Abstract
Modification of HIV-1 Gag with myristic acid, a saturated 14-carbon fatty acid (14:0), is essential for HIV-1 assembly. We recently showed that exogenous treatment of cells with unsaturated 14-carbon fatty acids, 5-cis-tetradecenoic acid (14:1n-9) and 5-cis,8-cis-tetradecadienoic acid (14:2n-6), reduces the affinity of some myristoylated proteins for plasma membrane rafts, membrane subdomains that have been shown to be required for efficient assembly of HIV. Here we demonstrate that treatment of cells with 14:1n-9 and 14:2n-6 fatty acids reduced the affinity of Gag for rafts but not membranes in general. Furthermore, treatment of cells with 14-carbon unsaturated fatty acids inhibited Gag-driven particle assembly. These effects most likely reflect covalent modification of Gag with unsaturated fatty acids. Treatment with 14:1n-9 and 14:2n-6 fatty acids did not alter intracellular protein trafficking, nor did it reduce cell viability. These studies suggest a strategy to attack HIV assembly by selectively altering the patterns of fatty acid modification.
Expression of the HIV-1 Gag protein is necessary and sufficient for the production of virus-like particles (VLPs; ref. 1). VLPs consist of a multimerized array of 1,000–1,500 Gag molecules surrounded by a lipid envelope obtained from the host cell plasma membrane (2). These particles resemble immature virions and can be isolated from the cell-culture medium. Cotranslational modification of Gag with the saturated fatty acid myristic acid (tetradecanoic acid, abbreviated here as 14:0) to the N-terminal glycine is required for the binding of Gag to cellular membranes as well as the subsequent assembly and budding of virions/VLPs (3).
N-myristoylation of proteins is catalyzed by the enzyme N-myristoyl transferase (NMT), which uses myristoyl-CoA as a substrate (4). Several studies have considered NMT as a potential drug target for the inhibition of retroviral assembly and as a general antimicrobial agent (4). For example, NMT inhibitors have been shown to prevent both membrane binding of Gag as well as virus assembly (5). In addition, heteroatom-substituted myristic acid analogs such as 12-methoxydodecanoic acid can be used by NMT as alternative substrates for covalent attachment to proteins. The hydrophilic nature of these compounds inhibits membrane binding and function of the modified proteins, including HIV-1 Gag. Inhibition of assembly in this manner has been shown to dramatically lower the required dose of the reverse-transcriptase inhibitor AZT to block HIV replication in infected lymphocytes (6). Although potentially useful in therapeutic strategies, NMT inhibitors and heteroatom-substituted myristic acid analogs are expected to affect a broad spectrum of cellular processes that depend on protein N-myristoylation for membrane binding, including G-protein signaling, Src-family tyrosine kinases, and vesicular transport.
An alternative strategy for perturbing the function of N-myristoylated proteins derives from the observation that a subset of retinal N-terminal fatty acylated proteins are modified specifically by lauric acid (12:0), 5-cis-tetradecenoic acid (14:1n-9), or 5-cis,8-cis-tetradecadienoic acid (14:2n-6) in place of 14:0 (7, 8). These fatty acids are less hydrophobic than 14:0 either because of a shorter hydrocarbon chain or the presence of cis double bonds. Attachment of unsaturated fatty acids has been proposed to increase the on/off rate of N-terminal fatty acylated proteins from membranes, with the potential to regulate the extremely rapid process of visual transduction (9). Although the distribution of fatty acyl-CoA pools in numerous cell types suggests that these “alternative” fatty acids are quite prevalent in the cytoplasm, heterogeneous N-terminal fatty acylation has been observed only in the retina (10). This suggests that some form of sequestration of fatty acyl-CoA species occurs such that only myristoyl-CoA is accessible to NMT in nonretinal cell types. This sequestration can be bypassed by exogenous treatment of cells with 14:1n-9 or 14:2n-6, resulting in the modification of N-terminal fatty acylated proteins with these unsaturated fatty acids. Such treatment inhibits specific cellular pathways mediated by Src-family kinases but has no apparent overall effect on cell proliferation (11).
Of particular interest is the mechanism by which these unsaturated fatty acids have been proposed to inhibit signaling by the Src-family kinase Fyn. Our laboratory recently showed that modification of Fyn with 14:1n-9 or 14:2n-6 reduces the efficiency of signaling by displacing Fyn from plasma membrane rafts (11). Raft microdomains are small semitransient assemblies of lipids and proteins at the plasma membrane (12, 13) that function to organize signaling complexes. Acylation of Fyn with unsaturated fatty acids most likely reduces the affinity of Fyn for the highly saturated, ordered core of the raft bilayer (11, 14).
