Abstract
Dogs with mucopolysaccharidosis VII (MPS VII) were injected intravenously at 2–3 days of age with a retroviral vector (RV) expressing canine β-glucuronidase (cGUSB). Five animals received RV alone, and two dogs received hepatocyte growth factor (HGF) before RV in an attempt to increase transduction efficiency. Transduced hepatocytes expanded clonally during normal liver growth and secreted enzyme with mannose 6-phosphate. Serum GUSB activity was stable for up to 14 months at normal levels for the RV-treated dogs, and for 17 months at 67-fold normal for the HGF/RV-treated dog. GUSB activity in other organs was 1.5–60% of normal at 6 months for two RV-treated dogs, which was likely because of uptake of enzyme from blood by the mannose 6-phosphate receptor. The body weights of untreated MPS VII dogs are 50% of normal at 6 months. MPS VII dogs cannot walk or stand after 6 months, and progressively develop eye and heart disease. RV- and HGF/RV-treated MPS VII dogs achieved 87% and 84% of normal body weight, respectively. Treated animals could run at all times of evaluation for 6–17 months because of improvements in bone and joint abnormalities, and had little or no corneal clouding and no mitral valve thickening. Despite higher GUSB expression, the clinical improvements in the HGF/RV-treated dog were similar to those in the RV-treated animals. This is the first successful application of gene therapy in preventing the clinical manifestations of a lysosomal storage disease in a large animal.
The mucopolysaccharidoses (MPS) are lysosomal storage diseases (LSD) that result from deficient activity of enzymes that degrade glycosaminoglycans (GAGs) (1, 2). The overall incidence of MPS is 1:27,000 live births (3). MPS VII (Sly syndrome) is due to deficient β-glucuronidase (GUSB; EC 3.2.1.31) activity and results in growth retardation, mobility problems, dysostosis multiplex, facial dysmorphia, hepatosplenomegaly, corneal clouding, cardiac valvular abnormalities, and mental retardation (4). MPS VII dogs are homozygous for an arginine to histidine substitution at amino acid 166 in the canine GUSB (cGUSB) protein (5, 6). Features in MPS VII dogs resemble those in humans, except mental retardation is difficult to assess, and hepatosplenomegaly is less severe.
The clinical manifestations of LSD can be reduced by enzyme replacement therapy (ERT), which involves i.v. injection of enzyme. Enzyme that contains mannose 6-phosphate (M6P) can be internalized via the M6P receptor (M6PR) present on the surface of most cells (7, 8). ERT has been therapeutic in mice with MPS VII (reviewed in ref. 9), large animals with MPS (10–12), and humans with LSD (13–15). The rationale for hepatic gene therapy is that the liver will continuously secrete GUSB with M6P into the blood, because most cells that produce functional GUSB secrete some enzyme that is phosphorylated.
We and others have demonstrated that Moloney murine leukemia virus (MLV)-based retroviral vectors (RV) can stably express proteins from the liver in rodents (reviewed in ref. 16). Although efficient transfer into hepatocytes of adults by using an MLV-based RV requires induction of replication with hepatocyte growth factor (HGF) (17) or other methods, the rapid liver growth allowed transduction to occur without any stimulus for hepatocyte replication in newborn mice (18) and dogs (19). We report the clinical improvements seen in six MPS VII dogs that were transduced with a cGUSB-expressing RV as neonates and followed for 6–17 months.
Materials and Methods
hAAT-cGUSB-WPRE contains the human α1-antitrypsin promoter upstream of the canine GUSB cDNA (19). For the RV-treated dogs, MPS VII dogs were injected with four doses of a 5-ml bolus of hAAT-cGUSB-WPRE over 1–2 min at 4-h intervals via an external jugular catheter at 2–3 days after birth as described (19) and summarized in Table 1. For the HGF/RV-treated dogs, eight equal doses of human HGF (20) were given every 3 h (cumulative dose 2.5 mg/kg) beginning at 2 days after birth. Beginning 3 h after the last dose of HGF, they each received four doses of RV (5 ml every 4 h) via the i.v. catheter.
Table 1.
