CASE PRESENTATION
Angela Shih, MD: A 35-year-old, previously healthy homosexual man was admitted because of anemia. He had had exertional dyspnea, palpitations, and fatigue for 3 months; burning substernal chest pain on exertion for 1 month; and fever and chills for 2 days. Outpatient blood test results included a hematocrit of 13%, a platelet count of 355 × 103/μL, and a white blood cell count of 4.4 × 103/μL. He denied any hematemesis, hematochezia, melena, or other bleeding.
The patient had had knee surgery at age 13 and allergic rhinitis. He was not taking any medications, and his family history was unknown, as he was adopted. He worked as a manager of a call center. He smoked cigarettes (less than a pack a day for 10 years), drank alcohol occasionally, and smoked marijuana. He never used intravenous drugs.
His temperature was 38.1°C (100.6°F), and his blood pressure was normal. He was pale but generally well appearing and in no acute distress. Examination of the head, eyes, ears, nose, and throat disclosed no abnormalities except for conjunctival pallor and pale mucous membranes. The thyroid gland was not enlarged, and no nodes were palpated in the neck. The chest was clear to auscultation. The heart rate and rhythm were regular, and a precordial 2/6 systolic murmur was heard. No hepatosplenomegaly was noted. Examination of the extremities, nervous system, and skin disclosed no abnormalities.
Admission laboratory results are summarized in the Table. The patient was transfused with several units of packed red blood cells. Additional laboratory studies were obtained, and a diagnostic procedure was performed.
Table.
Initial laboratory values
| Sodium | 139 mEq/L | White blood cell count | 4.0×103/μ |
| Potassium | 4.2 mEq/L | Differential | 1% myelocytes |
| Chloride | 103 mEq/L | 1% metamyelocytes | |
| Bicarbonate | 26 mEq/L | 11% bands | |
| Blood urea nitrogen | 18 mg/dL | 38% segs | |
| Creatinine | 0.9 mg/dL | 35% lymphocytes | |
| Glucose | 87 mg/dL | 11% monocytes | |
| Calcium | 9.7 mg/dL | 3% eosinophils | |
| Total protein | 6.8 g/dL | Hemoglobin | 4.0 g/dL |
| Albumin | 3.8 g/dL | Hematocrit | 12.2% |
| Total bilirubin | 0.3 mg/dL | Mean corpuscular volume | 87 fL |
| Alkaline phosphatase | 97 U/L | Platelet count | 319×103/μL |
| Aspartate aminotransferase | 14 U/L | Reticulocyte count | 0.1% |
| Alanine aminotransferase | 31 U/L | Folate | 4.0 ng/mL |
| Lactate dehydrogenase | 590 U/L | Vitamin B12 | 322 pg/mL |
| Erythrocyte sedimentation rate | 60 mm/hr | Iron | 66 μg/dL |
| Thyroid-stimulating hormone | 2.04 μIU/mL | Total iron-binding capacity (saturation, 26%) | 268 μg/dL |
| Ferritin | 360 ng/mL | ||
| Red blood cell morphology on peripheral smear: moderate anisocytosis, ovalocytosis, and mild rouleaux. | |||
DIFFERENTIAL DIAGNOSIS
Ruby E. Kassanoff, MD: Our patient is a 35-year-old white man with 3 months of fatigue and dyspnea on exertion, 1 month of chest pain, and 2 days of fever and chills. He has no history of chronic illnesses or blood loss and denies any use of medications. He has a low-grade fever and some physical signs of anemia, but no other abnormalities. He has a severe anemia with a hematocrit of 13%.
I want to discuss some factors important in determining the cause of an anemia and then see how they apply to this patient.
We want to know the family history, but that's not helpful here because the patient was adopted. We also want to know whether his anemia is acute or chronic. His seems to be fairly acute, because he had a normal blood count about a year earlier. We always want to know about any drug exposure, including recreational drugs such as alcohol; any chronic illnesses, such as HIV or cancer; and any evidence of blood loss. Our patient denies all of those except mild alcohol use.
On physical examination, we want to assess the vital signs to make sure the patient is hemodynamically stable, and then we can determine possible causes of the anemia. We look for lymphadenopathy, hepatomegaly or splenomegaly, jaundice, neurologic abnormalities, and fecal occult blood. We are not told this patient's fecal occult blood test result, but I assume that it was negative.