HIV-1 has been proposed recently to assemble in raft-like microdomains (15–17). This process requires modification of Gag with 14:0 as well as multimerization of Gag, thereby bringing multiple myristic acid moieties in close apposition (16). Because the N-terminal HIV-1 Gag sequence can be acylated with 14:1n-9 and 14:2n-6 in vitro (18), we reasoned that exogenous treatment with unsaturated fatty acids might alter the subcellular localization of HIV-1 Gag in vivo. Here we show that treatment of cells with 14:1n-9 and 14:2n-6 removes Gag from plasma membrane rafts. The mislocalization of Gag triggered by unsaturated fatty acids has functional consequences in that it results in strong inhibition of VLP production.
Materials and Methods
Antibodies and Reagents.
Rabbit anti-p24CA antiserum was used to immunoprecipitate Gag. Either the anti-p24CA serum or anti-p24CA monoclonal antibody (Advanced Biotechnologies) was used to detect Gag by Western blot. Protein A/G Plus agarose beads were purchased from Santa Cruz Biotechnology. The following antibodies were purchased as indicated: anti-Src monoclonal antibody (Upstate Biotechnology, Lake Placid, NY); anti-caveolin-1 polyclonal antibody (Santa Cruz Biotechnology); and anti-GFP monoclonal antibody (Roche, Indianapolis). Optiprep was purchased from GIBCO/BRL Life Technologies (Grand Island, NY). Triton X-100 (TX-100) was purchased from Fisher Biotech (Fair Lawn, NJ). Myristic acid (14:0), palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1n-9), arachidonic acid (20:4n-6), eicosapentenoic acid (20:5n-3), docosahexenoic acid (22:6n-3), and 2-hydroxymyristate were purchased from Sigma. 5-cis-tetradecenoic acid (14:1n-9) and 5-cis,8-cis-tetradecadienoic acid (14:2n-6) were a kind gift from Horst Schulz (City College, City University of New York, New York).
DNA Plasmids.
Pr55Gag was expressed from the Rev-independent vector pCMV5 Gag (19) in the absence of other HIV-1 gene products. pCMV5 GagΔp15 expresses a Gag protein truncated after the viral protease site at the C terminus of the p2 spacer peptide (16). The construction of pGagEGFP was as described (20). Construction of pVALO Gag69EGFP was performed as described (21) with the exception that the enhanced GFP-coding sequence was used (M. Tritel, unpublished results). CD4 was expressed from pEGFP-N2 CD4FL (a kind gift from John Wills, Pennsylvania State University College of Medicine, Hershey).
Cell Culture and Transfections.
For transfections, COS-1 cells were seeded to ≈25% density and allowed to grow to near confluency before transfection with 3–5 μg of plasmid DNA using Lipofectamine 2000 (GIBCO/BRL Life Technologies). For treatment of cells with fatty acids, cells were split 1:2 24 h after transfection. After the cells became adherent, the medium was replaced with DMEM supplemented with 2% dialyzed FBS and 0.5% defatted BSA complexed to 100 μM fatty acid as described (11).
Cell Labeling.
Cells transfected with pCMV5 Gag were starved for 1 h in DMEM containing 2% dialyzed FBS and then labeled for 4 h at 37°C with 50 μCi/ml (1 Ci = 37 GBq) 13-[125I]iodotridecanoic acid (IC13) in DMEM containing 2% dialyzed FBS and 0.5% defatted BSA (22). Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl/1.0% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS/50 mM Tris, pH 8.0) and centrifuged at 100,000 × g to obtain clarified supernatants. Immunoprecipitation then was performed in RIPA buffer by using anti-p24CA antiserum (19). Alternately, labeled cells were prepared according to the detergent-resistant membrane (DRM) protocol described below. Radiolabeled Gag was analyzed by SDS/PAGE and quantified by using a PhosphorImager.
Isolation of DRMs and Light Membrane Fractions.
DRMs were isolated as described (23). COS-1 cells were transfected with pCMV5 Gag and treated overnight with fatty acid. The following day, the cells were extracted on ice for 20 min in TNET buffer (50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/5 mM EDTA/0.5% TX-100) containing a protease-inhibitor mixture. Cells then were Dounce-homogenized, adjusted to 35% Optiprep, and placed at the bottom of an SW55 centrifuge tube. The lysate was overlayed with 3.5 ml of 30% Optiprep in TNET followed by 200 μl of TNET. After centrifugation at 170,000 × g at 4°C for 4 h, five equal fractions were collected from the top of the gradient. The fractions were adjusted to 1× RIPA buffer and clarified, and aliquots of each fraction were precipitated with 20% trichloroacetic acid or immunoprecipitated. Proteins were analyzed by SDS/PAGE followed by Western blotting. Light membrane fractions were isolated in similar fashion with the following modifications. TX-100 was omitted from all buffers. After Dounce homogenization, the cell extracts were adjusted to 50% Optiprep, overlayed with 1 ml each of 40, 30, and 20% Optiprep in TNET and finally with 400 μl of 10% Optiprep in TNET. After centrifugation, eight equal fractions were collected and analyzed as described above.
Preparation of Crude Cytosol (S100) and Membrane (P100) Fractions.