Summary of gene transfer and clinical effects in treated and control dogs
| Dog* | Dose of RV, rfu/kg, and time of first dose† | Percent liver cells transduced at 4M‡ | Liver GUSB, units/mg, at 4M§ | Serum GUSB, units/ml¶ | Percent serum GUSB with M6P‖ | Evaluation of gait, cornea, and heart at the indicated time after birth** |
|---|---|---|---|---|---|---|
| RV-treated MPS VII dogs | ||||||
| M1312 Cisco male | 3.5 × 109 at 48 h | 3.1% | 178 | 166 at 6M | 27.4 ± 9.3% (n = 3) | Ran with stiff gait, short stride, and a limp at 6M |
| M1328 Roy male | 3.6 × 109 at 48 h | 2.5% | 183 | 429 at 14M | 8.4 ± 3.7% (n = 2) | Runs with almost normal gait, mild right hind leg lameness, and mild bilateral tarsal valgus at 14M; slight corneal clouding at 8M; mild MR at 9M, but no murmur or MV thickening |
| M1332 Dale female | 3.7 × 109 at 48 h | 2.0% | 89 | 76 at 14M | 19.7 ± 1.6% (n = 2) | Runs with almost normal gait at 14M; no corneal clouding at 8M; no MV disease at 9M |
| M1337 Penny female | 3.3 × 109 at 72 h | 4.4% | 317 | 294 at 14M | 11.7 ± 2.7% (n = 2) | Runs with almost normal gait at 14M; has mild spina bifida occulta; no corneal clouding at 8M; no MV disease at 9M |
| M1339 Sky King male | 3.0 × 109 at 72 h | 2.1% | 144 | 132 at 6M | 8.3 ± 1.3% (n = 2) | Ran with stiff gait, short stride, a limp, and severe tarsal and carpal valgus at 6M |
| HGF/RV-treated MPS VII dogs | ||||||
| M1287 Preston male | 12 × 109 at 67 h | 19.4% | 2830 | 18,039 at 17M | 24.3 ± 7.5% (n = 2) | Runs with moderate tarsal valgus, stiff gait, short stride, a limp, and hind legs close together at 17M; has mild spina bifida occulta; no corneal clouding at 11M; no MV disease at 12M |
| M1288 male | 7.9 × 109 at 67 h | 10.1% at 5D | 863 at 5D | 8,617 at 5D | 4.5% at 5D | ND as he was killed at 8 days |
| Normal dogs | ||||||
| NA | NA | 0 | 475.1 ± 40.4 (n = 11) | 269 ± 16.6 (n = 10) | 36.8 ± 3.5% at >3M (n = 7); 5.5 ± 1.5% at 5D | Run normally with an occasional animal with a stiff gait due to hip dysplasia; no corneal clouding; no MV disease |
| Untreated MPS VII dogs | ||||||
| NA | NA | 0 | 0.3 ± 0.1 (n = 3) | 0.55 ± 0.05 (n = 6) | ND | Cannot stand or walk by 6 months of age; corneal clouding present by 4M; MV murmur, MV thickening, and MR present by 9 months |
The number, name, and sex of each treated dog. NA, not applicable.
The cumulative dose of RV in red-forming units (rfu)/kg and the age after birth in hours of the first dose of RV. The HGF/RV-treated dogs received HGF during the 24 h prior to RV injection, as detailed in the methods section.
The percentage of hepatocytes that were transduced was determined at 4 months (4M) after transduction for most animals. Evaluation was at 5 days (5D) after treatment for M1288.
The GUSB activity in liver homogenates was determined at 4M after transduction unless otherwise stated.
The GUSB activity in serum at the last time of analysis, which is indicated in months (M) or days (D) after transduction.
The percentage of the total GUSB in serum that bound to a M6PR column and eluted with M6P on the indicated number (n) of samples collected at 1–6 months after transduction unless otherwise stated. ND, not determined.
The gait, degree of corneal clouding, and mitral valve (MV) disease at the indicated time in months (M) after birth. Normal MV means no murmur of mitral valve regurgitation (MR) or echocardiographic evidence of MR or MV thickening.
Analysis of Animals.
Dogs were maintained at the School of Veterinary Medicine University of Pennsylvania under National Institutes of Health and U.S. Department of Agriculture guidelines for the care and use of animals in research. Liver biopsies were performed as described (19). Radiographs and aspiration of synovial joint fluid and aqueous humor were performed after i.m. injection of 0.02 mg/kg of atropine (Phoenix Pharmaceutical, St. Joseph, MO) and 0.1 mg/kg of hydromorphone (Elkins-Sinn, Cherry Hill, NJ), and an i.v. injection of 2 mg/kg of propofol (Abbott). For the ocular examination, the pupils were dilated with 1% tropicamide (Mydriacyl 1%, Alcon Laboratories, Ft. Worth, TX). Evaluations of radiographs, eyes, and echocardiograms were done by a radiologist (V.W.K.), an ophthalmologist (G.A.), and a cardiologist (M.S.), respectively, who were blinded to the genotype and treatment of the animals. Euthanasia was performed using 80 mg/kg of sodium pentobarbital (Veterinary Laboratories, Lenexa, KS) in accordance with American Veterinary Medical Association guidelines, and tissue samples were frozen immediately on dry ice. Dog M1339 died while under propofol anesthesia during removal of cerebrospinal fluid. He appeared to be in good health before the procedure. Dogs were perfused with two liters of cold saline before the collection of tissues. Averages ± the standard error of the mean (SEM) were calculated for all values, and comparisons were between two groups by using the Student's t test.
Analysis of Serum and Organs.
GUSB histochemical staining of liver was performed on 8-μm frozen sections by using conditions that reduced the activity, as detailed in ref. 19. GUSB activity was determined by incubating samples with 4-methylumbelliferyl β-d-glucuronide (Sigma) for 1–16 h, and measuring fluorescence (17). One unit of enzyme produces 1 nmol of product in 1 h. For organ extracts, activity was normalized to the protein concentration (Bradford assay kit, Bio-Rad). The percentage of transduced hepatocytes was calculated as detailed (19). To determine the percentage of M6P-containing enzyme, 500 μl of serum was loaded onto a 1 ml column containing the cationic independent M6PR coupled to Sepharose (7). The column was washed with 5 ml of PBS, then with 5 ml of 5 mM glucose 6-phosphate in PBS, and M6P-containing proteins were eluted with 5 ml of 10 mM M6P in PBS. The total enzyme activity in each fraction was then determined.