Appropriate laboratory tests would include a complete blood cell count to determine the degree of anemia and the presence of associated abnormalities of white blood cells or platelets. The mean corpuscular volume can be helpful in categorizing the anemia; our patient's value of 87 fL indicates that his red cells are normal in size. The lactate dehydrogenase level helps to evaluate for hemolysis, as can bilirubin and haptoglobin, but the haptoglobin test takes much longer to come back. The reticulocyte count is important in distinguishing between hyperproliferative anemias, such as those from blood loss or hemolysis, and hypoproliferative states, which suggest a bone marrow process. Our patient's reticulocyte count was 0.1%, which is very low and indicates a hypoproliferative anemia.
The peripheral blood smear should always be examined in patients with an unexplained anemia. Our patient's smear showed anisocytosis, or variation in size between individual red blood cells, which is very common in severe anemia and is fairly nonspecific. He had some ovalocytosis, or elongation of the red blood cells; this can be seen in a variety of conditions, including iron deficiency, thalassemias, hemoglobin S or C, hemolytic anemias, and primary elliptocytosis, but is not specific for any of those conditions. He also had mild rouleaux formation, or stacking of the red blood cells, which can reflect increased protein concentration such as that observed with multiple myeloma or Waldenström's macroglobulinemia, but it is not specific for those conditions. Spherocytes, which might suggest a hemolytic process, schistocytes to suggest a microangiopathic hemolytic anemia, or teardrop cells to suggest a bone marrow infiltrative or myelophthisic process were all notably absent from his smear. He also had no white blood cell or platelet abnormalities such as changes in their number, evidence of basophilic stippling to suggest a myelodysplastic syndrome, or hypersegmented neutrophils as seen in vitamin B12 deficiency.
On the basis of this patient's presentation, test results, and peripheral smear, I believe he has a hypoproliferative anemia, for which there is a large differential diagnosis. Iron deficiency is probably the most common type, usually from chronic blood loss; however, it generally produces microcytic indices, as do the thalassemias, making those less likely here. Vitamin B12 and folate deficiencies generally produce macrocytic indices; test results and the fact that his indices were normocytic rule out these causes. Chronic renal insufficiency from a decrease in erythropoietin production and anemia of chronic disease appear to be ruled out by this patient's lack of significant medical history. Myelodysplastic syndrome usually occurs in older patients and generally is associated with abnormalities of white blood cells or platelets. The last category of hypoproliferative anemias comprises the aplastic anemias—those involving failure of bone marrow production of one or more cell lines—and fits this case the best.
True aplastic anemia is associated with a marked decrease in erythroid, granulocytic, and megakaryocytic cells in the bone marrow and is seen as a pancytopenia on the peripheral blood smear. In pure red cell aplasia, selective destruction or inhibition of erythroid progenitors in otherwise normal marrow leads to an anemia on the peripheral smear, as in this case.
Red cell aplasia can be congenital or acquired and can be transient, which is more common in children, or chronic, which is more common in adults. Congenital red cell aplasia, known as Diamond-Blackfan anemia, is usually diagnosed within the first year of life and is associated with other physical anomalies in about 30% of patients. Acquired red cell aplasia is rare, and only a few hundred cases of the chronic form have been described. It generally occurs from the fifth to the seventh decade, but it can occur at any age and has no gender or racial predisposition. Organomegaly or lymphadenopathy typically is not seen unless it is associated with an underlying disease process.
Laboratory test results of patients with acquired red cell aplasia usually show normocytic, normochromic anemia, a reticulocyte count of <1%, a high erythropoietin level, and generally high iron levels with saturation of the iron-binding capacity.
Acquired red cell aplasia can be primary, or idiopathic, or secondary to a variety of conditions. Many drugs can cause acquired red cell aplasia, the most common being chloramphenicol, azathioprine, procainamide, phenytoin, and isoniazid. Nutritional deficiencies, including folic acid, vitamin C, or riboflavin deficiency and protein malnutrition, can cause acquired red cell aplasia, but these cases are rare. Because replacement of the deficient nutrient does not always improve the anemia, there is some question about the true causality. Pregnancy also has been linked to acquired red cell aplasia, as have several solid tumors, including those of the stomach, breast, gallbladder, lung, skin, and thyroid. Some autoimmune disorders have been seen in association, particularly lupus erythematosus and rheumatoid arthritis, as have some lymphoproliferative disorders, most commonly chronic lymphocytic leukemia.