Cells were lysed in hypotonic buffer and homogenized, and postnuclear supernatants were centrifuged at 100,000 × g to sediment cellular membranes (P100). For flotation of Gag-containing membrane fractions, cell homogenates were adjusted to 70% (wt/vol) sucrose in PBS and layered successively with 65% sucrose and 10% sucrose in SW40 tubes. Samples were centrifuged for 14 h at 100,000 × g. Fractions were collected from the top and analyzed by Western blotting (24).
Quantification of Gag in VLPs from Labeled Cells.
After overnight treatment with fatty acid, Gag-expressing COS-1 cells were starved for 1 h in DMEM lacking methionine and cysteine and supplemented with 2% dialyzed FBS and BSA–fatty acid complex. Cells then were labeled in the same medium containing 100 μCi/ml Tran35S-label (ICN) for 1, 2, or 4 h. Gag protein was immunoprecipitated from the medium containing VLPs and from the cell lysates (25). Radiolabeled Gag was analyzed by SDS/PAGE and quantified by using a PhosphorImager.
Immunofluorescence.
COS-1 or COS-7 transfected cells were grown on coverslips and processed 48 h posttransfection (20). Cells were incubated with a 1:500 dilution of anti-protein disulfide isomerase polyclonal antibody (StressGen Biotechnologies, Victoria, Canada) or anti-CD4 monoclonal antibody (Monoclonal Core Facility, Memorial Sloan–Kettering Cancer Center, New York) (surface anti-CD4 staining was performed without cell permeabilization) in PBS for 90 min. After four 5-min washes in PBS, cells were incubated with Cy5 anti-rabbit IgG (Jackson ImmunoResearch) or Alexa Fluor 594 goat anti-mouse (Molecular Probes) for 45 min. Cells then were stained for 5 min with 250 ng of Hoechst 33258/ml to label nuclei, washed four times for 5 min each with PBS, and mounted on microscope slides. Gag–enhanced GFP (EGFP) expression was visualized directly. In some experiments, cells were incubated on ice with 0.5% TX-100 in PBS for 20 min before fixation. Laser scanning confocal microscopy was performed on a Zeiss LSM510 confocal microscope equipped with an Axiovert 100M inverted microscope using an ×63, 1.2-numerical-aperture water-immersion lens for imaging.
Results
Unsaturated Fatty Acids Reduce Gag Partitioning to Rafts.
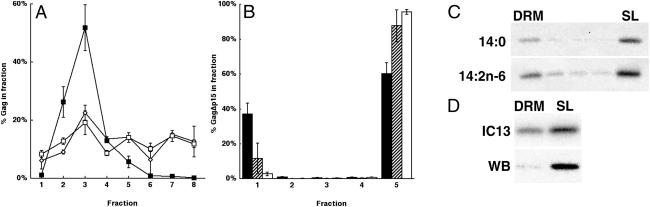
We have shown that exogenous addition of unsaturated fatty acids can alter the pattern of both S- and N-acylation of proteins in cells in culture (11). In light of these findings, we sought to determine the effects of such treatment on modification of myristoylated HIV-1 Gag protein. Modification of Gag-derived peptides by alternate NMT substrates (14:1n-9 and 14:2n-6) has been demonstrated previously (18). Myristoylation has been shown to be essential for the partitioning of Gag to plasma membrane rafts, an early step in the assembly process (16). We therefore examined the subcellular localization of Gag in cells treated with 14:1n-9 and 14:2n-6. Cells treated with 14:0 served as a control. Fig. 1A depicts the fractionation of Gag with a detergent-free discontinuous Optiprep gradient. We have shown previously that in the absence of detergent Gag partitions to a light membrane fraction that contains raft components and is distinct from nonraft plasma membrane fractions (16). When either 14:0 (this study) or no exogenous fatty acids (16) were added, Gag was found primarily in fractions 2–4, in the low-density portion of the gradient. In cells treated with 14:1n-9 or 14:2n-6, Gag shifted toward the denser gradient fractions, representing a shift out of plasma membrane rafts. In contrast, the distribution of caveolin-1, an endogenous raft marker protein, did not change (data not shown). Little or no change in cell viability was apparent when cells were incubated with 14:0 or 14:2n-6, as assessed by trypan blue exclusion and [3H]thymidine incorporation (ref. 26; Table 1).
Figure 1.
