Abstract
The inability of transplanted cells to proliferate in the normal liver hampers cell therapy. We considered that oxidative hepatic DNA damage would impair the survival of native cells and promote proliferation in transplanted cells. Dipeptidyl peptidase-deficient F344 rats were preconditioned with whole liver radiation and warm ischemia–reperfusion followed by intrasplenic transplantation of syngeneic F344 rat hepatocytes. The preconditioning was well tolerated, although serum aminotransferase levels rose transiently and hepatic injury was observed histologically, along with decreased catalase activity and 8-hydroxy adducts of guanine, indicating oxidative DNA damage. Transplanted cells did not proliferate in the liver over 3 months in control animals and animals preconditioned with ischemia–reperfusion alone. Animals treated with radiation alone showed some transplanted cell proliferation. In contrast, the liver of animals preconditioned with radiation plus ischemia–reperfusion was replaced virtually completely over 3 months. Transplanted cells integrated in the liver parenchyma and liver architecture were preserved normally. These findings offer a paradigm for repopulating the liver with transplanted cells. Progressive loss of cells experiencing oxidative DNA damage after radiation and ischemia–reperfusion injury could be of significance for epithelial renewal in additional organs.
Keywords: oxidative damage‖hepatocyte
Liver repopulation with transplanted cells is of considerable interest for cell and gene therapy (1). Transplanted hepatocytes integrate in the liver parenchyma, function normally, and survive life-long (2–4). However, transplanted cells do not proliferate in the normal adult liver, whereas specific therapies require a significant transplanted cell mass. Proliferation in transplanted cells depends on whether native cells are at survival/proliferation disadvantages, as suggested by animal studies using exogenous toxins or natural disease, e.g., fumaryl acetoacetate hydroxylase (FAH) mice (hereditary tyrosinemia type-1), Long-Evans Cinnamon (LEC) rats (Wilson's disease), P-glycoprotein-2 (Pgy-2) mutant mice (progressive familial intrahepatic cholestasis), etc. (5–12). Initial clinical studies in familial hypercholesterolemia (FH) or Crigler–Najjar syndrome substantiated these principles (13, 14).
Genotoxic liver injury is a potent stimulus for transplanted cell proliferation. Rats exposed to retrorsine, a pyrrolizidine alkaloid, or whole liver radiation (RT), which produce DNA adducts and oxidative injury, respectively, lead to extensive transplanted cell proliferation in conjunction with two-thirds partial hepatectomy (PH) (15, 16). Although PH induces hepatic DNA synthesis, its additional effects include oxidative DNA damage, senescence-type changes, including p21 expression, polyploidy, attenuated proliferation capacity, and hepatocyte apoptosis (17–19). Both retrorsine and RT increase PH-induced hepatic polyploidy and apoptosis (20, 21). Moreover, the thyroid hormone T3, which regulates PH-induced polyploidy, substitutes for PH in retrorsine and RT-preconditioning for liver repopulation (22, 23). As neither retrorsine nor T3 (in the large doses required) is appropriate for clinical use, we examined whether hepatic preconditioning with RT and warm ischemia–reperfusion (IRP), which also activates oxidative stress (24), could be effective for liver repopulation.
Methods
Animals.
The Animal Care and Use Committee at Albert Einstein College of Medicine approved animal protocols. F344 donor rats were from the National Cancer Institute (Bethesda). The Special Animal Core of the Liver Research Center provided 6- to 8-week-old dipeptidyl peptidase IV (DPPIV)-deficient (DPPIV−) F344 rats. Hepatocytes were isolated by collagenase digestion and cells were used when trypan blue was excluded by >85% (5). Hepatocytes (5 × 106) were injected into the splenic pulp, which deposits hepatocytes into liver sinusoids (2–5). The whole liver was radiated to 10–50 Gy with an orthovoltage x-ray machine (Philips Super M 100, Philips Medical Systems, Bothell, WA), as reported (16). To induce IRP, the left portal vein branch was isolated and occluded with an aneurysm clip for 30 to 90 min (25).
Flow Cytometry.
Cell nuclei were isolated with detergent/trypsin and stained with 0.04% propidium iodide for analysis using FACStarplus (Becton Dickinson) (18, 19).
Biochemical Assays.