Results
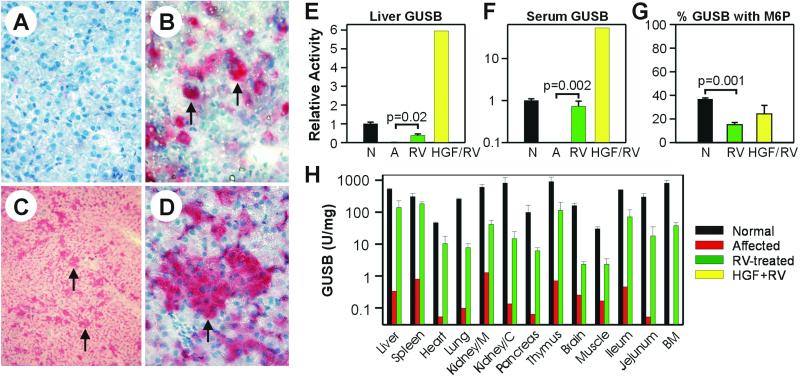
Five “RV-treated” MPS VII puppies received an i.v. injection of amphotropic RV within 3 days after birth (Table 1), as reported (19). Two “HGF/RV-treated” MPS VII dogs received human HGF, which induces replication of canine hepatocytes (21), followed by a 3-fold higher dose of RV than was given to the RV-treated dogs (Table 1). Histochemical staining for GUSB demonstrated that untreated affected dogs had no detectable activity (not shown), whereas activity was low in hepatocytes from normal animals (Fig. 1A). An average of 2.8% ± 0.4% (SEM) of hepatocytes from the RV-treated dogs had high GUSB activity at 4 months after treatment, and were presumably transduced (19). For the two HGF/RV-treated dogs, 10.1% and 19.4% of all hepatocytes were transduced at 1 week (Fig. 1B) and 4 months (Fig. 1 C and D), respectively. The large clusters present at 4 months likely derived from clonal expansion of transduced hepatocytes during normal growth. At 4 months, GUSB activity in liver homogenates was 182 ± 38 units/mg (38% of normal) for RV-treated dogs, and 2,830 units/mg (6-fold normal) for an HGF/RV-treated dog (Fig. 1E). Although the percentage of transduced hepatocytes was 7-fold and the liver GUSB activity 12-fold higher for the HGF/RV-treated than for the RV-treated dogs at 4 months, only one HGF/RV-treated dog was evaluated, and it is unclear whether the higher vector dose or the preadministration of HGF was responsible for the higher expression. Previously, prior administration of HGF resulted in a very modest and statistically insignificant increase in the percentage of neonatal hepatocytes that were transduced with an RV, although small numbers of animals were analyzed (19). Studies in additional animals will need to be performed to determine whether HGF potentiates transduction of hepatocytes from neonatal MPS VII dogs with an MLV-based vector.
Figure 1.
Expression and secretion of GUSB. (A–D) In situ GUSB staining of liver sections. Frozen liver sections were stained for GUSB activity (red cytoplasm) and counterstained with hematoxylin (blue nuclei). (A) Normal. Very little enzyme activity was present in hepatocytes after 1 h of staining. (Original magnification: 60×.) (B) HGF/RV-treated MPS VII dog at 5 days after treatment. The MPS VII dog M1288 was treated with HGF before the injection of RV as detailed in Table 1, and killed at 5 days after transduction. The arrows indicate transduced hepatocytes with high levels of enzyme activity in a 1-h stain. (Original magnification: 60×.) (C and D) HGF/RV-treated dog at 4 months. Liver biopsy of the HGF/RV-treated dog M1287 4 months after transduction. The arrows indicate clusters of hepatocytes with high levels of enzyme activity after a 25-min stain. (Original magnification: C, 10×; D, 60×.) (E) Liver GUSB. Livers from homozygous normal (N; n = 9), untreated affected MPS VII dogs (A; n = 3), RV-treated MPS VII dogs at 4 months (RV; n = 5), or an HGF/RV-treated MPS VII dog (HGF/RV; n = 1) at 4 months after transduction were homogenized and the GUSB-specific activity determined. The activity relative to that of homozygous normal dogs was determined and plotted as the average ± SEM. The P value for comparison of activity in affected and RV-treated dogs is shown. (F) Serum GUSB. Serum GUSB activity was measured 6 months after transduction and the activity relative to that in homozygous normal dogs was determined. (G) Percent GUSB with M6P. The percentage of GUSB that contained M6P for serum samples obtained at 1–6 months after birth for normal (N; n = 7), RV-treated dogs (RV; n = 11 because of analysis of samples at two or three times for each animal), or HGF/RV-treated dogs (HGF/RV; n = 2). (H) Organ GUSB activity. Organs from two homozygous normal dogs (black bars), one untreated affected dog (red bars), and two RV-treated MPS VII dogs (M1312 and M1339; green bars) at 6 months after birth were homogenized and the GUSB specific activity determined. Kidney/M is kidney medulla, Kidney/C is kidney cortex, and BM is bone marrow.