Unsaturated 14-carbon fatty acids alter the behavior of Gag on Optiprep density gradients. (A) After overnight treatment with fatty acid-supplemented medium, Dounce homogenates of COS-1 cells transfected with pCMV5 Gag were floated in a discontinuous Optiprep gradient. Fractions were collected such that fraction 1 is the top and fraction 8 is the bottom of the gradient. Trichloroacetic acid precipitates of each fraction were subjected to SDS/PAGE and Western blotting by using anti-p24CA antibody. The percent of total Gag protein in each fraction was quantitated from cells treated with 14:0 (closed squares), 14:1n-9 (open squares), or 14:2n-6 (open diamonds). (B) Cells expressing GagΔp15 were treated with fatty acid-supplemented medium and lysed at 4°C in 0.5% TX-100. The lysates were floated on discontinuous Optiprep gradients. Five fractions were collected from the top; fraction 1 contains DRMs and fraction 5 contains the soluble lysate (SL). Closed bars represent the percent of total GagΔp15 detected in each fraction from cells treated with 14:0, hatched bars 14:1n-9, and open bars 14:2n-6. (C) GagΔp15-expressing cells were treated with 14:0 or 14:2n-6 overnight and labeled the following day with IC13. After labeling, the cells were lysed and floated as described for B. Anti-p24CA immunoprecipitates were analyzed by SDS/PAGE, and Gag-specific bands were visualized by PhosphorImager analysis. Representative gradients are depicted, showing that approximately equal fractions of IC13-labeled GagΔp15 are recovered in DRMs in either condition. (D) A comparison of radiolabel (IC13) and Western blot (WB) signals from immunoprecipitates of the same gradient of 14:2n-6-treated cells. Only the DRM and soluble lysate fractions are shown.
Table 1.
Viability and growth of fatty acid-treated cells
| Fatty acid | % viable cells (normalized) | cpm, % of untreated cells |
|---|---|---|
| Trypan blue exclusion | ||
| 14:0 | 100 ± 0.5* | — |
| 14:2n-6 | 91.7 ± 0.6 | — |
| [3H]Thymidine incorporation | ||
| Untreated | — | 100 ± 14 |
| 14:0 | — | 85 ± 28 |
| 14:2n-6 | — | 94 ± 8 |
All errors are SD of n = 6 independent experiments.
Rafts typically are isolated as DRMs that maintain their buoyant density in density gradients after treatment with nonionic detergents at 4°C (27). Gag multimerization makes Gag-containing membrane fragments fractionate at densities higher than “classical” DRMs (16). However, truncation of most of the multimerization domains (NC-p1-p6) generates a Gag protein, GagΔp15, that can be isolated in DRMs by using standard techniques (16). As depicted in Fig. 1B, treatment of COS-1 cells expressing GagΔp15 with 14:1n-9 or 14:2n-6 greatly reduced the percentage of this protein isolated in DRMs.
One potential explanation for the ability of unsaturated fatty acids to reduce partitioning of proteins to rafts is that these fatty acids are incorporated into raft lipids, thereby perturbing raft structure (28). This model predicts that proteins modified with saturated fatty acids such as myristic acid or palmitic acid also will be shifted out of rafts. To test this hypothesis, GagΔp15-expressing cells were treated with 14:0 or 14:2n-6 and subsequently labeled with IC13, an iodinated myristic acid analog. Lysates were fractionated on density gradients to isolate DRMs (Fig. 1C, n = 3). Nearly identical amounts (≈25%) of 125I-radiolabeled Gag was found in DRMs derived from cells treated with either 14:0 or 14:2n-6. A similar experiment is depicted in Fig. 1D, in which only the top (DRM) and bottom (soluble lysate) fractions of 14:2n-6-treated cells were collected. Again, ≈25% of the radiolabeled protein was found in DRMs. In contrast, Western blotting of the identical fractions indicated that the total population of GagΔp15 protein was largely excluded from DRMs when cells were treated with 14:2n-6. Thus, the subpopulation of protein modified with the radiolabeled saturated fatty acid (IC13) is not shifted out of DRMs. These data provide strong evidence for a model in which the displacement of Gag proteins from DRMs (and rafts) is due to direct modification of Gag by unsaturated fatty acids.
Unsaturated Fatty Acids Do Not Prevent Gag Membrane Binding.
Displacement of a protein from rafts could be due to a shift to less-ordered regions of cellular membranes or a failure of that protein to bind membranes altogether. To distinguish between these alternatives, membrane binding of GagΔp15, Gag69EGFP (a protein consisting of the first 69 amino acids of Gag fused to EGFP), and the endogenous myristoylated protein c-Src was examined by using a sedimentation assay. The amount of c-Src, Gag69EGFP, or GagΔp15 present in membrane fractions was essentially the same in cells treated with 14:0 or 14:2n-6 (Table 2). Likewise, a membrane flotation assay revealed that the majority of full-length Gag was found in the membrane fraction in cells treated with either 14:0 or 14:2n-6. Thus, treatment with unsaturated fatty acids such as 14:2n-6 does not prevent Gag from binding to membranes but rather specifically shifts Gag out of raft membrane microdomains.
Table 2.
Membrane binding of myristoyl proteins in treated cells
| Protein | % in membranes (14:0) | % in membranes (14:2n-6) |
|---|---|---|
| Gag | 89 ± 9 | 91 ± 6 |
| GagΔp15 | 72 ± 16 | 85 ± 12 |
| Gag69eGFP | 80 ± 0.3 | 71 ± 6 |
| c-Src | 87 ± 22 | 78 ± 9 |
Data are means and SD of 2–4 independent experiments.
