Abstract
Approximately 3% of children in developed countries are born with nongenetic birth defects. However, the nature and mechanisms of teratogenesis are poorly understood. We investigated mechanisms of teratogen-mediated blockade of maternofetal transport by screening a combinatorial library for peptides that bind nonendothelial placental vasculature in pregnant mice. Here, we identified a peptide motif, TPKTSVT, that homes to the yolk sac, induces placental necrosis, and disrupts embryo development. We show that TPKTSVT promotes transcytosis of phage into the embryo and blocks the transplacental transport of immunoglobulins. Based on these data, we propose a model in which TPKTSVT targets a placental Fc receptor. Absence of TPKTSVT placental homing in mice lacking β2-microglobulin (β2m) suggests FcRn/β2m as a target for the TPKTSVT, which is unexpected, given the normal development of FcRn/β2m-deficient progeny. High-throughput screening for embryotoxins that target placental receptors could be developed to systematically identify and avoid exposure to teratogenic drugs.
Teratogens, substances that cause nongenetic fetal morbidity and mortality while being negligibly toxic to the mother, fall into two classes. The first class includes compounds that are actively or passively transferred through the maternofetal barrier and target fetal development by altering cell-signaling pathways controlling essential processes, such as angiogenesis, in the developing embryo (1, 2). Teratogens of the second class interfere with fetal development by affecting the delivery of nutrients to the embryo executed by the placenta (3, 4). In mammals, maternofetal molecule exchange occurs by filtration of blood from the maternal to the fetal side of the placenta through several distinct cell layers (4). Teratogens that target the placenta are thought to function by blocking receptors required for transport of nutrients to the fetus (3, 5). However, although many amino acid, fatty acid, nucleoside, and carbohydrate transporters are expressed in the placenta (6), the roles of specific receptors in the maintenance of maternofetal exchange and normal embryo development have not yet been established.
In vivo phage display, a method for studying interactions between receptors expressed preferentially in an organ of interest and their ligands (7, 8), is a prospective tool for molecular analysis of placental transport. Screenings of phage-displayed random peptide libraries in mice (9–12) and humans (13) have resulted in the isolation of ligands that selectively home to specific tissues when administered systemically. Homing ligands subsequently have been used to identify corresponding receptors differentially expressed in normal and tumor blood vessels (14–16). Here, we adapted this technology to select teratogenic ligands that bind nonendothelial receptors expressed on the villous placental epithelium, the site of maternofetal molecule exchange.
Experimental Procedures
Animals.
Staged pregnant, 18 days postconception (dpc) C57BL/6 female mice were purchased from Harlan Breeders (Indianapolis). Congenic pregnant, β2-microglobulin (β2m)-null females (stock 002087) mice were purchased from The Jackson Laboratory. All animal experiments involved standard procedures established and approved by the M. D. Anderson Cancer Center Animal Facility.
Phage Library Screening.
In vivo screening of an M13 phage-display library, CX7C (7, 13), for placenta-homing peptides was performed as described (7, 9) with the modifications described below. In each biopanning round, an 18-dpc C57BL/6 female was injected i.v. (tail vein) with 1010 transducing units (TU) of the library. Phage (103 TU in round 1 to 104 TU in round 4) were recovered from the placentas after 5 min of circulation and bulk-amplified for a subsequent round. In a procedure introduced here, the sublibrary amplified after the third round of panning was cleared of nonspecific binders in a subtraction step: a virgin C57BL/6 female was infused through the tail vein with 109 TU of phage selected in round 3. After 5 min, the unbound circulating phage were recovered from plasma. The plasma contained approximately 107 TU of precleared phage. Such phage population (1% of the injected pool) were recovered and amplified for the fourth (and final) round of biopanning.
Phage Recovery.
Mouse placentas and embryonic livers were weighed individually, ground with a glass Dounce homogenizer, suspended in 1 ml of DMEM containing proteinase inhibitors (DMEM-prin; 1 mM PMSF/20 μg/ml aprotinin/1 μg/ml leupeptin), vortexed, and washed three times with DMEM-prin. Tissue homogenates (or 10 μl of blood for normalization of phage titer in placenta against circulating phage titer) then were incubated with 1 ml of host bacteria (log-phase Escherichia coli K91kan; OD600 ≈ 2). Aliquots of the bacterial culture were plated onto Luria–Bertani agar plates containing 40 μg/ml tetracycline and 100 μg/ml kanamycin. Plates were incubated overnight at 37°C. Triplicate samples were processed for host bacterial infection, phage recovery, and histological analysis.