We next tested whether the liver secreted enzyme with M6P. Serum GUSB activity was 195 ± 36 units/ml (73% of normal) for the RV-treated group at 6 months (Fig. 1F) and was stable thereafter in three dogs (Table 1). For the HGF/RV-treated dogs, serum GUSB activity rose rapidly, and was 2,203 ± 51 units/ml (8.3-fold normal) at 2 days and 8,861 ± 244 units/ml (33-fold normal) at 5 days. The one HGF/RV-treated animal that was followed longer maintained stable levels of GUSB activity at 18,039 units/ml (67-fold normal) at 17 months. In normal dogs, 37% ± 4% of the serum GUSB contained M6P (Fig. 1G). Although this value was significantly lower (P = 0.001) for the RV-treated dogs, 16% ± 3% of their serum GUSB was mannose 6-phosphorylated. For the HGF/RV-treated dog, 24% of the serum GUSB contained M6P. GUSB activity was present in all organs at 1.5% to 60% of normal values for two RV-treated dogs that were killed at 6–7 months after transduction (Fig. 1H). Activity was probably not due to contamination with blood, because the animals were perfused with saline before the collection of tissues. The activity in spleen may derive in part from expression, because RV DNA sequences were identified in the spleen shortly after i.v. injection of an MLV-based RV into newborn dogs (19). However, activity in other organs was likely due to uptake from blood, because in our previous study 0.6% or fewer of the cells from other organs were transduced after i.v. injection of a higher dose of RV than was used here (19).
Growth, Gait, Joints, and Bones.
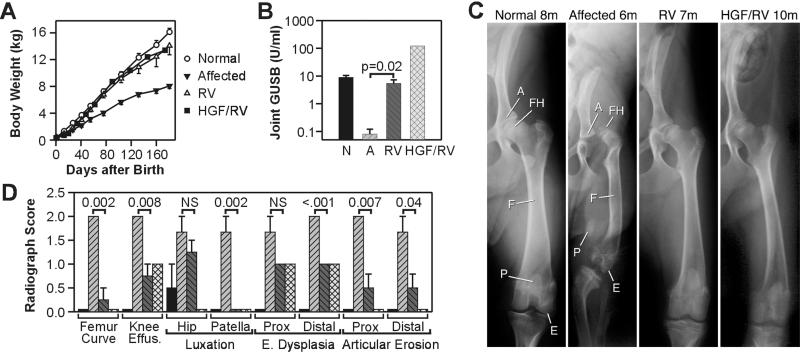
The average body weight of untreated affected dogs was only 50% that of normal littermates at 6 months (Fig. 2A; P < 0.0001 for affected vs. normal), whereas the weights of RV-treated dogs were 87% of normal (not significant for RV-treated vs. normal), and 1.7-fold that of untreated affected dogs (P = 0.003 for RV-treated vs. untreated dogs). The weight of the only HGF/RV-treated dog evaluated was 84% of normal at 6 months. Untreated affected dogs are unable to walk by 6 months, primarily because of orthopedic abnormalities, because neurological examinations are normal. In contrast, all transduced dogs remained able to run throughout the study period (6–17 months; see Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org, and Table 1). However, some treated animals had tarsal valgus (turning out of the hind foot) and mild to moderate gait abnormalities.
Figure 2.
Effect of RV-mediated gene therapy on growth, joints, and bones. (A) Growth curve. The body weights of six normal, five untreated MPS VII, five RV-treated MPS VII, and one HGF/RV-treated MPS VII dogs from the same three litters were plotted as the average ± SEM. (B) Synovial fluid GUSB. Synovial fluid was aspirated from the knee joint at 6–8 months after birth for normal (N; n = 7), 6–8 months for affected (A; n = 6), 6–8 months for RV-treated (RV; n = 7 because of inclusion of fluid from both knees in some animals), and 10 months for HGF/RV-treated (HGF/RV; n = 1) dogs. The averages ± SEM are shown. (C) Radiographs of the hind limb. Radiographs were obtained from a normal dog at 8 months (8m), an untreated affected dog at 6 months, an RV-treated MPS VII dog (RV; M1332) at 7 months, and the HGF/RV-treated MPS VII dog (HGF/RV; M1287) at 10 months. FH, femoral head; E, epiphysis; A, acetabulum; F, femur; P, patella. (D) Quantitation of radiograph changes. Radiographs of the upper leg from two normal dogs at 6–7 months, three untreated affected MPS VII dogs at 6–7 months, four RV-treated MPS VII dogs at 6–7 months, and one HGF/RV-treated MPS VII dog at 10 months were evaluated by a radiologist and scored as 0 (normal), 1 (mildly to moderately abnormal), or 2 (markedly abnormal), and plotted as the average ± SEM. Bars are shaded and appear in the same order as in B. The amount of curving of the femur (Femur Curve), effusion in the knee joint (Knee Effus.), luxation of the hip and of the patella, epiphyseal (E.) dysplasia of the proximal (Prox) and distal femur, and articular erosion of the proximal and distal femur are shown. The P values were obtained from statistical comparisons between values for untreated affected and RV-treated dogs.