Red cell aplasia is more commonly caused by viruses, such as the hepatitis viruses, HIV, Epstein-Barr virus, or human T-cell lymphotrophic virus type I. Parvovirus B19 can cause severe anemia in patients with chronic hemolytic anemias, such as spherocytosis, sickle cell anemia, or paroxysmal nocturnal hemoglobinuria, and severe anemia may be the first manifestation of well-compensated hemolytic disease. In patients without a chronic hemolytic anemia, parvovirus can cause an acquired red cell aplasia that is generally transient and is usually described as a viral prodrome followed by gradual recovery of blood cell counts within 7 to 10 days. But the red cell aplasia may become persistent, especially in patients who are immunosuppressed and particularly those infected with HIV.
About 30% to 50% of acquired red cell aplasias are associated with thymomas, and about 5% of patients with thymoma will develop pure red cell aplasia. The anemia generally resolves with removal of the thymoma, but radiation therapy has been unsuccessful in the past (1).
The last large category is idiopathic acquired red cell aplasia. It is difficult to determine what percentage of cases are truly idiopathic, since parvovirus B19 testing has not always been available.
The pathogenesis of suspected pure red cell aplasia involves several mechanisms: immunoglobulins directed against erythropoietin or the cells themselves; T-cell cytokines blocking response to erythropoietin, particularly in chronic lymphocytic leukemia–related aplasia; and direct lysis of erythroid cells by a virus. In parvovirus B19, the erythroid specificity is via the blood group P antigen 2.
Evaluation of a patient with suspected pure red cell aplasia involves first stopping all medications that the patient is taking and then obtaining a bone marrow biopsy to confirm the diagnosis. The marrow of patients with red cell aplasia is generally normocellular with a reduction only in the erythroid lines; the remaining erythroids appear immature but normal. There may be a slight increase in eosinophils and small mononuclear cells and a slight left shift in the myeloid series. If parvovirus B19 is the cause, scattered giant pronormoblasts can be seen through the bone marrow. Cytogenetic studies of marrow cells are typically normal. Other studies to consider would be chest x-ray or computed tomography to evaluate for thymoma, which can be treated and the aplasia cured, and to rule out any malignant cells. Other tests to consider in the appropriate clinical setting would be HIV testing, viral hepatitis testing, or screening for autoimmune disorders. Parvovirus B19 testing can be done by either immunoglobulin M (IgM) antibody testing or by parvovirus B19 DNA testing. The latter, which is available at Baylor through an outside laboratory and takes about a week to come back, may be a better test in patients who are immunosuppressed and cannot mount an appropriate antibody response. The immunoglobulin G (IgG) antibody test is not helpful, because more than 50% of the general population has parvovirus IgG antibodies.
In conclusion, I think our patient has an acquired red cell aplasia, and I think bone marrow biopsy was probably performed to confirm the diagnosis. Further testing could help determine whether the disorder is viral, autoimmune, thymoma associated, or idiopathic.
PATHOLOGY REPORT
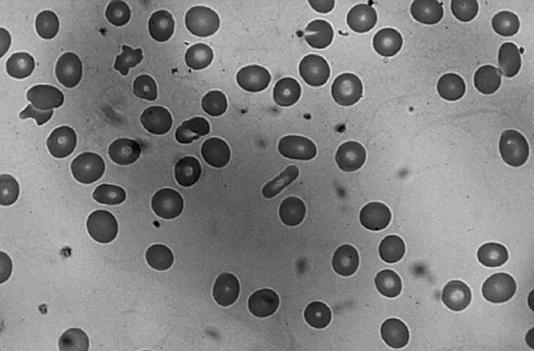
Basel Altrabulsi, MD: The peripheral blood smear from this patient showed a normocytic, normochromic anemia (Figure 1). There was a little variation in the red blood cell size, or mild anisocytosis, and rare elliptocytes. The platelets appeared normal; no giant forms were noted. The morphology of the white blood cells was unremarkable, and no hypersegmentation, immature white blood cells, or nucleated red blood cells were seen.
Figure 1.
The patient's peripheral blood smear showed a normocytic, normochromic anemia.