Fusion and Recombinant Peptides.
FITC-conjugated CTPKTSVTC or the control peptide CARAC, cyclized by the flanking cysteines, were synthesized chemically and HPLC-purified to >90% purity (Anaspec, San Jose, CA). The FITC-peptide stocks were made by dissolving lyophilized peptides in DMSO to a concentration of 20 mM, after which the peptides were diluted to 1 mM with PBS, and aliquots were frozen until use. The CTPKTSVTC peptide and an unrelated control peptide, CTREVHRSC, fused in-frame with GST at the amino terminus were purified to approximately 90% purity with the BugBuster GST Bind Kit (Novagen) and buffer-exchanged into PBS with Centricon PL-10 columns (Millipore), and the aliquots were frozen until use. For in vivo peptide homing validation, 10 μl of FITC-peptide stocks were diluted 20-fold with PBS or 250 μl of 5 mg/ml GST-peptides were injected. For phage-homing competition and IgG transcytosis-blocking experiments, 500 μl of 5 mg/ml GST-peptides was administered i.v.
Peptide Localization in Tissues.
Immunohistochemistry on sections of formalin-fixed, paraffin-embedded mouse tissue was performed as described (7, 9). For phage-peptide immunolocalization, a rabbit anti-fd phage antibody (Sigma) was used at 1:1,000 dilution and detected with a secondary horseradish peroxidase-conjugated antibody. For GST-peptide immunolocalization, a goat anti-GST antibody (Amersham Pharmacia) was used at 1:1,000 dilution and detected with a secondary alkaline phosphatase-conjugated antibody. For mouse IgG immunolocalization, the ARK Peroxidase kit (Dako) was used. All immunohistochemistry and FITC immunofluorescence images were captured by using an Olympus IX70 microscope and digital camera setup.
Peptide Embryotoxicity.
For peptide embryotoxicity studies, agents were injected at the following daily doses: GST-peptides, 0.1 mg (≈3 nM); FITC-peptides, 50 μg (≈30 nM); and phage-peptides, 1011 TU; all agents were dissolved in PBS. Mice were injected s.c. in the back (5–10 injections per course).
Results
Isolation of Placenta-Homing Peptides.
To isolate peptides that home to the placenta in vivo, we screened a phage library displaying a peptide insert with the structure CX7C (C, cysteine; X, any amino acid residue; refs. 7 and 13) in pregnant mice. After three rounds of panning, we used an in vivo subtraction procedure with nonpregnant mice to restrict ligands selected to those binding specifically to placenta (see Experimental Procedures). After subtraction, the relative amount of phage homing to the placenta increased substantially, suggesting that clearance of nonspecific binders enriched the pool of selected phage clones for those with tropism for placental cell surface proteins. We next sequenced the DNA encoding the corresponding phage-displayed peptides. Analysis of selected motifs with sas software (Version 8; SAS Institute, Cary, NC) revealed that four different motifs (TPKTSVT, RXXGXVR, LGLRSVG, and YIRPFTL) constituted 36.1% of clones. We confirmed by quantification of phage recovery that the four motifs homed to the mouse placenta relative to a control insertless phage (range, 2- to 9-fold; median, 4.9-fold).
Localization of the TPKTSVT Peptide in Mouse Placenta.