On physical examination, all untreated affected dogs had effusions in their knee and elbow joints by 6 months, whereas the treated dogs had no effusions throughout the study period. Synovial fluid from the knee contained 5.4 ± 2 units/mg (60% of normal) of GUSB activity for the RV-treated dogs and 121.4 units/mg (13-fold normal) for the HGF/RV-treated dog (Fig. 2B). Radiographs of the hind leg of affected dogs at 6 months of age demonstrated that the femoral head and the distal femur have bony mottling that result from epiphyseal dysplasia, the femoral head is luxated (separated) from the acetabulum, the femur is curved and appears shorter than in the normal animals, and the patella is medially luxated (Fig. 2C). Radiographs of the RV- and HGF/RV-treated dogs had fewer abnormalities than the untreated affected dogs (Fig. 2 C and D), although some manifestations of orthopedic disease in the hind leg were not prevented, and there was no amelioration of abnormalities in the cervical spine (not shown). These orthopedic improvements are likely to be responsible for the ability of the treated MPS VII dogs to walk.
Ocular Disease.
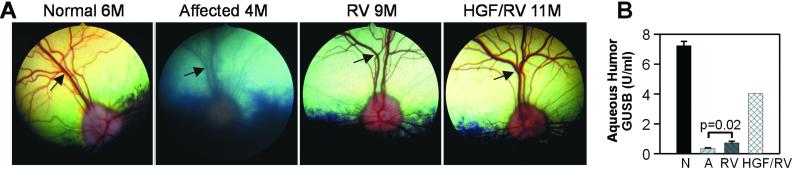
Lysosomal storage pathology occurs in the corneal stroma of affected dogs, which results in a cloudy ground-glass appearance (not shown) and blurring of the fundus image (Fig. 3A) by 4 months. In contrast, the fundi appeared sharp in all three RV-treated dogs and one HGF/RV-treated dog evaluated at 8–11 months (Fig. 3A), and little or no corneal clouding was present (not shown). The prevention of corneal clouding is likely due to uptake of enzyme from blood via the prelimbic capillaries by the corneal stromal cells (9, 22). The aqueous humor from RV-treated dogs had 0.7 units/ml of GUSB activity (10% of normal; Fig. 3B), whereas the aqueous humor from the HGF/RV-treated dog had 4 units/ml (56% of normal). Enzyme in the aqueous humor might reduce lysosomal storage material in the endothelium of the cornea (23).
Figure 3.
Effect of RV-mediated gene therapy on the eye. (A) Fundus. The fundus of a normal dog (Normal) at 6 months (6M), an untreated MPS VII dog (Affected) at 4 months, an RV-treated MPS VII dog (RV; M1332) at 8 months, and the HGF/RV-treated MPS VII dog (HGF/RV; M1287) at 11 months. Arrows identify the blood vessels in the retina, which are indistinct in the affected dog. (B) Aqueous humor GUSB. Aqueous humor was aspirated from normal dogs at 6–10 months (n = 5), untreated affected dogs at 6–7 months (n = 4), RV-treated dogs at 6–10 months (n = 5), and the HGF/RV-treated dog at 13 months (n = 1) and the GUSB activity in units/ml determined and plotted as the average ± SEM.
Heart Disease.
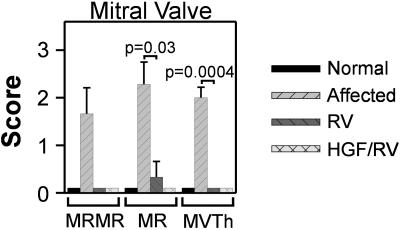
Accumulation of glycosaminoglycans (GAGs) in the heart values can result in cardiac disease, which is a major cause of death in MPS patients (1). By 8–9 months, 50% of untreated MPS VII dogs had murmurs of mitral valve regurgitation (MR), most had echocardiographic evidence of MR, and all had mitral valve thickening (Fig. 4). For the three RV-treated dogs evaluated at 9 months, none had murmurs, only one had mild MR, and none had mitral valve thickening. The HGF/RV-treated MPS VII dog had no cardiac abnormalities at 12 months. All treated dogs evaluated had normal thickness of the tricuspid, aortic, and pulmonary valves, no aortic valve insufficiency, and the aortic diameter was within normal limits. These parameters are usually abnormal in untreated affected dogs by this age (24).
Figure 4.
Effect of RV-mediated gene therapy on the mitral valve. Echocardiograms were evaluated at 8–9 months after birth for normal (n = 2), untreated affected (n = 5), and RV-treated dogs (n = 3), and at 12 months for the HGF/RV-treated dog (n = 1). The intensity of a murmur of mitral valve regurgitation (MRMR) was scored from 0 (none) to 5 (most severe). The severity of mitral valve regurgitation (MR) on echocardiography was scored from 0 (normal) to 3 (severe regurgitation), and the degree of mitral valve thickening on echocardiography was scored from 0 (normal) to 3 (markedly thickened). The P value obtained from statistical comparisons between the untreated affected and the RV-treated MPS VII dogs is shown.