Subcellular Localization of GagEGFP in Cells Treated with Unsaturated Fatty Acids.
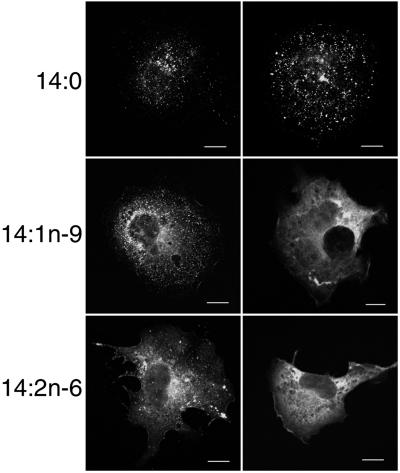
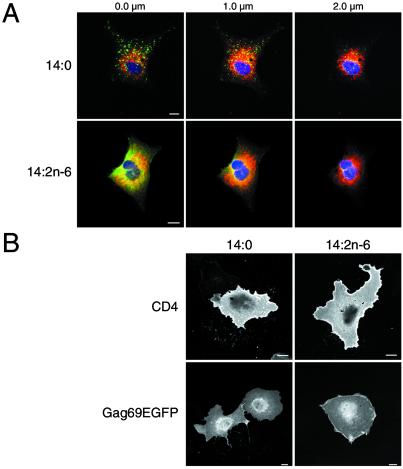
To study the effects of 14:1n-9 and 14:2n-6 treatment on Gag localization further, cells transfected with a GagEGFP expression vector were examined by laser scanning confocal microscopy. As depicted in Fig. 2, GagEGFP in cells treated with 14:0 exhibited a typical punctate fluorescent-staining pattern that represents VLP assembly sites at the plasma membrane (20). In contrast, the staining pattern of GagEGFP in cells treated with 14:1n-9 (Middle) and 14:2n-6 (Bottom) was different. Two patterns were similarly prevalent: cells that exhibited both punctate and diffuse fluorescent-staining patterns (Fig. 2 Left) and cells with only a diffuse pattern (Fig. 2 Right). Confocal analysis of successive focal planes was performed with GagEGFP-expressing cells costained with antibody to protein disulfide isomerase, an endoplasmic reticulum (ER) marker protein (Fig. 3A). In 14:0-treated cells, the GagEGFP signal was predominantly at the plasma membrane, as we have reported (20). In cells treated with 14:2n-6, much of GagEGFP remained at the plasma membrane (0 μm), although a portion of the protein was also visible in the focal plane with maximal ER staining (1 μm).
Figure 2.
Confocal analysis of the distribution of GagEGFP in cells treated with 14-carbon fatty acids. Cells transfected with pGagEGFP were grown on coverslips in medium supplemented with the indicated fatty acids. Cells then were fixed and prepared for confocal microscopy. Because there was a range of morphologies from cell to cell, two representative cells are shown for each condition. (Scale bars, 10 μm.)
Figure 3.
Membrane localization of Gag. (A) Confocal analysis of 14-carbon fatty acid-treated cells showing successive focal planes of a z stack. The point of attachment closest to the coverslip is 0 μm. GagEGFP fluorescence is green. Protein disulfide isomerase, a luminal ER marker labeled with rabbit anti-protein disulfide isomerase and Cy5 anti-rabbit IgG, is depicted in red. Nuclei are blue. (B) Surface immunolabeling of CD4 using anti-CD4 monoclonal antibody and Alexa Fluor 594 anti-mouse in transiently transfected COS-7 cells (Upper) and GFP fluorescence of Gag69EGFP-expressing cells (Lower). (Scale bars, 10 μm.)
To determine whether any gross abnormalities of intracellular trafficking of membrane proteins were caused by 14:2n-6 treatment, we examined COS-7 cells transiently transfected with a plasmid expressing human CD4. A comparison of surface labeling of CD4 using monoclonal anti-CD4 antibody (Fig. 3B Upper) revealed approximately equivalent intensity and distribution of CD4 at the plasma membrane in 14:0- and 14:2n-6-treated cells, indicating that the secretory apparatus was not impaired grossly. We also examined the distribution of Gag69EGFP, a myristoylated protein that shares some features of intracellular traffic with Gag (19, 21) but does not multimerize (19) or enter membrane rafts (our unpublished results). The fluorescence pattern of this protein was unchanged after treatment with the different fatty acids (Fig. 3B Lower), suggesting that early stages of Gag traffic before raft association are unaffected.