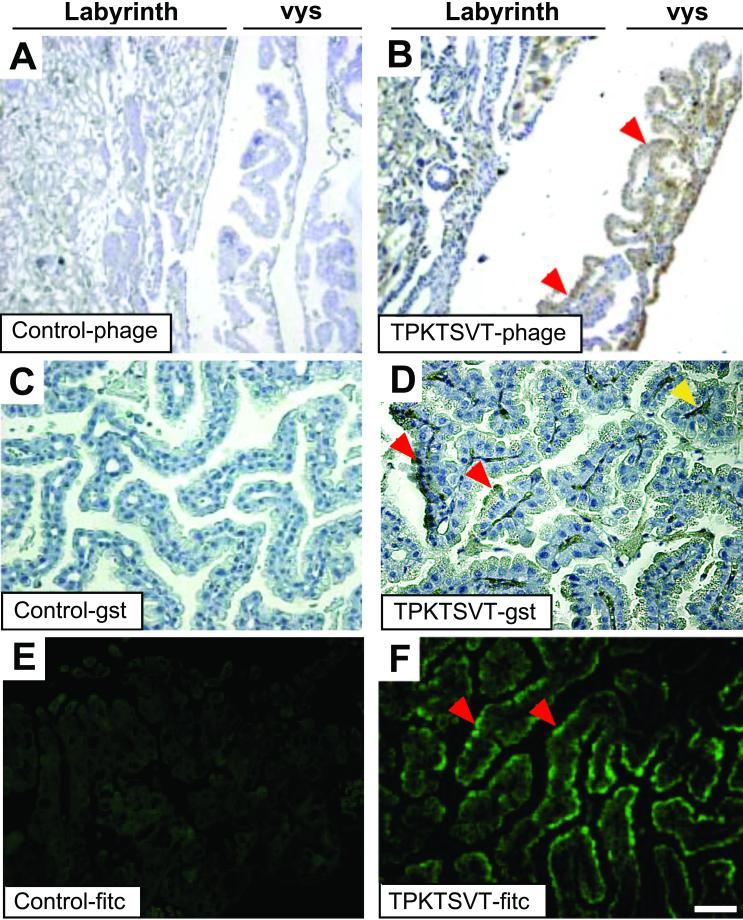
To gain insight into the molecular mechanisms of peptide homing to the placenta, we determined the tissue distribution of phage injected into pregnant mice by immunohistochemistry (Fig. 1). Whereas a control phage barely localized to placental tissues (Fig. 1A), the TPKTSVT-phage homed to the placenta, showing marked localization to the villi of the visceral yolk sac (vys) endoderm (Fig. 1B). The vys is the layer of epithelial cells that surrounds the embryonic microvasculature and functions as the final barrier during transport of molecules such as IgG from the labyrinth layer of the placenta into the fetus (4, 5, 17, 18). To verify that targeting of the TPKTSVT motif to the vys endoderm also occurs if the peptide is outside of the context of the phage, we tested the homing of TPKTSVT fused with GST protein to placenta. We injected either TPKTSVT-GST or a control TREVHRS-GST fusion (an unrelated peptide with a similar overall charge) into pregnant mice and examined the tissue distribution of each peptide. Although localization of the control GST fusion to the placenta was not observed (Fig. 1C), accumulation of the TPKTSVT-GST peptide in the apical cytoplasm of the vys was readily detectable (Fig. 1D) and matched that observed for TPKTSVT-phage (Fig. 1B). Similarly, FITC-conjugated TPKTSVT injected i.v. into pregnant mice also specifically localized to the apical vys cytoplasm (Fig. 1F), whereas a control FITC conjugate was not detectable in the placenta (Fig. 1E). Localization of TPKTSVT-phage, TPKTSVT-GST, or TPKTSVT-FITC to control organs, such as brain and pancreas, was not detected. Together, these data show that the TPKTSVT peptide targets the placenta, with the strongest homing noticed in the vys (Fig. 1 B, D, and F).
Figure 1.
In vivo homing of the TPKTSVT motif to the mouse vys. (A and B) Antiphage immunohistochemistry (brown staining). Anti-GST immunohistochemistry (black staining) (C and D) or FITC immunofluorescence (green fluorescence) (E and F) in paraffin sections of the placentas from 18-dpc pregnant mice injected i.v. 6 h before tissue processing with control insertless phage (A), TPKTSVT-phage (B), control GST-peptide (C), TPKTSVT-GST peptide (D), control FITC-peptide (E), or TPKTSVT-FITC peptide (F). Homing of the TPKTSVT peptide to the vys (red arrowhead) and translocation to the embryonic capillaries (yellow arrowhead) are indicated. Only the vys is shown in C–F. Hematoxylin counterstaining is blue (A–D). [Bars = 100 μm (A and B) and 20 μm (C–F).]
The TPKTSVT Peptide Binds to a Placental Transporter.
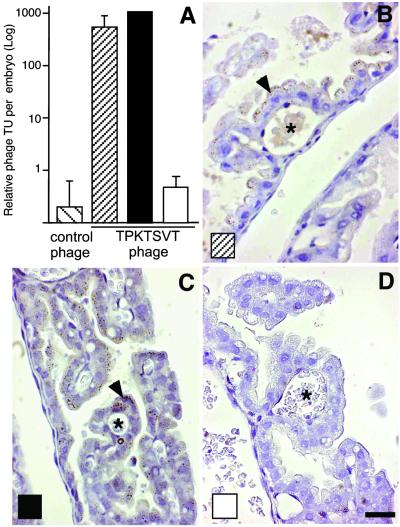
Given that the TPKTSVT peptide localizes to the vys, the tissue primarily responsible for maternofetal transport in mice (Fig. 1), we tested whether this motif would promote phage transport into the embryo. We injected TPKTSVT-phage or control phage into pregnant mice and determined the recovery of phage from embryos: specific accumulation of TPKTSVT-phage in embryos was up to 1,000-fold greater than that of the control (Fig. 2A). We also showed that the TPKTSVT peptide binds to a specific placental transporter: the maternofetal transfer of TPKTSVT-phage was blocked by an excess of coinjected TPKTSVT peptide but not the control GST fusion (Fig. 2A). Phage immunocytochemistry confirmed that the uptake of TPKTSVT-phage by the vys cells was not affected by the control GST fusion (Fig. 2 A– C); in contrast, TPKTSVT-GST prevented internalization of TPKTSVT-phage into the vys epithelium (Fig. 2D). Together, these results suggest that the TPKTSVT peptide is actively transported through the placenta into the embryo by binding to a receptor in the vys.