Discussion
MPS VII and related disorders might be treated in three ways: (i) ERT; (ii) bone marrow transplantation (BMT); and (iii) gene therapy. ERT has reduced many of the clinical manifestations of MPS in animals and humans (9–13), but is inconvenient to administer and is extremely expensive. BMT has lessened the clinical manifestations of MPS VII in mice (reviewed in ref. 9), and MPS in large animals (24–26) and humans (27–29), but has substantial risks for patients and a compatible donor is often not available. Gene therapy of hematopoietic cells has improved pathology in mice (30, 31), but has not been effective in large animals (32, 33).
Most systemic gene therapy approaches for MPS VII (reviewed in ref. 34) were designed to allow modified cells to secrete enzyme into the blood. Hepatic gene therapy with MLV-based RV (17), lentiviral-based RV (35), adeno-associated virus (AAV) vectors (36–38), or adenoviral vectors (39, 40) allowed the liver to secrete the large (≈300 kDa) GUSB protein into the blood in mice, and resulted in substantial clinical benefit. Gene therapy of muscle with AAV vectors (34), or with fibroblasts using different approaches (41, 42), resulted in lower serum GUSB activity than liver-directed gene therapy in mice. For the RV-treated dogs, GUSB activity in liver, serum, and other organs was similar to the highest long-term (>6 months) expression reported previously in MPS VII mice with gene therapy (37). The GUSB activity in the HGF/RV-treated dog exceeded that reported previously in any animal model. In contrast, neonatal i.v. injection of an AAV vector expressing cGUSB or hGUSB from the CMV-β-actin promoter failed to result in significant levels of expression in MPS VII dogs for reasons that are unclear (M. Sands and M.E.H., unpublished data). Although ex vivo gene therapy of fibroblasts resulted in low-level expression and improvements in pathology in the liver in MPS VII dogs, there was no improvement in clinical features (43).
In this study, the average mannose 6-phosphorylated-GUSB in serum was 32 units/ml for the RV-treated dogs. Because the plasma volume in liters is 4% of the body weight in kg, and the half-life of lysosomal enzymes in blood of large animals (10, 11) or humans (13–15) is less than 1 h, it can be calculated that at least 640 units of phosphorylated GUSB activity per kg is secreted into the blood every hour, or 107,520 units/kg/week in the RV-treated dogs. This is similar to the dose used for ERT in humans with MPS I (125,000 units/kg/week; ref. 13). The HGF/RV-treated dog is calculated to secrete 12,000,000 units/kg/week. No antibodies were produced to the cGUSB (not shown), and the absence of a cytotoxic T lymphocyte response was inferred from the stable expression from the liver. The lack of an immune response may be because wild-type GUSB differs at only one position from the mutant protein. A single amino acid change should allow most of the epitopes of the protein to be presented during development and result in tolerization. Alternatively, this lack may be due to performing gene therapy at an age when the immune system was relatively immature.
Implications for Gene Therapy.
The prevention of abnormalities in growth, mobility, and many bone manifestations resemble what was observed when BMT or high-dose ERT were performed relatively early in life in MPS VII mice (9), or in large animals (10–12) and humans (13) with MPS. The failure to prevent some aspects of bone disease was seen with all treatments, and may be due to abnormal bone formation in utero or the inability of GUSB to reach some sites. Our gene therapy approach almost completely prevented cardiovascular disease, a major cause of death in patients with MPS, and corneal clouding was markedly reduced. In contrast, some animals and patients that receive ERT or BMT have only partial reductions in cardiovascular disease and corneal clouding (13, 24–29, 44), which may be due in part to the later age of treatment. Two RV-treated dogs had low (1.5% of normal) GUSB enzyme activity and reductions in cytoplasmic vaculation in the brain at 6 months (K.P.P. and M.E.H., unpublished work), and all treated dogs appeared to be neurologically normal throughout the study period. The enzyme activity in the brain may have been taken up from blood before the formation of the blood—brain barrier, because in MPS VII mice enzyme can reach the brain if injected within 2 weeks after birth (9). We remain concerned, however, that this systemic gene therapy approach may not result in sustained improvements in the pathological manifestations of MPS VII in the brain. Brain pathology and neurological function will need to be evaluated long-term in these dogs.
The clinical effect on heart, joint, and eye abnormalities was similar for the RV- and HGF/RV-treated dogs, suggesting that achieving near-normal serum GUSB levels at an early age is sufficient to prevent these manifestations. Neonatal humans should have sufficient hepatocyte replication for gene transfer, because they grow rapidly. Although currently most patients with MPS are not diagnosed at birth, implementation of newborn screening (45) may help to identify newborns with LSD in the future. We are also testing whether gene transfer into older MPS VII dogs can be achieved. This will likely require HGF or some other treatment to augment hepatocyte replication, because hepatocyte replication is very low in adult animals (17), and only 2.3% of hepatocytes were replicating at 6 days after birth in dogs (19). Although the risks of germline transmission and insertional mutagenesis are low, we will analyze animals for vector sequences in germ cells, and for neoplasia. Previously, we did not detect RV sequences in the gonads following neonatal gene transfer (19). If no adverse effects are observed, this approach may be an effective and simple treatment for patients with LSD, deficiencies of blood proteins such as hemophilia, or deficiencies of other liver proteins.