Chemicals were from Sigma. Samples from both ischemic and nonischemic liver lobes were stored at −70°C. DPPIV activity was measured by homogenizing 1:10 (wt/vol) tissues in 0.25 M sucrose in PBS, pH 7.4. Forty-microliter extracts were incubated with 900 μl of 75 mM glycine, pH 8.7 with NaOH/500 μg of Gly-Pro-p-nitroanilide hydrochloride at room temperature. The change in absorbance at 405 nm visible light was measured over 15 min. Purified p-nitroaniline was used as standard and data were normalized with protein content measured by the Bradford assay (Bio-Rad). To quantitate liver repopulation, F344 and DPPIV− rat liver homogenates were mixed together in various proportions. Data were expressed as μmol of p-nitroaniline per mg of tissue per min. Catalase activity was measured as described (26) and results were normalized to protein content. Each assay was in triplicate.
Tissue Analysis.
Samples from ischemic (median liver lobe) and nonischemic liver areas (right posterior lobe) were frozen to −70°C and 5-μm cryostat sections were made. To show oxidative DNA damage, sections were fixed in 70% ethanol and stained with anti-8-oxo-2′dG (Trevigen 4355-MC-100, Gaithersburg, MD). Sections were digested with RNase (100 μg/ml) for 1 hr at 37°C and DNA was denatured in 4 M HCl for 7 min followed by neutralization with 50 mM Tris base. Tissues were blocked with 10% FBS and incubated with 1:300 antibody at 37°C for 16 hr. Tissues were quenched with 3% H2O2 in methanol for 30 min, incubated with biotinylated multilink secondary antibody (BioGenex Laboratories, San Ramon, CA), and visualized with diaminobenzidine (DAKO) by using Vectastain ABC system (Vector Laboratories). A commercial kit was used to analyze apoptosis [terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay, Roche Molecular Biochemicals]. TUNEL-positive nuclei were counted under ×400 magnification in 20–30 random areas per tissue. Transplanted cells were localized in the liver with DPPIV histochemistry (2–5). F344 rat liver and untreated DPPIV− rat liver served as positive and negative controls, respectively. The number of transplanted cells was counted in 50 consecutive high-power fields (×200). Additional tissues were stained histochemically for DPPIV plus glycogen and glucose-6-phosphatase (3).
Blood Tests.
Serum was stored at −20°C and analyzed for total bilirubin, alanine aminotransferase, albumin, alkaline phosphatase, and lactate dehydrogenase with an automated clinical microsystem (Bayer-Chem-1, Bayer Corp. Diagnostics Division, Tarrytown, NY).
Statistical Analysis.
Data are expressed as means ± SD. Statistical analysis used Student's t test, χ2 test, Mann–Whitney U rank correlation test, one-way analysis of variance (ANOVA), and Dunn's test to isolate groups differing from others (Jandel Scientific, San Rafael, CA). P values <0.05 were considered significant.
Results
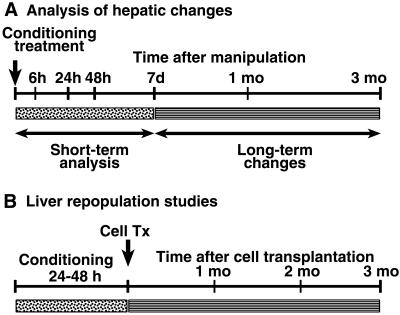
The experimental design is shown in Fig. 1. Multiple animal groups were established with 3–4 rats at each time-point studied.
Figure 1.
Experimental design. (A) Short-term analysis of animals extended for 7 days after manipulations and included serological, tissue, and cell-ploidy assays. Long-term analysis was for up to 3 months after preconditioning with cell-ploidy and tissue analysis involving untreated DPPIV− rats, and rats treated with 50 Gy of RT alone, IRP alone, and 50 Gy of RT plus IRP. (B) For liver repopulation, hepatocytes were transplanted within 24–48 hr after hepatic preconditioning followed by analysis after 1, 2, and 3 months. Animals received 50 Gy of RT alone, IRP alone, and 10, 20, 30, 40, and 50 Gy of RT plus IRP.
Analysis of Liver Repopulation.
