Abstract
C-reactive protein (CRP) is an acute-phase protein that binds specifically to phosphorylcholine (PC) as a component of microbial capsular polysaccharide and participates in the innate immune response against microorganisms. CRP elevation also is a major risk factor for cardiovascular disease. We previously demonstrated that EO6, an antioxidized LDL autoantibody, was a T15 clono-specific anti-PC antibody and specifically binds to PC on oxidized phosphatidylcholine (PtC) but not on native PtC. Similarly, EO6 binds apoptotic cells but not viable cells. In addition, such oxidized phospholipids are recognized by macrophage scavenger receptors, implying that these innate immune responses participate in the clearance because of their proinflammatory properties. We now report that CRP binds to oxidized LDL (OxLDL) and oxidized PtC (OxPtC), but does not bind to native, nonoxidized LDL nor to nonoxidized PtC, and its binding is mediated through the recognition of a PC moiety. Reciprocally, CRP binds to PC, which can be competed for by OxLDL and OxPtC but not by native LDL, nonoxidized PtC, or by oxidized phospholipids without the PC headgroup. CRP also binds to apoptotic cells, and this binding is competed for by OxLDL, OxPtC, and PC. These data suggest that CRP binds OxLDL and apoptotic cells by recognition of a PC moiety that becomes accessible as a result of oxidation of PtC molecule. We propose that, analogous to EO6 and scavenger receptors, CRP is a part of the innate immune response to oxidized PC-bearing phospholipids within OxLDL and on the plasma membranes of apoptotic cells.
Keywords: atherosclerosis‖innate immunity‖scavenger receptors‖autoantibody EO6
C-reactive protein (CRP) is an acute-phase reactant, which belongs to the highly conserved pentraxin family of plasma proteins and serves as a pattern-recognition molecule in the innate immune system (for review, see ref. 1). CRP typically binds in a calcium-dependent manner to phosphorylcholine (PC) as a component of capsular polysaccharide of many microorganisms. In addition, CRP was reported to bind to apoptotic cells and to enhance their clearance (2), although the ligand to which CRP bound was not defined. CRP promotes the clearance of CRP-opsonized particles by its direct binding to Fcγ receptors (3, 4). Moreover, CRP bound to multivalent ligands activates a classical complement pathway, which in turn enhances phagocytosis via phagocytic complement receptors (5). Thus, the main biological function of CRP is considered to be a first-line innate host defense, mediating clearance of pathogens and apoptotic or necrotic cells.
There is now much evidence that, once initiated, atherosclerosis displays many of the characteristics of a chronic inflammatory state (6–8). Substantial evidence suggests that oxidized low density lipoprotein (OxLDL) and oxidized phosphatidylcholines (PtC, PC-containing phospholipids), prominent components of OxLDL, have many proinflammatory and proatherogenic properties (9, 10). One might expect, therefore, that there would be host defenses against such products. We recently cloned a panel of murine IgM anti-OxLDL autoantibodies from the spleens of hypercholesterolemic apolipoprotein E-deficient mice. These antibodies, as exemplified by EO6, bind OxLDL but not native LDL (11). A subsequent characterization of EO6 revealed that it binds exclusively to oxidized PtC (OxPtC), such as POVPC [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine] or to POVPC-protein adducts, but not to the native, nonoxidized PtC (12). Recent studies demonstrate that the PC headgroup is the obligatory moiety for EO6 binding (13). EO6 can block the binding and uptake of OxLDL by macrophage scavenger receptors, such as CD36 and SR-B1, as can its model epitope POVPC covalently linked to BSA (12, 14, 15). EO6 also binds to the surface of apoptotic cells but not viable cells (16). Similarly, EO6 and POVPC-BSA can effectively block the uptake of apoptotic cells by macrophages (16). These data imply that apoptotic cells also present OxPtC on their membranes, and recent studies demonstrate an enrichment of OxPtC in such apoptotic cells (ref. 17, and M.-K.C., C.J.B., Y. Miller, G. Subbanagounder, J. A. Berliner, G. Silverman, and J.L.W., unpublished observations). Recently, the antigen recognition domain of EO6 has been demonstrated to be identical to that of the highly conserved T15 clono-specific natural antibody that specifically recognizes PC (18, 19). EO6/T15 specifically bind to oxidized PC-containing phospholipids, such as POVPC or POVPC-protein adducts, but do not bind to the same nonoxidized phospholipids, even though they contain the same PC moiety. Thus, the PC headgroup is a cryptic epitope in native LDL that is revealed after oxidation-induced conformational changes in the PtC molecule, which in turn makes the PC moiety accessible for EO6 binding. By analogy, when cells undergo apoptosis, which is associated with oxidative events, the PC headgroup of OxPtC on their membranes also becomes accessible for EO6 binding. In contrast, EO6 always binds to PC when present as a conjugate to a protein, such as KLH, or lipoteichoic acid on capsular polysaccharide.