Supplementary Material
Acknowledgments
We thank Paula Henthorn for the canine GUSB cDNA, Stuart Kornfeld and Walter Gregory for assistance with the mannose 6-phosphate receptor column experiments, William Sly, Stuart Kornfeld, and Mark Sands for discussions of the manuscript, James Hayden for radiograph illustrations, and veterinary students and the University Laboratory Animal Resources staff for assistance with animal care. This work was supported by National Institutes of Health Grants DK54061 and K02 DK02575 (to K.P.P.), DK54481 and RR02512 (to M.E.H.), and DK46637 and Training Grant RR07063 (to J.H.W.), and Washington University Digestive Diseases Research Core Center Grant P30 52574.
Abbreviations
- GUSB
β-glucuronidase
- cGUSB
canine GUSB
- M6P
mannose 6-phosphate
- MPS
mucopolysaccharidosis
- LSD
lysosomal storage disease
- ERT
enzyme replacement therapy
- HGF
hepatocyte growth factor
- RV
retroviral vector
- MLV
Moloney murine leukemia virus
- MR
mitral regurgitation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Neufeld E F, Meunzer J. In: Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 2001. pp. 3421–3452. [Google Scholar]
- 2.Haskins M E, Giger U. In: Clinical Biochemistry of Domestic Animals. Kaneko J J, Harvey J W, Bruss M L, editors. New York: Academic; 1997. pp. 741–761. [Google Scholar]
- 3.Meikle P J, Hopwood J J, Claue A E, Carey W F. J Am Med Assoc. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 4.Sly W S, Quinton B A, McAlister W H, Rimoin D L. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 5.Haskins M E, Desnick R J, DiFerrante N, Jezyk P F, Patterson D F. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Ray J, Bouvet A, DeSanto C, Fyfe J C, Xu D, Wolfe J H, Aguirre G D, Patterson D F, Haskins M E, Henthorn P S. Genomics. 1998;48:248–253. doi: 10.1006/geno.1997.5189. [DOI] [PubMed] [Google Scholar]
- 7.Sands M S, Vogler C A, Ohlemiller K K, Roberts M S, Grubb J H, Levy B, Sly W S. J Biol Chem. 2001;276:43160–43165. doi: 10.1074/jbc.M107778200. [DOI] [PubMed] [Google Scholar]
- 8.Kornfeld S. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 9.Vogler C, Barker J, Sands M S, Levy B, Galvin N, Sly W S. Pediatr Dev Pathol. 2001;4:421–433. doi: 10.1007/s10024001-0079-1. [DOI] [PubMed] [Google Scholar]
- 10.Shull R M, Kakkis E D, McEntee M F, Kania S A, Jonas A J, Neufeld E F. Proc Natl Acad Sci USA. 1994;91:12937–12941. doi: 10.1073/pnas.91.26.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawley A C, Brooks D A, Muller V J, Petersen B A, Isaac E L, Bielicki J, King B M, Boulter C D, Moore A J, Fazzalari N L, et al. J Clin Invest. 1996;97:1864–1873. doi: 10.1172/JCI118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakkis E D, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson C W, O'Malley T, Weil M A, Aguirre G A, et al. Mol Genet Metab. 2001;72:199–208. doi: 10.1006/mgme.2000.3140. [DOI] [PubMed] [Google Scholar]
- 13.Kakkis E D, Muenzer J, Tiller G E, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, et al. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 14.Eng C M, Guffon N, Wilcox W R, Germain D P, Lee P, Waldek S, Caplan L, Linthorst G E, Desnick R J. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 15.Van den Hout J M, Reuser A J, de Klerk J B, Arts W F, Smeitink J A, Van der Ploeg A T. J Inherited Metab Dis. 2001;24:266–274. doi: 10.1023/a:1010383421286. [DOI] [PubMed] [Google Scholar]
- 16.Ponder K P. Trends Cardiovasc Med. 1999;9:158–162. doi: 10.1016/s1050-1738(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 17.Gao C, Sands M S, Haskins M E, Ponder K P. Mol Ther. 2000;2:233–244. doi: 10.1006/mthe.2000.0121. [DOI] [PubMed] [Google Scholar]
- 18.VandenDriessche T, Vanslembrouck V, Goovaerts I, Zwinnen H, Vanderhaeghen M L, Collen D, Chuah M K. Proc Natl Acad Sci USA. 1999;96:10379–10384. doi: 10.1073/pnas.96.18.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Haskins M E, Gao C, Weil M A, O'Malley T M, Melniczek J R, O'Donnell P A, Mazrier H, Ellinwood N M, Ponder K P. Mol Ther. 2002;5:141–153. doi: 10.1006/mthe.2002.0527. [DOI] [PubMed] [Google Scholar]