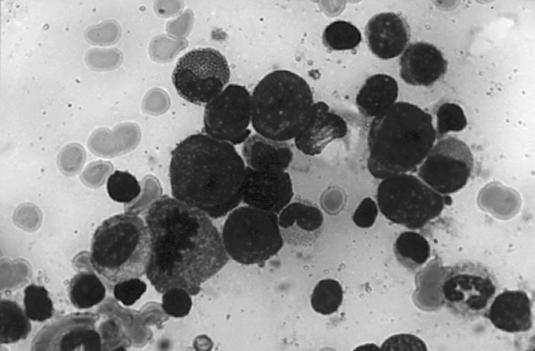
The patient's bone marrow smear revealed a myeloiderythroid ratio of 2 or 3 to 1 (Figure 2). White blood cells showed normal maturation without dyspoiesis; bands and neutrophils were present. Megakaryocytes also appeared normal. The prominent finding was erythroid hyperplasia, demonstrated by an increased number of red cell precursors: pronormoblasts and few polychromatophilic normoblasts and orthochromic erythroblasts. Erythroblasts have dense, blue, immature cytoplasm and large nuclei with prominent nucleoli. No inclusions were seen in these cells. Very few of the pronormoblasts were maturing. The mature forms showed minimal if any dyspoiesis.
Figure 2.
The patient's bone marrow smear revealed a myeloid-erythroid ratio of 2 or 3 to 1. Erythroid hyperplasia was the prominent finding.
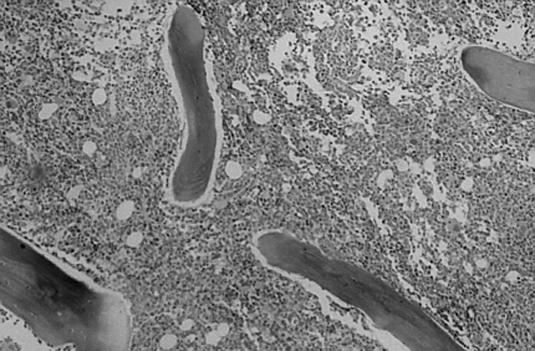
The bone marrow biopsy was very hypercellular (approximately 90% cellularity) (Figure 3). There was focal clustering of the megakaryocytes. The biopsy contained large, immature cells corresponding to the pronormoblasts seen on the bone marrow smear. The iron studies demonstrated adequate iron stores, and no ringed sideroblasts were seen.
Figure 3.
The patient's bone marrow biopsy was hypercellular, with approximately 90% cellularity.
The features seen in the biopsy bring to mind the myelodysplastic syndromes, although more dyspoiesis would be expected in addition to ringed sideroblasts and immature myeloid precursors. Myelocytic leukemia is a possibility, but by definition, more than 30% of the cells in the aspirate would be blasts, which were not present in this case. Megaloblastic anemia is in the differential, but we would expect to see more megaloblastic changes, such as giant bands and hypersegmented neutrophils. Again, these were not seen.
Viral infection, especially with parvovirus, is on the differential, too, but it prompts an interesting question: Is it possible to have patients with parvovirus infection who present with hypercellular rather than hypocellular marrow? The answer, at least in our experience, is no. Most patients with parvovirus infection have hypocellular marrow and classic giant pronormoblasts with intranuclear inclusions.
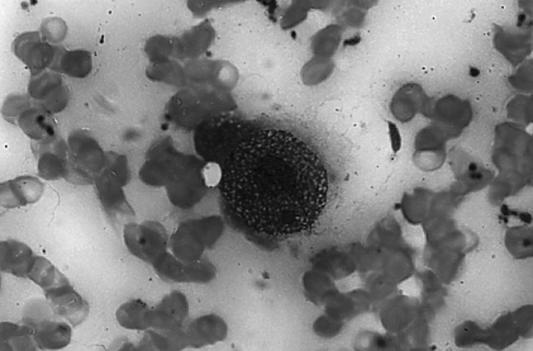
In a typical case of parvovirus infection, anemic patients have a bone marrow cellularity of about 15% to 20%. The presence of classic giant pronormoblasts with eosinophilic inclusions pushing the chromatin to the side, or margination of the chromatin, is characteristic of and diagnostic for parvovirus in specimens stained with hematoxylin and eosin (Figure 4).
Figure 4.
Bone marrow aspirate showing a giant pronormoblast with eosinophilic inclusions pushing the chromatin to the side, a feature that is diagnostic for parvovirus in specimens stained with hematoxylin and eosin.
A recent article described immunodeficient patients, especially those with AIDS, who were infected with parvovirus and presented with hypercellular marrow (3). However, all of the cases in this study showed the classic intranuclear inclusions. A recent abstract described hypercellular marrow in a patient infected with parvovirus (4). In 2 cases, the classic intranuclear inclusions were not seen, and the parvovirus infection was confirmed using immunohistochemical stains.
At Baylor, we are using a monoclonal IgG antibody against the capsid viral proteins VP1 and VP2. The stain should be cytoplasmic or nuclear. The patient had hypercellular marrow with erythroid hyperplasia and positive staining for parvovirus.