We hypothesized that, analogous to the studies with EO6, CRP would recognize OxLDL and apoptotic cells but not native LDL or viable cells, and that the binding moiety for CRP would be PC, exposed by the oxidation of PtC. In the present study, we report that CRP binds to OxLDL but not to native LDL in its natural configuration, and that the binding of CRP to OxLDL is mediated through recognition of PC. In addition, we show that CRP binding to apoptotic cells is competed for by OxLDL and OxPtC, but not by nonoxidized PtC. These data demonstrate that PC exposed by oxidation of PtC on the surface of OxLDL or cells undergoing apoptosis is the ligand for the binding of CRP. We propose that, like EO6 and certain scavenger receptors of macrophages, CRP is a part of an innate immune response to oxidized PC-bearing phospholipids.
Materials and Methods
Materials.
Details on materials are published in supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Chemiluminescent Immunoassay for CRP Binding to Antigens.
CRP binding to antigens was determined by using a chemiluminescent immunoassay (20). In brief, antigens in PBS containing 0.27 mM EDTA were added to each well of a 96-well, white, round-bottomed microtitration plate (Dynex Technologies, Chantilly, VA) and incubated overnight at 4°C. For CRP binding to phospholipid, phospholipid dissolved in 100% ethanol was added into each well (200 μg/ml, 25 μl per well), dried under N2, and then exposed to air for the indicated times at room temperature to induce oxidation. In some experiments, LDL was captured by anti-human apoB100 monoclonal antibody (MB47) coated on microtiter wells (21). To determine the extent of CRP binding, antigens in the wells were blocked with 2% BSA/PBS, and then CRP diluted in 1% BSA/buffer A (10 mM TBS/2 mM CaCl2/1 mM MgCl2, pH 7.4) was incubated with antigens for 1 h. After washes, CRP binding was detected by incubation with rabbit anti-human CRP antibody (IgG); binding of anti-human CRP antibody was then detected by incubation with alkaline phosphatase-labeled goat anti-rabbit IgG. These antibodies were diluted according to manufacturer's protocol. After further washes, 25 μl of 50% solution of Lumi-Phos 530 was added to each well and incubated for 1–2 h. Luminescence was determined by using a Dynatech luminometer (Dynex Technologies). CRP binding was measured as relative light units measured over 100 ms.
The specificity of binding of CRP to various antigens was determined by competition immunoassay in the absence or presence of increasing concentrations of competitors (12, 13). In the case of phospholipid, indicated phospholipid was diluted in 100% chloroform in glass tubes to a final volume of 100 μl, dried under N2, and, in some cases, exposed to air to induce air-oxidation. CRP diluted in 1% BSA/buffer A was incubated with competitors for 1 h. After incubation, CRP-competitor complexes were pelleted by centrifugation at 1,800 × g, and supernatants were tested for remaining CRP-binding activity to indicated antigens by using the chemiluminescent immunoassay or to cells by using flow cytometry.
Induction of Apoptosis in Jurkat T Cells and Flow Cytometry Analysis of CRP Binding.
CRP binding to Jurkat T cells induced to undergo apoptosis was determined as described in supporting Text, which is published on the PNAS web site.
Immunhistochemisty of Coronary Artery Specimens.
Human coronary atherosclerotic lesions were used for immunohistochemical staining for endogenous CRP and OxPtC epitopes by using monoclonal CRP-8 and EO6, respectively, as described in detail in supporting Text, which is published on the PNAS web site.
Results
CRP Binds to OxLDL but Not to Native LDL in Its Natural Configuration in a Calcium-Dependent Manner.
We demonstrated previously that oxidative modification of LDL exposes PC on its surface, as demonstrated by the binding of PC-specific T15 clono-specific antibodies, such as EO6. Based on the known ability of CRP to bind to PC in the presence of calcium (1), we hypothesized that, analogous to EO6, CRP also would bind to OxLDL but not to native LDL, and that this would occur through the recognition of PC exposed by oxidation of PtC on the OxLDL.
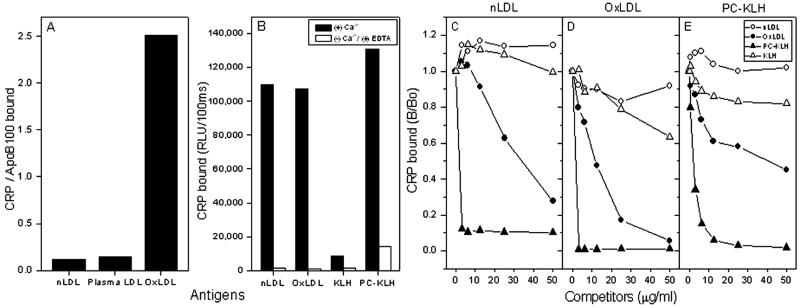
To test CRP binding, native LDL or OxLDL was captured by MB47, a monoclonal anti-human apoB100 antibody plated on the well, and then CRP binding to the captured LDL was evaluated in the presence of calcium. As shown in Fig. 1A, CRP did not bind to native, isolated LDL or to the LDL captured directly from human plasma. In contrast, there was a strong binding of CRP to the captured OxLDL.
Figure 1.