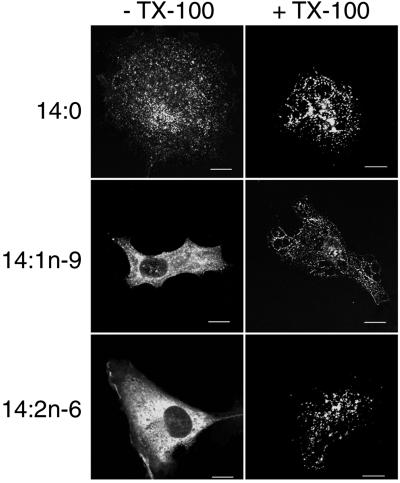
One of the hallmarks of raft association is the resistance of raft proteins to solubilization by nonionic detergents. Raft association can be assayed via fluorescence microscopy by extracting cells with PBS containing TX-100 before fixation and staining (29). COS-1 cells transfected with GagEGFP were treated with 14:0, 14:1n-9, or 14:2n-6 and then extracted with PBS or PBS plus TX-100. In 14:0-treated cells, the punctate fluorescence pattern was largely preserved in either condition (Fig. 4), consistent with Gag remaining raft-associated. In both 14:1n-9- and 14:2n-6-treated cells, the majority of the fluorescent signal was lost after detergent addition, consistent with the protein having been solubilized by TX-100 and removed from rafts. However, some cells retained a weak fluorescent signal that resembled the punctate pattern found in 14:0-treated cells, which likely represents the population of multimerized Gag that is myristoylated with 14:0 and hence retains raft association.
Figure 4.
Treatment with unsaturated 14-carbon fatty acids increases the detergent solubility of Gag. Cells were transfected with pGagEGFP and grown on coverslips in medium supplemented with the indicated fatty acids. Before fixation the cells were treated with 0.5% TX-100 in PBS or with PBS alone on ice for 20 min.
Unsaturated Fatty Acids Inhibit VLP Production by Gag.
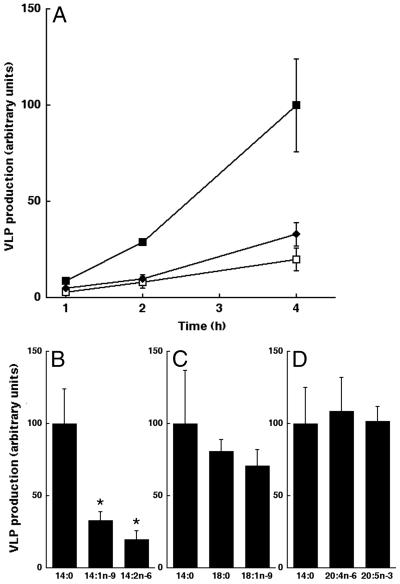
If Gag association with rafts is functionally important, then removal of Gag from plasma membrane rafts by treatment with unsaturated fatty acids should inhibit the formation of VLPs. To test this hypothesis, COS-1 cells transfected with Gag were treated with different fatty acids. Myristic acid (14:0) was used as a positive control in all experiments, because posttranslational modification of Gag with this fatty acid is essential for assembly of VLPs. After overnight incubation, the cells were metabolically labeled with Tran35S-label, and Gag was immunoprecipitated from the cell lysates and media at various time points. For each time point the ratio of radiolabeled Gag in the medium (a measure of VLPs) to radiolabeled Gag in cells was calculated to obtain the efficiency of VLP production. As depicted in Fig. 5 A and B, addition of the unsaturated fatty acids 14:1n-9 and 14:2n-6 significantly reduced the rate of VLP production from transfected COS-1 cells, with the latter having a stronger effect.
Figure 5.
Reduced VLP production by exogenous addition of unsaturated 14-carbon fatty acids. (A) COS-1 cells were transfected with pCMV5 Gag. On the following day, the medium was replaced with a fat-free medium supplemented with the indicated fatty acid. After overnight treatment, cells were radiolabeled with Tran35S-label. VLPs and whole-cell lysates were collected at 1-, 2-, and 4-h time points. Relative VLP production was quantitated from cells treated with 14:0 (closed squares), 14:1n-9 (closed diamonds), or 14:2n-6 (open squares). Error bars represent SD (n = 4). (B) Data from the 4-h time point in A represented as a bar graph, highlighting differences in VLP production (*, P < 0.005). (C and D) Other fatty acids do not affect VLP production significantly. Data are presented as described for B, showing results from experiments conducted with cells treated with 18:0, 18:1n-9, 20:4n-6, and 20:5n-3. 14:0 was used as a control in all experiments.
To determine whether the effects of 14:1n-9 and 14:2n-6 on VLP production were general properties of unsaturated fatty acids, we compared the effects of several other fatty acids. For ease of comparison, only the 4-h time points are shown in bar-graph form. VLP production after treatment with the saturated fatty acid stearic acid (18:0) and the monounsaturated fatty acid oleic acid (18:1n-9) was essentially the same as that of the positive control (14:0; Fig. 5C). The polyunsaturated fatty acids, arachidonic acid (20:4n-6) and eicosapentenoic acid (20:5n-3), also showed no significant effect on VLP production (Fig. 5D). These results demonstrate that unsaturated fatty acids in general do not have an inhibitory effect on VLP production. Rather, only those fatty acids that serve as substrates for NMT have the ability to reduce Gag-mediated particle formation.