DISCUSSION
Angela Shih, MD: The patient was indeed HIV positive. He had a positive antibody test and Western blot confirmation. His CD4 cell count on presentation was 31, and his viral load was 70,000. He did not have any detectable parvovirus B19 IgG or IgM titers, but his serum parvovirus B19 DNA by polymerase chain reaction assay was positive. His bone marrow, as already stated, showed erythroid hyperplasia, but the immunostains were positive for parvovirus B19. Thus, he was diagnosed with pure red cell aplasia secondary to parvovirus B19 infection, and this was actually his initial manifestation of HIV infection.
Parvovirus B19 is a small, single-stranded DNA virus first discovered in 1975. As already mentioned by Dr. Kassanoff, it has a high seroprevalence, with >50% of adults showing evidence of past infection with positive IgG antibodies. Most of these infections are asymptomatic. The virus is spread mostly through the respiratory route, although there have been reports of patients developing parvovirus B19 infection after receiving blood products. It infects actively replicating cells, specifically erythroid progenitor cells, and causes lysis of these red cell precursors, resulting in decreased red cell production.
In a typical course of infection, viremia occurs about 6 to 8 days after exposure. If patients are symptomatic, they usually have symptoms such as fever, chills, and headache. Most patients develop a transient reticulocytopenia, although this is usually clinically inapparent. By 2 to 3 weeks after exposure, patients usually mount an immune response with specific antibody production that results in clearance of the viremia and recovery of red cell production. At this time, patients may develop the classic rash and joint symptoms associated with parvovirus B19; these are thought to be due to immune complex formation.
Clinical manifestations are influenced by patient age and hematologic and immune condition. In utero infections result in severe anemia and hydrops fetalis. In children, the disease usually manifests as erythema infectiosum, or fifth disease, with the classic “slapped cheek” rash. Adults who are symptomatic usually have a nonspecific, viral-like syndrome with fever, malaise, headache, and arthralgias rather than the rash. In patients with increased demand for red cell production, such as those with hemolytic anemias, infection can result in an aplastic crisis. In immunocompromised patients, persistent infection can cause pure red cell aplasia and chronic severe anemia.
Pure red cell aplasia is characterized by an isolated decrease in red cell production by the bone marrow. It is usually a normocytic and reticulocytopenic anemia. Causes include drugs, autoimmune diseases, hematologic malignancies, and viral infections such as chronic parvovirus B19 infection.
It is thought that pure red cell aplasia secondary to parvovirus B19 develops in immunocompromised patients because they are unable to produce effective neutralizing antibodies and thus are unable to effectively clear the viral infection. Chronic infection results in persistent marrow suppression and severe reticulocytopenic anemia.
A few reports exist of parvovirus B19 causing pure red cell aplasia in supposedly immunocompetent patients. However, most cases involve immunocompromised patients. The disorder was first described in a child with congenital B- and T-cell immunodeficiency and decreased immunoglobulin production. It has since been described in transplant patients on immunosuppressive therapy, patients with lymphoproliferative disorders, and, as in our case, patients with AIDS. It has been described as the initial presentation of HIV infection (5).
Anemia is extremely common in HIV-infected patients and has multiple causes, including medications, malignancies, infections, and even HIV itself. The importance of parvovirus-associated pure red cell aplasia as a cause of anemia in HIV patients is unknown. The reported prevalence varies from only 0.5% of HIV-infected patients in some studies to up to 24% of patients with HIV having severe transfusion-dependent anemia (6). Whatever the exact prevalence, pure red cell aplasia is a treatable cause of anemia and should be considered in these patients.
Diagnosis of parvovirus B19 infection in immunocompetent patients is based on detecting IgM antibodies in acute infection. However, serologic tests are unreliable in immunocompromised patients, who often don't produce these specific antibodies. In addition, as Dr. Altrabulsi discussed, immunocompromised patients may not have the typical bone marrow morphology. Specific tests for parvovirus antigen or DNA are the best methods for diagnosing parvovirus B19 in immunocompromised patients.
Typical bone marrow features include erythroid hypoplasia with decreased red cell precursors in the marrow and rare giant pronormoblasts showing cytopathic effect with nuclear inclusions. Electron microscopy can also show intranuclear viral particles. However, in patients with HIV and in other immunocom- promised patients, giant pronormoblasts may not be present—in some studies, this is only 63% sensitive—and patients may actually have erythroid hyperplasia (7). The study referred to by Dr. Altrabulsi identified several patients with immunocompromised states and erythroid hyperplasia. The investigators postulated that this finding might be due to viral tolerance that allows cell development past the pronormoblast stage, although with decreased production overall (3).