Figure 2.
The TPKTSVT peptide specifically binds a placental transporter. (A) Recovery of indicated phage from embryos carried by 18-dpc pregnant mice injected i.v. (tail vein) with 1010 TU of the indicated phage 6 h before phage recovery or immunohistochemistry. ▨, TPKTSVT-phage administered alone; ■, control-GST coadministered; □, TPKTSVT-GST coadministered. Shown are coadministered. mean ± SEM from different embryos. (B–D) Antiphage HRP immunohistochemistry (brown staining, arrowheads) in paraffin sections of the vys from the corresponding mice, as indicated. *, Embryonic capillaries. Tissue components counterstained with hematoxylin appear blue. (Bar = 20 μm.)
The TPKTSVT Peptide Blocks the Placental IgG Transport.
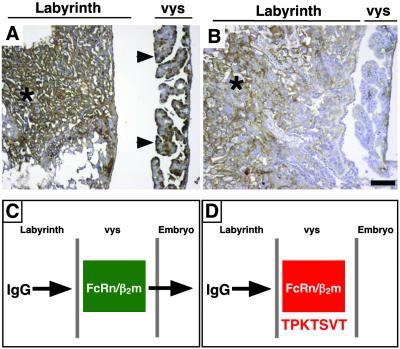
The pattern of the TPKTSVT localization to the placenta (Fig. 1) is reminiscent of that observed for IgG in that tissue (19); moreover, both IgG and TPKTSVT appear to undergo a receptor-mediated transport into the embryo during pregnancy. Thus, we hypothesized that IgG and TPKTSVT bind to a common receptor in the placenta. Here, we show that the TPKTSVT peptide competes for the placental transport of IgG. Intravenous administration of a control GST-fused peptide did not affect the placental transfer of IgG to the vys (Fig. 3A), whereas coadministration of an equimolar dose of TPKTSVT-GST blocked translocation of IgG through the placenta (Fig. 3B). Although IgG localization to the labyrinthine blood vessels in the embryo-distal placental compartments was still clearly detectable (Fig. 3B, asterisks), IgG staining in the vys epithelium was markedly decreased. Indeed, the vys levels of IgG were similar to what is observed in β2m-null mice, which are deficient in IgG transcytosis (20, 21). Based on these data, we proposed a model in which the TPKTSVT peptide selectively blocks transport through an Ig Fc receptor that mediates the uptake of IgG by the yolk sac (Fig. 3 C and D).
Figure 3.
The TPKTSVT peptide blocks placental IgG transcytosis. An 18-dpc pregnant mouse was injected i.v. with 100 μg of control GST fusion (A) or TPKTSVT-GST (B). The IgG distribution in the placenta after 6 h of peptide circulation was detected by anti-mouse IgG immunohistochemistry in paraffin sections (brown staining). Strong immunostaining is noted in the vys of mice injected with a control GST fusion (arrowheads) and in the labyrinth of wild-type mice injected with either TPKTSVT-GST or control GST fusion (*) but not in the vys of TPKTSVT-GST fusion (B). Hematoxylin counterstaining is blue. (Bar = 100 μm.) (C and D) A hypothetical model for the TPKTSVT peptide function. Normally, the FcRn/β2m complex transports IgG from maternal circulation through the labyrinth layer and then the yolk sac placenta (vys) and into the embryo (C). Targeting of the FcRn/β2m with TPKTSVT peptide can block the receptor complex and the transport of both IgG (B) or phage (Fig. 2D) into the embryo (D).
The TPKTSVT Receptor Is β2m-Associated.