Discussion
Several lines of evidence suggest that the mechanism by which unsaturated fatty acids inhibit VLP assembly is via direct covalent attachment to the N terminus of the Gag protein. Of the several classes of fatty acids tested, including saturated fatty acids of varying chain length, and unsaturated fatty acids of the n-3, n-6 and n-9 series, only those which have been shown previously to serve as alternative NMT substrates inhibited VLP production. Moreover, both 14:1n-9 and 14:2n-6 serve as NMT substrates that can be acylated to a peptide derived from the N terminus of Gag in vitro (18). Although we do not know the stoichiometry of Gag modification by unsaturated 14-carbon fatty acids in vivo, these modifications must occur to a significant extent, because Gag remains membrane-bound in 14:2n-6-treated cells. Gag mutants that cannot be N-terminally fatty acylated do not bind to membranes. Finally, we recently obtained direct biochemical evidence for in vivo modification of the N terminus of Fyn by 14:1n-9 and 14:2n-6 using mass spectrometry (11). Isolation of HIV-1 Gag-derived N-terminal peptides proved to be more difficult, and this approach remains an ongoing pursuit. Taken together, the data presented in this study and Liang et al. (11) strongly support the hypothesis that N-myristoylated proteins can be heterogeneously fatty acylated with 14:1n-9 and 14:2n-6.
Several recent reports have proposed a role for plasma membrane rafts in HIV-1 assembly (15–17). Myristoylated Gag proteins can be isolated in DRMs (15). The Gag-containing subdomains exhibit a higher density than classical rafts, and we have proposed the name “barges” to denote these high-density DRMs (16). Gag enters rafts/barges shortly after binding to membranes (17), and the density of barges resembles that of VLPs (16). Our working hypothesis is that barges are assembly complexes of multimerized Gag (16), which is supported by the finding that depletion of cholesterol disrupts rafts/barges and impairs VLP production (17). However, cholesterol depletion has pleiotropic effects on cells [see, e.g., Chen and Resh (30)]. The results presented in this work provide complementary evidence to support a role for rafts/barges in HIV-1 assembly. Treatment of cells with 14-carbon unsaturated fatty acids prevents Gag association with rafts, most likely because of the decreased affinity of unsaturated fatty acid chains for the liquid-ordered environment of the raft lipids (11, 14). It is possible also that acylation with unsaturated 14-carbon fatty acids perturbs Gag-protein structure in a manner that inhibits Gag multimerization and thereby inhibits raft association. In either case, mislocalization of Gag leads to inhibition of assembly. It is important to note that in 14:1n-9- and 14:2n-6-treated cells, Gag proteins that are modified with the saturated fatty acid (IC13) retain their affinity for rafts, indicating that the 14-carbon unsaturated fatty acids do not elicit any gross perturbations in raft structure.
One potential interpretation to account for the inhibition of VLP production is that unsaturated fatty acids lead to a reduction in the amount of Gag at the plasma membrane. Confocal imaging of sequential optical slices (z stacks) revealed the presence of Gag in cells treated with 14:1n-9 and 14:2n-6 at both the plasma membrane as well as the ER. In some cells, raft domains comprise the majority of the plasma membrane surface area (31). Thus, Gag displaced from rafts that cannot be accommodated in the bulk plasma membrane regions may partition randomly to the internal membrane with the largest surface area—i.e., the ER. This relocalization may prevent accumulation of a critical mass of Gag at the plasma membrane that is necessary for VLP production.
Our findings raise the possibility that certain classes of unsaturated fatty acids might be used as a dietary strategy to combat HIV-1 replication. Common dietary unsaturated fatty acids such as oleic acid (18:1n-9) and linoleic acid (18:2n-6) can be retroconverted to 14:1n-9 and 14:2n-6, respectively, by the peroxisomal pathway (9). We have shown, at least in principle, that long-chain n-9 and n-6 fatty acids are unlikely to have an inhibitory effect on HIV-1 assembly (Fig. 5), which indicates that fatty acyl-CoA produced by the retroconversion pathway is not readily available for use by NMT for posttranslational modification of proteins. Instead, direct introduction of 14:1n-9 and 14:2n-6 into the diet might be a useful therapeutic tool. 14:1n-9 is known commonly as physeteric acid and is abundant in whale oil (9). 14:2n-6 is known commonly as goshuyic acid and can be found in Evodia rutaecarpa, a traditional Chinese medicinal plant (9, 32). There is currently no medical literature on the pharmacologic effects of either of these fatty acids. Moreover, with the exception of retinal proteins, which are naturally heterogeneously N-acylated (7, 8), long-term ingestion of unsaturated 14-carbon fatty acids may have undesirable effects on N-myristoylated signaling proteins such as Src, Gα proteins, and Arf. Nonetheless, natural sources or organic synthesis could be used potentially for future in vivo studies directed at therapeutic uses for these fatty acids in the treatment of HIV infection.