Binding of CRP to LDL and competition immunoassay. Chemiluminescent immunoassays for CRP binding to captured or plated antigens were performed as described in Materials and Methods. (A) CRP binding to captured LDL. Isolated LDL (nLDL), plasma (plasma LDL), or OxLDL was added to microtiter wells coated with anti-apoB-100 monoclonal antibody MB47; CRP (0.5 μg/ml) then was incubated with the captured LDLs. The binding of CRP was determined as described in Materials and Methods. In parallel wells, the extent of binding of biotin-labeled antibodies to human apoB-100 was measured to normalize the amount of LDL captured in each well. The mean of triplicate determinations was taken, and data were expressed as the ratio of binding of CRP to binding of detecting antibodies to captured LDL. (B) CRP binding to plated antigens of native LDL (nLDL), oxidized LDL (OxLDL), PC conjugated with keyhole limpet hemocyanin (PC-KLH), and KLH. CRP (0.1 μg/ml) was incubated with indicated antigens in the presence of calcium or in the absence of calcium plus 10 mM EDTA. Each point is the mean of triplicate determinations. For competition immunoassays, CRP was diluted at 0.5 μg/ml for binding to native LDL (C) and OxLDL (D), and at 0.1 μg/ml for binding to PC-KLH (E). CRP was then incubated in the absence or presence of indicated concentrations of competitors for 1 h. After the incubation, CRP-competitor complexes were pelleted by centrifugation, and supernatants were tested for CRP-binding activity to indicated plated antigens. Data are the mean of triplicate determinations, expressed as a ratio of CRP binding to antigen in the presence of competitor to the binding in the absence of competitor (B/Bo).
Next we tested CRP binding to LDL in parallel with PC, a prototypic ligand of CRP, by plating antigens directly on the well. As shown in Fig. 1B, CRP bound to OxLDL as well as to PC conjugated with keyhole limpet hemocyanin (PC-KLH) but not KLH; CRP binding to these antigens was calcium-dependent, as binding could almost be abolished by the deletion of calcium and the addition of EDTA. Unexpectedly, CRP also bound to native LDL directly plated on the wells. We speculated that the plating procedure, or the adherence of native LDL on the well itself, might have initiated oxidation of the LDL or altered its structure respectively, so as to expose PC, to which CRP bound. In separate experiments, we demonstrated that, although plating LDL in the presence of the antioxidant BHT slightly reduced the binding of CRP, it did not eliminate it (data not shown). The finding that CRP bound to native LDL plated in the presence of antioxidants but that it did not bind to the same native LDL when captured by MB47 suggested that structural changes induced by adherence of LDL might have exposed PC for the binding of CRP.
To characterize the specificity of CRP binding to these antigens plated on the wells, a competition immunoassay was performed in the presence of increasing concentrations of competitors. Binding of CRP to OxLDL (Fig. 1D) or PC-KLH (Fig. 1E) was competed for by both OxLDL and PC-KLH but not by native LDL or KLH, implying that PC mediates CRP binding to OxLDL. However, CRP binding to the native LDL plated on the well was not competed for by native LDL itself, but was competed for very efficiently by OxLDL and PC-KLH (Fig. 1C).
Taken together, these findings suggest that CRP binds OxLDL or native LDL with altered structure, but not to native LDL in its natural configuration, and that this binding is mediated by the PC moiety, which is exposed either by oxidation or by other structural changes of the LDL particle.
CRP Binds to Oxidized PtC but Not to Nonoxidized PtC or Other Oxidized Phospholipids.
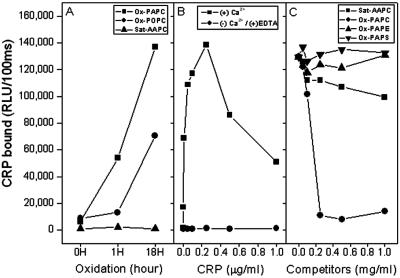
To test directly whether the oxidation of PtC is required for CRP binding, PtC containing all saturated fatty acids, which cannot undergo oxidation, or PtC with unsaturated fatty acids were exposed to increasing degrees of oxidation and then tested for CRP binding by using a chemiluminescent immunoassay. Fig. 2A shows that CRP bound only to oxidized, unsaturated PtC in proportion to the duration of air oxidation and the degree of unsaturation. In contrast, CRP did not bind to PtC containing only saturated fatty acids (saturated-AAPC, i.e., diC20:0-PC). CRP binding to oxidized PtC was calcium-dependent, as CRP binding to oxidized PAPC was abolished by the deletion of calcium and the addition of EDTA (Fig. 2B). A competition immunoassay was performed to demonstrate if the oxidation of unsaturated PtC rendered the PC headgroup available for CRP binding, similar to the situation with EO6. Thus, we tested if the ability of CRP to bind to PC-KLH could be competed for by oxidized PtC, and if the PC headgroup was obligatory for CRP binding. Fig. 2C shows that CRP binding to PC-KLH was competed for almost completely by oxidized PAPC. In contrast, saturated PtC (saturated-AAPC) or other phospholipids that do not contain the PC headgroup (PAPE and PAPS), but contain the same unsaturated carbon chains as PAPC, did not compete for CRP binding to PC-KLH.
Figure 2.