FcRn, a β2m-associated class I MHC (MHC-I) homologue, is an Fc receptor, which appears to regulate placental IgG transport (22, 23). This notion is supported by the fact that IgGs incapable of binding FcRn are not transported across the human placenta in an ex vivo model (24). In addition, FcRn expression pattern in the placenta (25) resembles that of IgG localization. Interestingly, a search of the mouse protein database by using blast software (National Center for Biotechnology Information; www.ncbi.nlm.nih.gov/blast/) revealed similarity of the selected motif TVSTKPT (the reverse TPKTSVT) to the sequence PPKTTVT (amino acids 192–198 of the mouse MHC-I; GenBank accession no. AAD43175). Moreover, the corresponding conserved human MHC-I sequence, PPKTHVT, is exposed on the surface of the MHC-I α3 chain immediately adjacent to H-192 residue, a known β2m-interaction site (26). Because FcRn is an MHC-I family member, we set out to test whether TPKTSVT motif targets the FcRn/β2m receptor complex in the placenta.
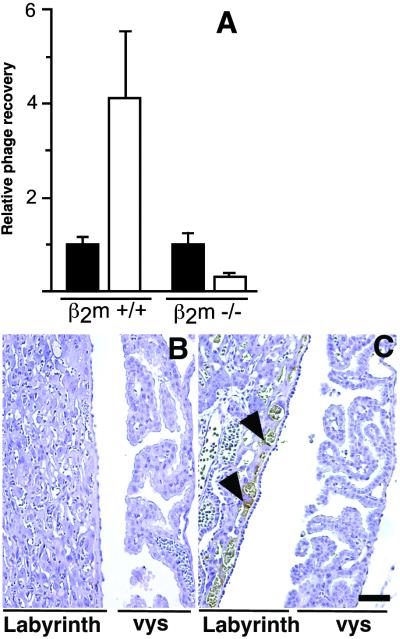
The β2m-deficient mouse strain, in which FcRn is not functional (20, 21), provided an animal model to test whether the TPKTSVT peptide is indeed a ligand for the FcRn/β2m receptor complex. We administered phage displaying the TPKTSVT peptide i.v. into pregnant β2m-null mice and assayed the recovery of phage from the placenta (Fig. 4). Although the TPKTSVT-phage homed to the wild-type placenta relative to control phage, such homing was not detectable in β2m-deficient mice (Fig. 4A). Immunohistochemical analysis of phage accumulation in β2m wild-type (Fig. 1B) and β2m-null (Fig. 4B) placentas confirmed that TPKTSVT-phage were not taken up by the vys epithelium in the β2m-deficient mice. In contrast, in vivo localization of a control phage displaying a different placenta-homing peptide, YIRPFTL, which, unlike TPKTSVT peptide, homes to the vasculature of the labyrinthine placenta—rather than to the vys—was not affected in β2m-null mice (Fig. 4C). Together, these observations strongly suggest that the TPKTSVT motif targets the placenta by binding to FcRn/β2m.
Figure 4.
Placental targeting by the TPKTSVT peptide requires a functional FcRn/β2m receptor complex. (A) Relative phage recovery (placenta-to-blood ratio; TU/ml) from placentas derived from 18-dpc pregnant wild-type or β2m-deficient mice injected with 1010 TU of control insertless phage (■) or TPKTSVT-phage (□) 6 h before phage recovery or immunohistochemistry. Shown are mean ± SEM from individual placentas. (B and C) Antiphage immunohistochemistry (brown) in paraffin sections of the placenta from 18-dpc pregnant β2m-null mice injected i.v. with TPKTSVT-phage (B) or control placenta-homing phage displaying the YIRPFTL peptide (C) 6 h before tissue processing. Staining of a placenta-homing phage displaying the unrelated peptide YIRPFTL is detected in the labyrinth blood vessels, as indicated (arrowheads). Hematoxylin counterstaining is blue. (Bar = 100 μm.)
The TPKTSVT Peptide Interferes with Mouse Pregnancy.
