. AIDS. 1999;13:S121–S136. [PubMed] [Google Scholar]
- 3.Oscherwitz J, Gotch F M, Cease K B, Berzofsky J A. AIDS. 1999;13:S163–S174. [PubMed] [Google Scholar]
- 4.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 5.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, et al. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berzofsky J A, Ahlers J D, Belyakov I M. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Liu Y. Curr Biol. 1994;4:499–505. doi: 10.1016/s0960-9822(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 8.Whitmire J K, Flavell R A, Grewal I S, Larsen C P, Pearson T C, Ahmed R. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- 9.Keene J A, Forman J. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirai M, Pendleton C D, Ahlers J, Takeshita T, Newman M, Berzofsky J A. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 11.Ahlers J D, Takeshita T, Pendleton C D, Berzofsky J A. Proc Natl Acad Sci USA. 1997;94:10856–10861. doi: 10.1073/pnas.94.20.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrowski M A, Justement S J, Ehler L, Mizell S B, Lui S, Mican J, Walker B D, Thomas E K, Seder R, Fauci A S. J Immunol. 2000;165:6133–6141. doi: 10.4049/jimmunol.165.11.6133. [DOI] [PubMed] [Google Scholar]
- 13.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalams S A, Walker B D. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belyakov I M, Hel Z, Kelsall B, Kuznetsov V A, Ahlers J D, Nacsa J, Watkins D, Allen T M, Sette A, Altman J, et al. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 16.Thornton A M, Shevach E M. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann T R, Coffman R L. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Kuchroo V K, Inobe J-i, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 19.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson D D, Carbone D P, Paul W E, Berzofsky J A. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 20.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang Z, Ertl H C J. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 22.Disis M L, Bernhard H, Shiota F M, Hand S L, Gralow J R, Huseby E S, Gillis S, Cheever M A. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 23.Sin J-I, Kim J J, Ugen K E, Ciccarelli R B, Higgins T J, Weiner D B. Eur J Immunol. 1998;28:3530–3540. doi: 10.1002/(SICI)1521-4141(199811)28:11<3530::AID-IMMU3530>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 24.Ahlers J D, Dunlop N, Alling D W, Nara P L, Berzofsky J A. J Immunol. 1997;158:3947–3958. [PubMed] [Google Scholar]
- 25.Ahlers J D, Belyakov I M, Matsui S, Berzofsky J A. Int Immunol. 2001;13:897–908. doi: 10.1093/intimm/13.7.897. [DOI] [PubMed] [Google Scholar]
- 26.Belyakov I M, Ahlers J D, Clements J D, Strober W, Berzofsky J A. J Immunol. 2000;165:6454–6462. doi: 10.4049/jimmunol.165.11.6454. [DOI] [PubMed] [Google Scholar]
- 27.Ahlers J D, Belyakov I M, Thomas E K, Berzofsky J A. J Clin Invest. 2001;108:1677–1685. doi: 10.1172/JCI13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett S R M, Carbone F R, Karamalis F, Flavell R A, Miller J F A P, Heath W R. Nature (London) 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 29.Ridge J P, Di Rosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 30.Schoenberger S P, Toes R E M, van der Voort E I H, Offringa R, Melief C J M. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 31.Kuniyoshi J S, Kuniyoshi C J, Lim A M, Wang F Y, Bade E R, Lau R, Thomas E K, Weber J S. Cell Immunol. 1999;193:48–58. doi: 10.1006/cimm.1999.1469. [DOI] [PubMed] [Google Scholar]
- 32.Gurunathan S, Irvine K R, Wu C Y, Cohen J I, Thomas E, Prussin C, Restifo N P, Seder R A. J Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 33.Mendoza R B, Cantwell M J, Kipps T J. J Immunol. 1999;159:5777–5781. [PubMed] [Google Scholar]
- 34.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo M P. J Exp Med. 1999;190:125–133. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smiley S T, Kaplan M H, Grusby M J. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 36.Berzofsky J A, Pendleton C D, Clerici M, Ahlers J, Lucey D R, Putney S D, Shearer G M. J Clin Invest. 1991;88:876–884. doi: 10.1172/JCI115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi H, Cohen J, Hosmalin A, Cease K B, Houghten R, Cornette J, DeLisi C, Moss B, Germain R N, Berzofsky J A. Proc Natl Acad Sci USA. 1988;85:3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlers J D, Pendleton C D, Dunlop N, Minassian A, Nara P L, Berzofsky J A. J Immunol. 1993;150:5647–5665. [PubMed] [Google Scholar]
- 39.Myers G, Josephs S F, Berzofsky J A, Rabson A B, Smith T F, Wong-Staal F. Human Retroviruses and AIDS 1989. Los Alamos, NM: Los Alamos National Laboratory; 1989. [Google Scholar]
- 40.Takeshita T, Takahashi H, Kozlowski S, Ahlers J D, Pendleton C D, Moore R L, Nakagawa Y, Yokomuro K, Fox B S, Margulies D H, Berzofsky J A. J Immunol. 1995;154:1973–1986. [PubMed] [Google Scholar]
- 41.Earl P L, Hugin A W, Moss B. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahlers J D, Dunlop N, Pendleton C D, Newman M, Nara P L, Berzofsky J A. AIDS Res Hum Retroviruses. 1996;12:259–272. doi: 10.1089/aid.1996.12.259. [DOI] [PubMed] [Google Scholar]
- 43.French R R, Chan H T, Tutt A L, Glennie M J. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 44.Sonoda K H, Exley M, Snapper S, Balk S P, Stein-Streilein J. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, Taniguchi M, Ikeda S. J Clin Invest. 2000;105:1761–1767. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seino K-I, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, Iwakura Y, Van Kaer L, Takeda K, Nakayama T, et al. Proc Natl Acad Sci USA. 2001;98:2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finkelman F D, Wynn T A, Donaldson D D, Urban J F. Curr Opin Immunol. 1999;11:420–426. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 48.Ruedl C, Kopf M, Bachmann M F. J Exp Med. 1999;189:1875–1883. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Z, Yuan L, Zhou X, Sotomayor E, Levitsky H I, Pardoll D M. J Exp Med. 2000;191:541–550. doi: 10.1084/jem.191.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackey M F, Gunn J R, Maliszewski C, Kikutani H, Noelle R J, Barth R J., Jr J Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]