Binding of CRP to PtCs and competition for the binding of CRP to PC (PC-KLH) by various phospholipids. The extent of CRP binding was determined by chemiluminescent immunoassay. (A) CRP binding to variously oxidized PtCs. Unsaturated and saturated PtC were plated in microtiter wells and air oxidized for the times indicated; CRP binding was then measured. Ox-PAPC, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine; Ox-POPC, oxidized 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; Sat-AAPC, saturated 1,2-arachidonyl-sn-glycero-3-phosphocholine (i.e., di20:0-PC). (B) Effect of calcium on the binding of CRP to oxidized PAPC. PAPC was air- oxidized in microtiter wells for 18 h, and the extent of CRP binding to oxidized PAPC was determined in the presence of calcium or in the absence of calcium and with the addition of 10 mM EDTA. (C) Competition immunoassays for binding of CRP to PC-KLH. Various phospholipids were air-oxidized for 18 h and used as competitors. Ox-PAPE, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphatidylethanolamine; Ox-PAPS, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphatidylserine. CRP (0.2 μg/ml in 1% BSA-TBS) was incubated in the absence or presence of indicated concentrations of phospholipids for 1 h. CRP-phospholipid complexes were pelleted by centrifugation at 1,800 × g for 30 min, and supernatants were tested for CRP-binding activity to PC-KLH. Each point is the mean of triplicate determinations.
In summary, CRP binds to oxidized PtC but not to nonoxidized PtC or to other phospholipids without a PC headgroup. Thus, these data demonstrate that the PC headgroup is a prerequisite for CRP binding, and that oxidation of PtC is required for exposure of the PC.
CRP Binding to PC Is Reduced by Competition with Monoclonal Antibody EO6, Which Is Specific for PC of Oxidized PtC.
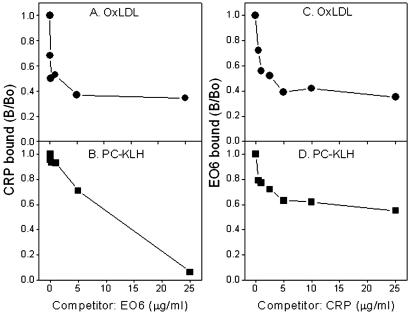
Because both EO6 and CRP recognize PC, we tested whether they can compete with each other for binding to PC-KLH or PC in the context of OxLDL. As shown in Fig. 3, under the conditions used, EO6 successfully competed for ≈60% of binding of CRP to OxLDL (A) and all of the binding to PC-KLH (B). Reciprocal competition also was observed in that CRP could compete for ≈60% of EO6 binding to OxLDL (C) or ≈40% of binding to PC-KLH (D). EO6 F(ab)2 fragments also were tested in this assay instead of EO6 because of the possible hindering effect of intact IgM molecule of EO6 (≈950 kDa). EO6 F(ab′)2 fragments, which have a similar molecular mass (≈150 kDa) as CRP in the usual pentamer form (≈115 kDa), also gave almost the same results as the intact EO6 molecule (data not shown).
Figure 3.
Competition immunoassay for binding of CRP or EO6 to antigens. Fixed and limiting concentrations of EO6 (0.1 μg/ml) or CRP (0.2 μg/ml) were incubated with plated antigens in the absence or presence of indicated concentrations of CRP or EO6. Binding of CRP to OxLDL (A) or to PC-KLH (B). Binding of EO6 to OxLDL (C) or to PC-KLH (D). Each point is the mean of triplicate determinations.
CRP Binds to Apoptotic Cells via PC.
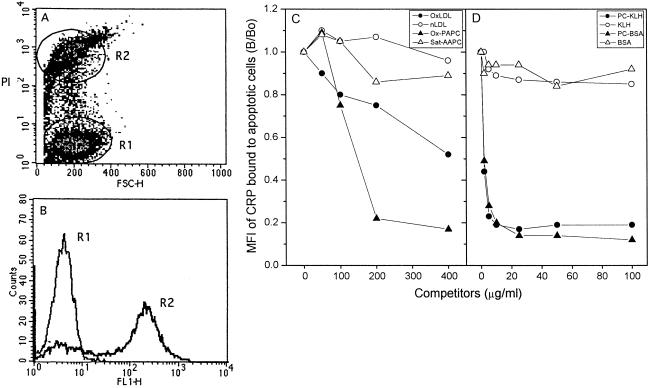
We have shown that T15 anti-PC antibodies including EO6 and T15 recognize the membrane of cells undergoing apoptosis (16, 18). Such apoptotic cells have an increased content of oxidized PtC (ref. 17, and M.-K.C., C.J.B., Y. Miller, G. Subbanagounder, J. A. Berliner, G. Silverman, and J.L.W., unpublished observations). The binding of EO6 suggests that apoptotic cells present PC, in analogy to what happens when LDL is oxidized. Based on these findings, we tested whether the binding of CRP to apoptotic cells occurs through the recognition of PC moiety. Fig. 4 A and B demonstrates that CRP displayed calcium-dependent binding to apoptotic Jurkat T cells, at a later stage of apoptosis, as judged by bright PI staining, which was gated and marked “R2.” CRP binding to the cells in R2 was competed for by OxLDL, oxidized PAPC, PC-KLH, or by PC-BSA in a dose-dependent manner (Fig. 4 C and D). In contrast, native LDL, saturated AAPC, KLH, or BSA did not compete at all. Taken together, these findings imply PC exposed by oxidation of PtC on the cell membrane serves as a ligand for binding of CRP to apoptotic cells, which are known to be under increased oxidative stress.