Acknowledgments
We thank Dr. Xiquan Liang for many helpful discussions, Dr. Horst Schulz for kind gifts of 14:1n-9 and 14:2n-6, and Raya Louft-Nisenbaum for technical assistance. This work was supported by National Institutes of Health Grant CA72309. O.W.L. is a Dorris J. Hutchison Graduate Fellow and is supported by a Frank L. Horsfall Graduate Fellowship.
Abbreviations
- VLP
virus-like particle
- NMT
N-myristoyl transferase
- TX-100
Triton X-100
- EGFP
enhanced GFP
- IC13
13-[125I]iodotridecanoic acid
- DRM
detergent-resistant membrane
- ER
endoplasmic reticulum
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 2.Layne S P, Merges M J, Dembo M, Spouge J L, Conley S R, Moore J P, Raina J L, Renz H, Gelderblom H R, Nara P L. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 3.Gottlinger H G, Sodroski J G, Haseltine W A. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson D R, Bhatnagar R S, Knoll L J, Gordon J I. Annu Rev Biochem. 1994;63:869–914. doi: 10.1146/annurev.bi.63.070194.004253. [DOI] [PubMed] [Google Scholar]
- 5.Bryant M, Ratner L. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant M L, Ratner L, Duronio R J, Kishore N S, Devadas B, Adams S P, Gordon J I. Proc Natl Acad Sci USA. 1991;88:2055–2059. doi: 10.1073/pnas.88.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dizhoor A M, Ericsson L H, Johnson R S, Kumar S, Olshevskaya E, Zozulya S, Neubert T A, Stryer L, Hurley J B, Walsh K A. J Biol Chem. 1992;267:16033–16036. [PubMed] [Google Scholar]
- 8.Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Nature (London) 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- 9.DeMar J C, Jr, Rundle D R, Wensel T G, Anderson R E. Prog Lipid Res. 1999;38:49–90. doi: 10.1016/s0163-7827(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 10.DeMar J C, Jr, Anderson R E. J Biol Chem. 1997;272:31362–31368. doi: 10.1074/jbc.272.50.31362. [DOI] [PubMed] [Google Scholar]
- 11.Liang X, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh M D. J Biol Chem. 2001;276:30987–30994. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- 12.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 13.Pralle A, Keller P, Florin E L, Simons K, Horber J K. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb Y, Hermida-Matsumoto L, Resh M D. J Biol Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen D H, Hildreth J E. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindwasser O W, Resh M D. J Virol. 2001;75:7913–7924. doi: 10.1128/JVI.75.17.7913-7924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono A, Freed E O. Proc Natl Acad Sci USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatnagar R S, Schall O F, Jackson-Machelski E, Sikorski J A, Devadas B, Gokel G W, Gordon J I. Biochemistry. 1997;36:6700–6708. doi: 10.1021/bi970311v. [DOI] [PubMed] [Google Scholar]
- 19.Tritel M, Resh M D. J Virol. 2000;74:5845–5855. doi: 10.1128/jvi.74.13.5845-5855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermida-Matsumoto L, Resh M D. J Virol. 2000;74:8670–8679. doi: 10.1128/jvi.74.18.8670-8679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, Resh M D. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van't Hof W, Resh M D. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manes S, Mira E, Gomez-Mouton C, Lacalle R A, Keller P, Labrador J P, Martinez A C. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spearman P, Horton R, Ratner L, Kuli-Zade I. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weldon R A, Jr, Erdie C R, Oliver M G, Wills J W. J Virol. 1990;64:4169–4179. doi: 10.1128/jvi.64.9.4169-4179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang X, Wisniewski D, Strife A, Shivakrupa, Clarkson B, Resh M D. J Biol Chem. 2002;277:13732–13738. doi: 10.1074/jbc.M200277200. [DOI] [PubMed] [Google Scholar]
- 27.Brown D A, Rose J K. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 28.Stulnig T M, Huber J, Leitinger N, Imre E M, Angelisova P, Nowotny P, Waldhausl W. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 29.Nichols B J, Kenworthy A K, Polishchuk R S, Lodge R, Roberts T H, Hirschberg K, Phair R D, Lippincott-Schwartz J. J Cell Biol. 2001;153:529–541. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Resh M D. J Biol Chem. 2001;276:34617–34623. doi: 10.1074/jbc.M103995200. [DOI] [PubMed] [Google Scholar]
- 31.Hao M, Mukherjee S, Maxfield F R. Proc Natl Acad Sci USA. 2001;98:13072–13077. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurono G, Aburano S, Yamada N, Nishikawa Y. Yakugaku Zasshi. 1973;93:691–692. doi: 10.1248/yakushi1947.93.5_691. [DOI] [PubMed] [Google Scholar]