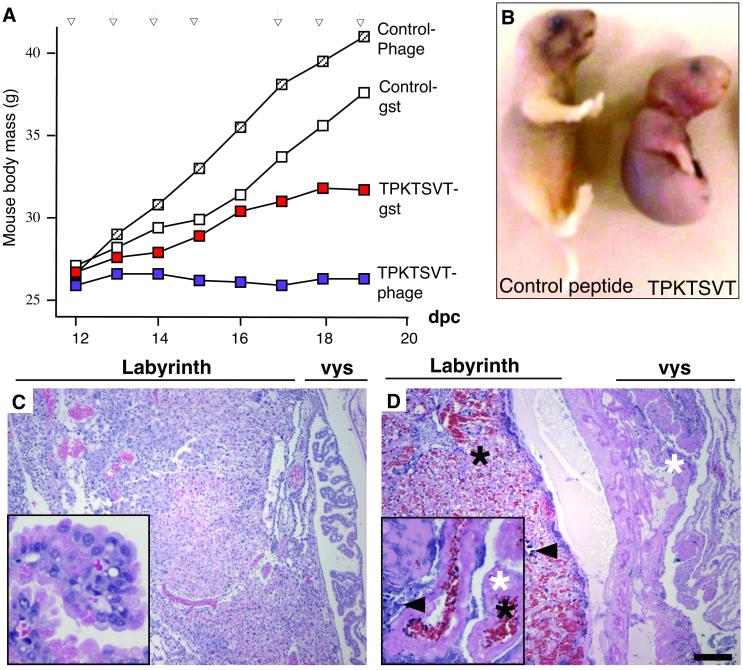
Because FcRn/β2m regulates maternofetal exchange, we reasoned that TPKTSVT might interfere with placental conductivity and tested whether it would affect embryonic development. We injected TPKTSVT peptide s.c. in three different contexts (displayed on the phage capsid, fused with GST, or to FITC) into pregnant mice and compared the progression of pregnancy with that in mice injected with control phage, control peptides, or saline. Repeated injections of the TPKTSVT peptide starting at midpregnancy (≈12 dpc) in doses nontoxic to the mother inhibited pregnancy progression, as evidenced by diminished mouse weight gain (Fig. 5A). In contrast, pregnancy courses in mice injected with similar doses of control peptides were indistinguishable from those in saline-injected mice (Fig. 5A). Examination of embryos on the 20th day of pregnancy (the delivery day for the control) revealed that the TPKTSVT peptide has a severe effect on embryonic development. Typically, in mice injected with either TPKTSVT-phage, TPKTSVT-GST, or TPKTSVT-FITC, growth-retarded, dead, or partially resorbed embryos were observed (Fig. 5B). Administration of TPKTSVT fusions to pregnant mice resulted in complete embryo (43%) resorption and in conceptuses (21%) that were delivered dead and/or malformed (Fig. 5B). The extent of embryo resorption and frequency of complete pregnancy abortion increased with prolonged TPKTSVT treatment, suggesting that the peptide effect is dose-dependent. Embryonic death or morbidity was not observed in any of the control groups.
Figure 5.
TPKTSVT peptide inhibits mouse pregnancy and is teratogenic. (A) Pregnancy courses (representative from five independent experiments) in mice injected s.c. with the indicated phage or peptides were monitored by weighing the mice daily on each injection (arrows). (B) Appearance of a normally developed, 20-dpc embryo for control GST fusion treatment as compared with a representative 20-dpc embryo resulted from TPKTSVT-GST fusion treatment (severe teratogenic phenotype). (C and D) Hematoxylin/eosin staining of 20-dpc, paraffin-embedded placentas derived from mice injected for 7 days with the control GST fusion (C) or the TPKTSVT-GST (D). Note hemorrhage (black asterisks), necrosis (white asterisks), and fibrosis (arrowheads). [Bar = 500 μm (50 μm for Insets).]
Morphologic inspection of tissues from TPKTSVT peptide-injected mice revealed that, in contrast to controls, placentas were edematous and grossly deformed. Histopathological examination of the placentas showed that the TPKTSVT treatment induced massive dilation of blood vessels and intraplacental bleeding, as well as widespread hemorrhagic necrosis (Fig. 5D). Hematoxylin staining of the placental epithelium after 7 days of peptide administration showed that most nuclei in the yolk sac and many in the labyrinthine compartment were degraded. Also, massive fibrosis was evident in the yolk sac cavity and in the labyrinthine portion of the placenta. In contrast, placentas from mice injected with control peptides (Fig. 5C) were indistinguishable from untreated placentas at corresponding stages of pregnancy. The effect of TPKTSVT peptide on the reproductive system was strikingly specific, because histological examination of control organs revealed no pathological changes or necrosis, and no signs of peptide toxicity to the mother were observed.