Figure 4.
Binding of CRP to apoptotic Jurkat T cells and competition immunoassay. Jurkat T cells were treated with staurosporine to induce apoptosis, and then stained with CRP and PI as described in Materials and Methods. Binding of CRP was measured by using flow cytometry. (A) Apoptosis-induced Jurkat T cells were gated into two populations according to the intensity of PI staining. Region 1: normal, viable cells and/or cells at very early stages of apoptosis. Region 2: cells at late stage of apoptosis with bright PI staining. (B) CRP-binding to cells in region 1 (R1) and region 2 (R2). (C and D) Aliquots of CRP (0.2 μg/ml in 1% BSA-TBS) were incubated in the absence or presence of oxidized PtC and PC-KLH as competitors. After the incubations, CRP-competitor complexes were pelleted by centrifugation at 1,800 × g for 30 min, and supernatants were tested for remaining CRP-binding activity to apoptotic Jurkat T cells by using flow cytometry. For the competition by native and OxLDL, CRP was incubated with apoptotic cells in the absence or presence of increasing concentrations of the LDLs. Mean fluorescence intensity (MFI) of CRP binding to the cells in region 2 was measured and expressed as the ratio of CRP binding to the cells in the presence of competitor to the binding in the absence of competitor (B/Bo). Two additional experiments gave similar results.
CRP Frequently Colocalizes with the EO6 Epitope in Human Atherosclerotic Lesions.
Because the ligands for both EO6 and CRP are OxPtC, we examined the ability of CRP and EO6 to bind to atherosclerotic lesions, which are known to contain such epitopes in vivo (11). Indeed, there was colocalization of endogenous CRP and EO6 epitopes in the intima and extracellular components of the lipid cores of lesions (for detailed description, see Fig. 5 and supporting Text, which are published as supporting information on the PNAS web site).
Discussion
Oxidation of LDL generates a variety of oxidatively modified molecules, including oxidized phospholipids (9, 10, 22). Oxidized phospholipids and other oxidation-specific epitopes are also formed on the surface membranes of cells undergoing apoptosis (refs. 16 and 17, and M.-K.C., C.J.B., Y. Miller, G. Subbanagounder, J. A. Berliner, G. Silverman, and J.L.W., unpublished observations). There is now considerable evidence that such oxidized phospholipids are proinflammmatory and proatherogenic. For example, they induce monocyte–endothelial interactions by stimulating endothelial cells to express monocyte-specific adhesion molecules and chemokines such as MCP-1. In addition, oxidized phospholipids are precursors of bioactive fatty acids that have many reported effects on the vascular wall (reviewed in refs. 9, 10, 22, and 23).
Adaptive immunity provides protection to the host by the generation of a large number of specific, high-affinity receptors against “pathogens” through somatic mutations. However, such immunity is delayed in onset and is not transferable from one generation to the next. In contrast, the innate immune system provides a rapid immune response to pathogens without the need for the somatic recombination of receptors that characterizes adaptive immunity. Of necessity, such receptors are of far more limited number and selected to be generalized rather than highly specific. Our laboratory has recently shown that there are both humoral and cellular innate immune responses to oxidized phospholipids (12, 14, 15). As noted at the beginning of this paper, we have shown that T15 clono-specific natural autoantibodies, such as EO6, which bind specifically to the PC moiety as a component of microbial capsular polysaccharide, also bind both OxLDL and apoptotic cells but not either native LDL or viable cells. A subsequent characterization of EO6 revealed that it specifically binds to the PC headgroup of PtC, but only when PtC are subjected to oxidizing conditions (12, 18). We have proposed that the PC headgroup is a cryptic epitope in PtC in the context of LDL or cell membranes, but oxidation-induced conformational changes in the PtC molecule itself, or in the surrounding lipid environment in which PtC resides, “exposes” PC, rendering it accessible for EO6 binding. Thus, when LDL undergoes oxidation, or when cells undergo apoptosis, which is associated with oxidative events, oxidation of PtC would be one mechanism that makes PC epitopes accessible for binding by EO6. Because these germ-line antibodies are naturally selected and are present within the first week of life even in mice grown in germ-free conditions (24), we have postulated that they are positively selected by apoptotic cells and/or oxidized phospholipids present on cellular debris or oxidized lipoproteins (18). Only later in life would such clones be expanded in response to microbial PC. In addition, these oxidized phospholipids serve as ligands mediating recognition of OxLDL and apoptotic cells by macrophage scavenger receptors, such as CD36 and SR-B1 (12, 14–16). These scavenger receptors are typical of so-called “pattern recognition receptors,” which are cellular receptors of the innate immune system and mediate binding to common ligands, termed “pathogen associated molecular patterns” (PAMP; refs. 25–27). Thus, the PC moiety is such a PAMP, whether present on microbes, OxLDL, or apoptotic cells.