- 20.Gao C, Jokerst R, Cai S R, Kennedy S C, Flye M W, Ponder K, P. Hepatology. 1999;30:1405–1416. doi: 10.1002/hep.510300602. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi Y, Hamanoue M, Ueno S, Aikou T, Tanabe G, Mitsue S, Matsumoto K, Nakamura T Y. Biochem Biophys Res Commun. 1996;220:7–12. doi: 10.1006/bbrc.1996.0347. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Davidson B L. Proc Natl Acad Sci USA. 1995;92:7700–7704. doi: 10.1073/pnas.92.17.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollard R J, Telegan P, Haskins M E, Aguirre G. Cornea. 1996;15:25–35. [PubMed] [Google Scholar]
- 24.Sammarco C, Weil M, Just C, Weimelt S, Hasson C, O'Malley T, Evans S M, Wang P, Casal M L, Wolfe J, Haskins M. Bone Marrow Transplant. 2000;25:1289–1297. doi: 10.1038/sj.bmt.1702448. [DOI] [PubMed] [Google Scholar]
- 25.Breider M A, Shull R M, Constantopoulos G. Am J Pathol. 1989;134:677–692. [PMC free article] [PubMed] [Google Scholar]
- 26.Norrdin R W, Simske S J, Gaarde S, Schwardt D D, Thrall M A. Bone. 1995;17:485–489. doi: 10.1016/8756-3282(95)00333-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly W S, Kondo N, Orii T. Bone Marrow Transplant. 1998;21:629–634. doi: 10.1038/sj.bmt.1701141. [DOI] [PubMed] [Google Scholar]
- 28.Vellodi A, Young E, Cooper A, Lidchi V, Winchester B, Wraith J E A. J Inherit Metab Dis. 1999;22:638–648. doi: 10.1023/a:1005525931994. [DOI] [PubMed] [Google Scholar]
- 29.Whitley C B, Belani K G, Chang P N, Summers C G, Blazar B R, Tsai M Y, Latchaw R E, Ramsay N K, Kersey J H. Am J Med Genet. 1993;46:209–218. doi: 10.1002/ajmg.1320460222. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe J H, Sands M S, Barker J E, Gwynn B, Rowe L B, Vogler C A, Birkenmeier E H. Nature (London) 1992;360:749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
- 31.Marechal V, Naffakh N, Danos O, Heard J M. Blood. 1993;82:1358–1365. [PubMed] [Google Scholar]
- 32.Lutzko C, Kruth S, Abrams-Ogg A C, Lau K, Li L, Clark B R, Ruedy C, Nanji S, Foster R, Kohn D, et al. Blood. 1999;93:1895–1095. [PubMed] [Google Scholar]
- 33.Simonaro C M, Haskins M E, Abkowitz J L, Brooks D A, Hopwood J J, Zhang J, Schuchman E H. Gene Ther. 1999;6:107–113. doi: 10.1038/sj.gt.3300797. [DOI] [PubMed] [Google Scholar]
- 34.Sands M S, Wolfe J H, Birkenmeier E H, Barker J E, Vogler C, Sly W S, Okuyama T, Freeman B, Nicholes A, Muzyczka N, et al. Neuromuscular Disord. 1997;7:352–360. doi: 10.1016/s0960-8966(97)00061-8. [DOI] [PubMed] [Google Scholar]
- 35.Stein C S, Kang Y, Sauter S L, Townsend K, Staber P, Derksen T A, Martins I, Qian J, Davidson B L, McCray P B., Jr Mol Ther. 2001;3:850–856. doi: 10.1006/mthe.2001.0325. [DOI] [PubMed] [Google Scholar]
- 36.Watson G L, Sayles J N, Chen C, Elliger S S, Elliger C A, Raju N R, Kurtzman G J, Podsakoff G M G. Gene Ther. 1998;5:1642–1649. doi: 10.1038/sj.gt.3300775. [DOI] [PubMed] [Google Scholar]
- 37.Daly T M, Ohlemiller K K, Roberts M S, Vogler C A, Sands M S. Gene Ther. 2001;8:1291–1298. doi: 10.1038/sj.gt.3301420. [DOI] [PubMed] [Google Scholar]
- 38.Daly T M, Vogler C, Levy B, Haskins M E, Sands M S. Proc Natl Acad Sci USA. 1999;96:2296–2300. doi: 10.1073/pnas.96.5.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosuga M, Takahashi S, Sasaki K, Li X K, Fujino M, Hamada H, Suzuki S, Yamada M, Matsuo N, Okuyama T. Mol Ther. 2000;1:406–413. doi: 10.1006/mthe.2000.0067. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi T, Watabe K, Uehara K, Sly W S, Vogler C, Eto Y. Proc Natl Acad Sci USA. 1997;94:1287–1292. doi: 10.1073/pnas.94.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moullier P, Bohl D, Heard J M, Danos O. Nat Genet. 1993;4:154–159. doi: 10.1038/ng0693-154. [DOI] [PubMed] [Google Scholar]
- 42.Ross C J, Bastedo L, Maier S A, Sands M S, Chang P L. Hum Gene Ther. 2000;11:2117–2127. doi: 10.1089/104303400750001426. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe J H, Sands M S, Harel N, Weil M A, Parente M K, Polesky A C, Reilly J J, Hasson C, Weimelt S, Haskins M E. Mol Ther. 2000;2:552–561. doi: 10.1006/mthe.2000.0202. [DOI] [PubMed] [Google Scholar]
- 44.Gullingsrud E O, Krivit W, Summers C G. Ophthalmology. 1998;105:1099–1105. doi: 10.1016/S0161-6420(98)96014-6. [DOI] [PubMed] [Google Scholar]
- 45.Meikle P J, Ranieri E, Ravenscroft E M, Hua C T, Brooks D A, Hopwood J J. Southeast Asian J Trop Med Public Health. 1999;30:104–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.