Discussion
Here, we used complementary approaches to demonstrate that a peptide motif, TPKTSVT, targets the vys through the FcRn/β2m receptor complex required for maternofetal IgG transport (22, 23). This conclusion is consistent with our finding that the TPKTSVT homology region exposed on the MHC-I surface immediately adjacent to H-192 is a β2m contact site in the α3-domain of MHC-I homologues (26). This makes the TPKTSVT motif an apparent mimetope of FcRn. Homing of the TPKTSVT peptide to the placenta, despite expression of FcRn in other tissues (22, 23), may be explained by placenta-specific presentation of the receptor complex because of either differential association of additional receptor subunits or by altered accessibility of the receptor to the circulating ligand in the placenta. The paradigm of such a tissue-specific presentation of ubiquitously expressed receptors is illustrated by recent reports on CD13 targeted by the NGR motif in tumors (15, 27) and on IL-11 targeted by the RRAGGS motif in human prostate (12) but not in other tissues. The placenta-specific binding of the TPKTSVT motif to the FcRn/β2m complex proposed here is also consistent with previously reported findings of FcRn interacting with IgG in the placenta through a mechanism different from that in other tissues (22, 23).
Targeting of FcRn/β2m transporter with TPKTSVT peptide delayed embryogenesis and caused embryo resorption and abortion. Importantly, the mouse MHC-I fragment apparently mimicked by the TPKTSVT motif (PPKTTVT) is virtually identical to the corresponding fragment of human MHC-I (PPKTHVT) (26). Thus, the human homologue of the murine receptor recognized by the selected TPKTSVT motif, FcRn/β2m, is also a potential target for teratogenesis. The observed teratogenicity likely is not caused by disruption of the FcRn/β2m receptor function, because fertility of mice is not affected significantly by the β2m deficiency (21). More likely, the embryotoxicity of TPKTSVT is secondary to the evident placental thrombosis and ischemia. This interpretation is consistent with results of several reports on targeting placental cell surface molecules with antibodies (28–30). Another, although not mutually exclusive, mechanism of placental dysfunction could be activation of complement and an immune response against the peptide itself. The possibility of peptide-directed inflammation is consistent with TPKTSVT-FITC being a less potent embryotoxin than the more immunogenic TPKTSVT-GST and TPKTSVT-phage. There is active research addressing the role of MHC-I in immune tolerance of the fetus during pregnancy (31). Along the lines of our findings, embryo resorption was observed on expression of transgenic MHC- I molecules early in embryogenesis (32). Therefore, it is possible that TPKTSVT similarity to MHC-I contributes to placental inflammation. A direct toxic effect of TPKTSVT on embryogenesis is unlikely because the number of TPKTSVT-phage particles accumulated on transcytosis is only in the range of 103 per embryo, and no accumulation of TPKTSVT peptide was detectable in any of the embryonic organs on administration.
In this study, we screened a phage-display library on nonendothelial vasculature in vivo. Inclusion of an in vivo subtraction step allowed us to isolate peptides targeting placenta with remarkable specificity. The results of this work have important implications for the development of pregnancy-safe therapeutics, because it appears that a substance can cause embryotoxicity by merely homing to a placental cell surface marker and not necessarily by inactivating the receptor function. Our study creates a basis for high-throughput identification of placental receptors prone to teratogen targeting. Systematic screening of compounds for tropism to these receptors could greatly decrease the risk of nongenetic birth defects.
Acknowledgments
We thank Dr. Corazon D. Bucana for expert assistance with immunostainings and Dr. Richard Sidman for critical reading of the manuscript. This study was supported in part by National Institutes of Health Grants CA90270 and CA8297601 (to R.P.) and CA90270 and CA9081001 (to W.A.), and awards from the Gilson–Longenbaugh Foundation and The V Foundation (to R.P. and W.A.) and the Department of Defense (BCTR 2000 30616 to M.G.K.). M.G.K. is a fellow of the Susan G. Komen Breast Cancer Foundation (BC996405). The University of Texas and R.P. and W.A. have equity in NTTX Biotechnology, which is subjected to certain restrictions under the university policy; the university manages the terms of these arrangements according to its conflict-of-interest policies.