CRP is an acute-phase reactant that belongs to a highly conserved pentraxin family of plasma proteins. It was initially characterized by its binding to the PC moiety of microbial capsular polysaccharide and has been demonstrated to play a role in rapid innate host defenses against bacteria (1). We now show that, analogous to certain natural antibodies and scavenger receptors, CRP binds both OxLDL and apoptotic cells, but not native LDL in its natural configuration or viable cells. This CRP binding to OxLDL and apoptotic cells is mediated through the recognition of PC moieties on their surface. Presumably, PC is exposed by oxidation of PtC, as the CRP binding is competed for by oxidized PtC but not by nonoxidized PtC, even though both oxidized and native PtC contain the same PC headgroup. Thus, CRP represents a third and even more primitive tier of innate immunity against the pathogenic expression of the PC moiety by OxLDL or apoptotic cells. We would speculate that, analogous to the T15 clono-specific natural antibodies, the PC moieties present on oxidized phospholipids of oxidized lipoproteins and apoptotic cells also have exerted positive evolutionary pressure to conserve this protein (18, 19).
CRP displays very similar binding characteristics, as does EO6. CRP bound to OxLDL, and the specificity of this binding through the recognition of PC exposed on its particle was demonstrated by the ability of even the lowest doses of soluble PC-KLH to fully compete. Soluble OxLDL also fully competed for binding, although likely with lower affinity (Fig. 1D). In turn, the binding of CRP to PC-KLH, which most likely occurs with higher affinity, was fully competed for by PC-KLH and by nearly 60% by OxLDL (Fig. 1E). In addition, CRP only bound to unsaturated PtC in proportion to their degree of oxidation and unsaturation (Fig. 2A) and did not bind to the saturated PtC even if exposed to the same oxidizing conditions (Fig. 2A). Similarly, the binding of CRP to PC-KLH could be fully competed for by oxidized PAPC but not by saturated AAPC that could not undergo oxidation. The absolute requirement for the presence of the PC headgroup for CRP binding was demonstrated by the failure of oxidized phosphatidylserine or phosphatidylethanolamine to prevent binding of CRP to PC-KLH (Fig. 2C). The finding that EO6 could substantially compete for the binding of CRP to OxLDL and PC-KLH (Fig. 3 A and B) strongly suggests that they share many common configurational epitopes of PC on these two structures. However, there is not an absolute immunological identity of all PC epitopes, as CRP only competed for 40–60% of the binding of EO6 to PC-KLH and OxLDL, respectively (Fig. 3 C and D). The latter results are undoubtedly influenced by issues of affinity and valence of the two binding proteins, EO6 and CRP.
It was reported that CRP displayed calcium-dependent binding to native LDL and VLDL (28). Indeed, we also observed such calcium-dependent binding, but only when the LDL was directly plated on microtiter wells (Fig. 1B). However, this binding to the plated native LDL was not competed for by the presence of a large excess of soluble native LDL, but was competed for efficiently by OxLDL and with even higher affinity by PC-KLH (Fig. 1C). We speculate that by adhering on the well, the native configuration of LDL may have been altered so as to subtly expose the PC moiety, thus mimicking PC exposure that occurs in OxLDL, and/or a subtle degree of oxidation occurred despite efforts to prevent this from happening. Indeed, when LDL in solution was captured by an anti-apoB-100 monoclonal antibody, CRP did not bind to the captured LDL, nor did it bind to LDL directly captured from human plasma (Fig. 1A). Moreover, we also observed a 2-fold greater binding of CRP to aggregated, native LDL (generated by vortexing) than to monomeric native LDL (data not shown). Thus, we postulate that the alteration to LDL caused by adherence on the well, or by aggregation, exposed the PC moiety, mimicking the PC exposure that occurs when LDL is oxidized. Indeed, other modifications of LDL also could alter its normal configuration, leading to PC exposure and CRP binding. For example, Bhakdi et al. (29) have shown that CRP binds in vitro to enzymatically degraded LDL via PC moiety, implying that such enzymatic degradation of LDL is yet another mechanism by which PC exposure occurs, leading to CRP binding. Thus, in addition to oxidation of PtC in OxLDL, structural changes of LDL caused by adherence, aggregation, or enzymatic modification might play a role in the exposure of PC.