Parvovirus B19 infection is best confirmed by identifying the viral antigen or DNA in serum or bone marrow. As was done in our case, immunohistochemical stains of bone marrow using monoclonal antibodies can be performed. Other studies include DNA hybridization, either dot blot in serum or in situ hybridization in bone marrow. The most sensitive diagnostic method is polymerase chain reaction amplification of DNA in either serum or bone marrow. This method is important because patients with chronic infections may have only low-titer viremias, which may not be detected by other means.
Once parvovirus B19 is diagnosed, it can usually be effectively treated with immunoglobulin therapy. Because most adults have been exposed to parvovirus B19 in the past and have protective IgG antibodies, it is thought that commercial immunoglobulin preparations contain these neutralizing antibodies and thus suppress the infection. Immunoglobulin doses from 0.4 to 1 g/kg/day are given intravenously for 2 to 5 days. Patients who relapse can be reinducted and then started on maintenance therapy of 0.4 g/ kg monthly. Most patients with HIV and with CD4 cell counts <100 can be expected to relapse within 6 months if they are not started on maintenance therapy; however, patients with CD4 cell counts >300 usually do not require maintenance therapy (8).
Although most patients treated with immunoglobulin therapy will have a hematologic recovery and resolution of their anemia, viral DNA usually can still be detected by polymerase chain reaction in these patients; thus, maintenance therapy may be needed. A recent case report showed that highly active antiretroviral therapy in an HIV-infected patient resulted in immune reconstitution and thus production of neutralizing antibodies and improvement in anemia (9).
The patient in this case was successfully treated with immunoglobulin therapy at 0.6 g/kg/day for 3 days. He did respond with improvement in his anemia. One month later, his hematocrit was 37%. He was also started on highly active antiretroviral therapy.
References
- 1.Hirst E, Robertson TI. The syndrome of thymoma and erythroblastopenic anemia. A review of 56 cases including 3 case reports. Medicine (Baltimore) 1967;46:225–264. doi: 10.1097/00005792-196705000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 3.Crook TW, Rogers BB, McFarland RD, Kroft SH, Muretto P, Hernandez JA, Latimer MJ, McKenna RW. Unusual bone marrow manifestations of parvovirus B19 infection in immunocompromised patients. Hum Pathol. 2000;31:161–168. doi: 10.1016/s0046-8177(00)80215-4. [DOI] [PubMed] [Google Scholar]
- 4.Bunyi-Teopengco E, Harihan S, Chang C, Machhi J, Shidham VB, Eshoa C, Kampalath B. Morphological and immunohistochemical study of bone marrow in adult immunosuppressed patients with parvovirus infection [poster]. 90th Annual Meeting, United States and Canadian Academy of Pathology, Atlanta, Ga, 2001.
- 5.Gottlieb F, Deutsch J. Red cell aplasia responsive to immunoglobulin therapy as initial manifestation of human immunodeficiency virus infection. Am J Med. 1992;92:331–333. doi: 10.1016/0002-9343(92)90085-p. [DOI] [PubMed] [Google Scholar]
- 6.Brown KE, Young NS, Liu JM. Molecular, cellular and clinical aspects of parvovirus B19 infection. Crit Rev Oncol Hematol. 1994;16:1–31. doi: 10.1016/1040-8428(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 7.Frickhofen N, Chen ZJ, Young NS, Cohen BJ, Heimpel H, Abkowitz JL. Parvovirus B19 as a cause of acquired chronic pure red cell aplasia. Br J Hematol. 1994;87:818–824. doi: 10.1111/j.1365-2141.1994.tb06743.x. [DOI] [PubMed] [Google Scholar]
- 8.Koduri PR, Kumapley R, Valladares J, Teter C. Chronic pure red cell aplasia caused by parvovirus B19 in AIDS: use of intravenous immunoglobulin—a report of eight patients. Am J Hematol. 1999;61:16–20. doi: 10.1002/(sici)1096-8652(199905)61:1<16::aid-ajh4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Arribas JR, Pena JM, Echevarria JE. Parvovirus B19–related anemia in an HIV-infected patient: rapid control after production of neutralizing antibodies during highly active antiretroviral therapy. Ann Intern Med. 2000;132:1011. doi: 10.7326/0003-4819-132-12-200006200-00036. [DOI] [PubMed] [Google Scholar]