Abbreviations
- β2m
β2-microglobulin
- DMEM-prin
DMEM containing proteinase inhibitors
- dpc
days postconception
- fd
fd bacteriophage
- TU
transducing units
- vys
visceral yolk sac
References
- 1.D'Amato R J, Loughnan M S, Flynn E, Folkman J. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnell R H. J Allergy Clin Immunol. 1999;103:337–342. doi: 10.1016/s0091-6749(99)70259-9. [DOI] [PubMed] [Google Scholar]
- 3.Maranghi F, Macri C, Ricciardi C, Stazi A V, Mantovani A. Adv Exp Med Biol. 1998;444:129–136. doi: 10.1007/978-1-4899-0089-0_15. [DOI] [PubMed] [Google Scholar]
- 4.Rugh R. The Mouse: Its Reproduction and Development. Oxford: Oxford Science Publications; 1990. [Google Scholar]
- 5.Beckman D A, Koszalka T R, Jensen M, Brent R L. Teratology. 1990;41:395–404. doi: 10.1002/tera.1420410405. [DOI] [PubMed] [Google Scholar]
- 6.Knipp G T, Audus K L, Soares M J. Adv Drug Deliv Rev. 1999;38:41–58. doi: 10.1016/s0169-409x(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 7.Pasqualini R, Arap W, Rajotte D, Ruoslahti E. In: Phage Display: A Laboratory Manual. Barbas C, Burton D, Silverman G, Scott J, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 22.1–22.24. [Google Scholar]
- 8.Kolonin M G, Pasqualini R, Arap W. Curr Opin Chem Biol. 2001;5:308–313. doi: 10.1016/s1367-5931(00)00207-6. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualini R, Ruoslahti E. Nature (London) 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 10.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. J Clin Invest. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arap W, Pasqualini R, Ruoslahti E. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 12.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby H M, Bredesen D E, Pasqualini R, Ruoslahti E. Proc Natl Acad Sci USA. 2002;99:1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arap W, Kolonin M G, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano R J, Mintz P J, Ardelt P U, Yao V J, Vidal C I, et al. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 14.Rajotte D, Ruoslahti E. J Biol Chem. 1999;274:11593–11598. doi: 10.1074/jbc.274.17.11593. [DOI] [PubMed] [Google Scholar]
- 15.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun R A, Shapiro L H, Arap W, Ruoslahti E. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 16.Essler M, Ruoslahti E. Proc Natl Acad Sci USA. 2002;99:2252–2257. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyden T W, Robinson J M, Tridandapani S, Teillaud J L, Garber S A, Osborne J M, Frey J, Budde P, Anderson C L. J Immunol. 2001;166:3882–3889. doi: 10.4049/jimmunol.166.6.3882. [DOI] [PubMed] [Google Scholar]
- 18.Jollie W P. Teratology. 1990;41:361–381. doi: 10.1002/tera.1420410403. [DOI] [PubMed] [Google Scholar]
- 19.Parr E L, Parr M B. J Reprod Immunol. 1985;8:153–171. doi: 10.1016/0165-0378(85)90038-5. [DOI] [PubMed] [Google Scholar]
- 20.Israel E J, Patel V K, Taylor S F, Marshak-Rothstein A, Simister N E. J Immunol. 1995;154:6246–6251. [PubMed] [Google Scholar]
- 21.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. Nature (London) 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 22.Ghetie V, Ward E S. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 23.Simister N E, Story C M. J Reprod Immunol. 1997;37:1–23. doi: 10.1016/s0165-0378(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 24.Firan M, Bawdon R, Radu C, Ober R J, Eaken D, Antohe F, Ghetie V, Ward E S. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 25.Saji F, Samejima Y, Kamiura S, Koyama M. Rev Reprod. 1999;4:81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 26.Tysoe-Calnon V A, Grundy J E, Perkins S J. Biochem J. 1991;277:359–369. doi: 10.1042/bj2770359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curnis F, Arrigoni G, Sacchi A, Fischetti L, Arap W, Pasqualini R, Corti A. Cancer Res. 2002;62:867–874. [PubMed] [Google Scholar]
- 28.Wang X, Campos B, Kaetzel M A, Dedman J R. Am J Obstet Gynecol. 1999;180:1008–1016. doi: 10.1016/s0002-9378(99)70674-5. [DOI] [PubMed] [Google Scholar]
- 29.Foidart J M, Yaar M, Figueroa A, Wilk A, Brown K S, Liotta L A. Am J Pathol. 1983;110:346–357. [PMC free article] [PubMed] [Google Scholar]
- 30.Knezevic N, Nikolic B, Brajsa K, Spaventi R, Jonjic N, Jonjic S, Marusic S. Am J Reprod Immunol. 1999;41:217–223. doi: 10.1111/j.1600-0897.1999.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 31.Carosella E D, Rouas-Freiss N, Paul P, Dausset J. Immunol Today. 1999;20:60–62. doi: 10.1016/s0167-5699(98)01387-5. [DOI] [PubMed] [Google Scholar]
- 32.Ait-Azzouzene D, Langkopf A, Cohen J, Bleux C, Gendron M C, Kanellopoulos-Langevin C. Mol Reprod Dev. 1998;50:35–44. doi: 10.1002/(SICI)1098-2795(199805)50:1<35::AID-MRD5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]