The similarity of the binding properties of CRP to those of EO6 also extends to apoptotic cells. We have previously shown that EO6 binds the surface membrane of apoptotic cells but not viable cells (16). We also demonstrated that there is an enhanced content of oxidized PtC in such apoptotic cells (ref. 17, and M.-K.C., C.J.B., Y. Miller, G. Subbanagounder, J. A. Berliner, G. Silverman, and J.L.W., unpublished observations). We now show that CRP binding to apoptotic cells is competed by OxLDL, but not native LDL (Fig. 4C). Similarly, oxidized PAPC is an effective competitor, but saturated AAPC, even though it contains the same PC headgroup, is not (Fig. 4C). Further evidence that this binding is mediated by PC comes from experiments demonstrating that PC conjugated with KLH or BSA are high affinity competitors for this binding (Fig. 4D). Thus, we propose that the generation of oxidized PtC on the cell surface, and/or an alteration in the lipid milieu of the membrane in which PtC are found (as noted below) lead to exposure of PC moieties, which could mediate CRP binding. CRP has been reported to bind to cell membranes, as well as to intracellular components such as chromatin-containing H1 and small nuclear ribonucleoprotein (30–32). Initial evidence for the binding specificity of CRP to cells was provided by immunohistochemical studies demonstrating that CRP was associated with cell membranes of damaged and necrotic cells, but not normal cells, at sites of inflammation and tissue necrosis (30). Recently, Gershov et al. (2) reported that CRP binds to the surface membrane of apoptotic cells and subsequently promotes clearance, but the ligand mediating such binding was not characterized. Volanakis et al. (33) proposed a potential mechanism by which CRP recognizes cell membranes. They showed that a prerequisite for the binding of CRP to artificial lipid membranes composed of PtC was the prior incorporation of lysophosphatidylcholine (LPC) into the membrane (33). Based on this finding, they postulated that a disturbance of surface molecular organization by LPC would be necessary for CRP binding. In support of this hypothesis, they demonstrated that CRP could bind to red blood cells only after the addition of LPC to cells, or treatment of cells with snake phosphospholipase A2 (PLA2) that hydrolyzes PtC and provides LPC (34). They proposed that in humans, secretory PLA2 may generate LPC, which in turn disturbs the molecular organization of the cell membrane leading to CRP binding. This proposal is of considerable interest in that oxidation of LDL generates considerable amounts of LPC as a consequence of an intrinsic PLA2 activity associated with LDL that preferentially hydrolyzes the shortened sn2 fatty acid of phospholipids after they have undergone oxidative-induced alterations in their composition (35). Similarly, we have recently shown that the content of LPC is increased 3- to 4-fold in the lipid extracts from the membranes of apoptotic cells (M.-K.C., C.J.B., Y. Miller, G. Subbanagounder, J. A. Berliner, G. Silverman, and J.L.W., unpublished observation). Thus, disturbed molecular organization of the cell membrane by an increased content of LPC or oxidation of PtC on the cell membrane could lead to the exposure of the PC moiety, which then mediates CRP binding. Thus, the PC moiety is a cryptic epitope on viable cells that is made accessible for CRP binding by enzymatic or oxidative modification associated with programmed cell death.
CRP has been demonstrated to be an independent and powerful risk factor for atherosclerotic events such as myocardial infarction, stroke, and peripheral vascular disease (reviewed in refs. 7 and 36). Several studies have been undertaken to elucidate the role of CRP in atherogenesis. For example, CRP colocalized with terminal complement complexes in early atherosclerotic lesions, suggesting that CRP may promote atherosclerotic lesion formation by activating the complement system (37). Recently, Zwaka et al. (38) reported that CRP could opsonize native LDL and enhance its uptake by macrophages, mediated via FcγRII (CD32), and thus, theoretically, contribute to foam cell formation. However, the ligand for CRP on native LDL was not characterized. In light of our data, we speculate that Zwaka et al., who used LDL from a commercial source, might have studied LDL that was mildly oxidized and/or altered in its physical state, so as to make PC accessible for CRP binding. As noted in our study, CRP did not bind to native LDL in its natural configuration. However, CRP did bind to native LDL plated in microtiter wells through the recognition of PC. It would be interesting to investigate whether a similar phenomenon of PC exposure occurs when native LDL binds to the extracellular matrix in the artery wall. Indeed, in this setting, LDL is also known to be aggregated (39), enzymatically modified (40), and oxidized (11). As noted in Fig. 5, we have now shown that there is substantial colocalization in atherosclerotic lesions of CRP and oxidized phospholipid epitopes of EO6. Furthermore, we have also observed colocalization of EO6 immunoreactivity and that of enzymatically degraded LDL (M.T., M.-K.C., C.J.B., E. Miller, S. Tsimikas, G. Schmitz, and J.L.W., unpublished observation).
Our studies have not yet addressed the consequences of CRP binding to OxLDL and apoptotic cells. CRP participates as a first line, innate host defense against pathogens by promoting the uptake and clearance of microorganisms through the recognition of PC. It will be of considerable interest to investigate whether, analogous to exogenous pathogens, the binding of CRP to endogenous ligands of OxLDL and apoptotic cells also can exert such biological effects.
Supplementary Material
Acknowledgments
We thank Dr. D. Steinberg for advice and support, and J.-I. Lee for his technical assistance. These studies were supported by National Institutes of Health Grants HL56989 (La Jolla SCOR), HL57505, and HL69464. M.-K.C. was supported by a postdoctoral fellowship grant from the Tobacco Related Disease Research Program and the Sam and Rose Stein Institute for Research on Aging faculty startup grant. C.J.B. was supported by a Ph.D. scholarship from Boehringer Ingelheim Fonds and a scholarship from the Austrian Academy of Science. M.T. was supported by the Regensburger Forschungsförderung in der Medizin (ReForM B) and the Deutsche Forschungsgemeinschaft (TO 228/2–1).
Abbreviations
- CRP
C-reactive protein
- PC
phosphorylcholine
- OxLDL
oxidized low density lipoprotein
- PtC
phosphatidylcholine
- OxPtC
oxidized phosphatidylcholine
- PC-KLH
PC conjugated with KLH
- LPC
lysophosphatidylcholine
Footnotes
See commentary on page 12515.
References
- 1.Volanakis J E. Mol Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 2.Gershov D, Kim S, Brot N, Elkon K B. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mold C, Gresham H D, Du Clos T W. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 4.Bharadwaj D, Stein M P, Volzer M, Mold C, Du Clos T W. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mold C, Gewurz H, Du Clos T W. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Ridker P M, Maseri A. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 8.Glass C K, Witztum J L. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 9.Witztum J L, Berliner J A. Curr Opin Lipidol. 1998;9:441–448. doi: 10.1097/00041433-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Berliner J A, Subbanagounder G, Leitinger N, Watson A D, Vora D. Trends Cardiovasc Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 11.Palinski W, Hörkkö S, Miller E, Steinbrecher U P, Powell H C, Curtiss L K, Witztum J L. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hörkkö S, Bird D A, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner J A, Friedman P, Dennis E A, Curtiss L K, et al. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman P, Hörkkö S, Steinberg D, Witztum J L, Dennis E A. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 14.Boullier A, Gillotte K L, Hörkkö S, Green D R, Friedman P, Dennis E A, Witztum J L, Steinberg D, Quehenberger O. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 15.Gillotte-Taylor K, Boullier A, Witztum J L, Steinberg D, Quehenberger O. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 16.Chang M-K, Bergmark C, Laurila A, Hörkkö S, Han K H, Friedman P, Dennis E A, Witztum J L. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum J L, Binder B R, Leitinger N. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 18.Shaw P X, Hörkkö S, Chang M-K, Curtiss L K, Palinski W, Silverman G J, Witztum J L. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearney J F. J Clin Invest. 2000;105:1683–1685. doi: 10.1172/JCI10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hörkkö S, Miller E, Dudl E, Reaven P, Curtiss L K, Zvaifler N J, Terkeltaub R, Pierangeli S S, Branch D W, Palinski W, et al. J Clin Invest. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih D M, Xia Y R, Wang X P, Miller E, Castellani L W, Subbanagounder G, Cheroutre H, Faull K F, Berliner J A, Witztum J L, et al. J Biol Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 22.Marathe G K, Harrison K A, Murphy R C, Prescott S M, Zimmerman G A, McIntyre T M. Free Radical Biol Med. 2000;28:1762–1770. doi: 10.1016/s0891-5849(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 23.Marathe G K, Prescott S M, Zimmerman G A, McIntyre T M. Trends Cardiovasc Med. 2001;11:139–142. doi: 10.1016/s1050-1738(01)00100-1. [DOI] [PubMed] [Google Scholar]
- 24.Sigal N H, Gearhart P J, Klinman N R. J Immunol. 1975;114:1354–1358. [PubMed] [Google Scholar]
- 25.Thomas C A, Li Y, Kodama T, Suzuki H, Silverstein S C, el Khoury J. J Exp Med. 2000;191:147–156. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Doi T, Hamakubo T, Kodama T. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medzhitov R, Janeway C A., Jr Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 28.Rowe I F, Soutar A K, Trayner I M, Baltz M L, de Beer F C, Walker L, Bowyer D, Herbert J, Feinstein A, Pepys M B. J Exp Med. 1984;159:604–616. doi: 10.1084/jem.159.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhakdi S, Torzewski M, Klouche M, Hemmes M. Arterioscler Thromb Vasc Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- 30.Kushner I, Kaplan M H. J Exp Med. 1961;114:961–974. doi: 10.1084/jem.114.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Clos T W, Zlock L T, Rubin R L. J Immunol. 1988;141:4266–4270. [PubMed] [Google Scholar]
- 32.Du Clos T W. J Immunol. 1989;143:2553–2559. [PubMed] [Google Scholar]
- 33.Volanakis J E, Wirtz K W. Nature (London) 1979;281:155–157. doi: 10.1038/281155a0. [DOI] [PubMed] [Google Scholar]
- 34.Narkates A J, Volanakis J E. Ann NY Acad Sci. 1982;389:172–182. doi: 10.1111/j.1749-6632.1982.tb22135.x. [DOI] [PubMed] [Google Scholar]
- 35.Parthasarathy S, Steinbrecher U P, Barnett J, Witztum J L, Steinberg D. Proc Natl Acad Sci USA. 1985;82:3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake G J, Ridker P M. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 37.Torzewski J, Torzewski M, Bowyer D E, Frohlich M, Koenig W, Waltenberger J, Fitzsimmons C, Hombach V. Arterioscler Thromb Vasc Biol. 1998;18:1386–1392. doi: 10.1161/01.atv.18.9.1386. [DOI] [PubMed] [Google Scholar]
- 38.Zwaka T P, Hombach V, Torzewski J. Circulation. 2001;103:1194–1197. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 39.Tabas I. Annu Rev Nutr. 1999;19:123–139. doi: 10.1146/annurev.nutr.19.1.123. [DOI] [PubMed] [Google Scholar]
- 40.Torzewski M, Klouche M, Hock J, Messner M, Dorweiler B, Torzewski J, Gabbert H E, Bhakdi S. Arterioscler Thromb Vasc Biol. 1998;18:369–378. doi: 10.1161/01.atv.18.3.369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.