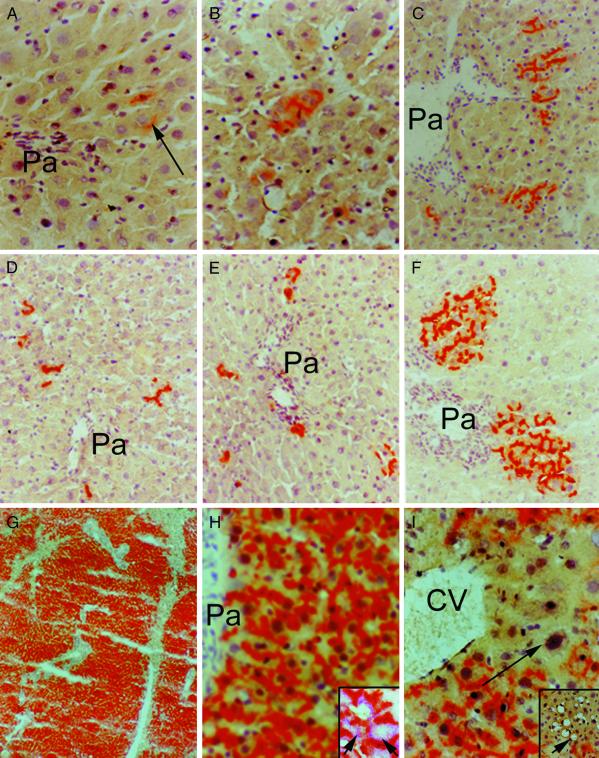
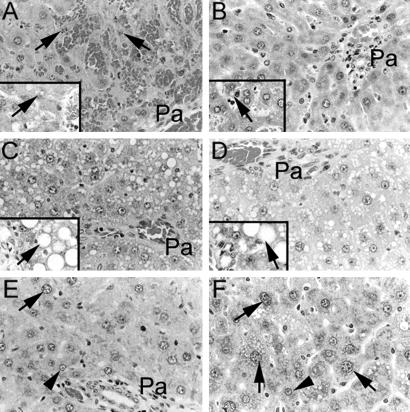
Transplanted cells were observed to engraft in animals after RT and/or IRP, including in ischemic and nonischemic liver lobes. After 2 weeks, animals conditioned with RT and IRP showed some transplanted cell proliferation, although this was generally limited. However, the situation was quite different after 3 months (Fig. 2). Control animals and animals conditioned with IRP alone showed clusters of 1–3 transplanted cells, whereas animals treated with 50 Gy of RT alone showed clusters of 5–15 transplanted cells. In contrast, animals subjected to 50 Gy of RT plus IRP showed extensive transplanted cell proliferation with virtually complete liver repopulation. Both ischemic and nonischemic liver lobes were repopulated in these animals. Transplanted cells displayed a reconstitution of bile canaliculi with normal glycogen and glucose-6-phosphatase content. Moreover, the liver architecture in repopulated areas was entirely normal, without any biliary proliferation or fibrosis, although unrepopulated perivenous areas of the liver lobule showed native hepatocytes with megalonuclei, steatosis, and apoptosis.
Figure 2.
Histological analysis of liver repopulation in DPPIV− rats 3 months after cell transplantation. (A) The liver of an animal without any preconditioning. The arrow points to a transplanted cell. Pa, portal area. Transplanted cells were arranged in clusters of one or two cells each and were observed in periportal areas only. (B) The effect of IRP alone on transplanted cell proliferation. Transplanted cells did not proliferate significantly and were arranged in cell clusters containing one or two cells. (C) An animal conditioned with 50 Gy of RT alone with some transplanted cell proliferation. (D–G) The effect of IRP plus 10, 20, 30, and 50 Gy of RT, respectively. The liver was virtually completely repopulated after preconditioning with IRP plus 50 Gy of RT. Both ischemic and nonischemic liver lobes were repopulated similarly. (H) A higher-magnification view of transplanted cells with normal liver architecture. The Inset shows dual stainings for DPPIV to localize transplanted cells and for glycogen (arrows) in the cytoplasm of transplanted cells. (I) The abnormal morphology in residual endogenous cells near a central vein (CV), with polyploid nuclei (arrow), as well as fatty change and areas of cell losses (Inset, arrow).
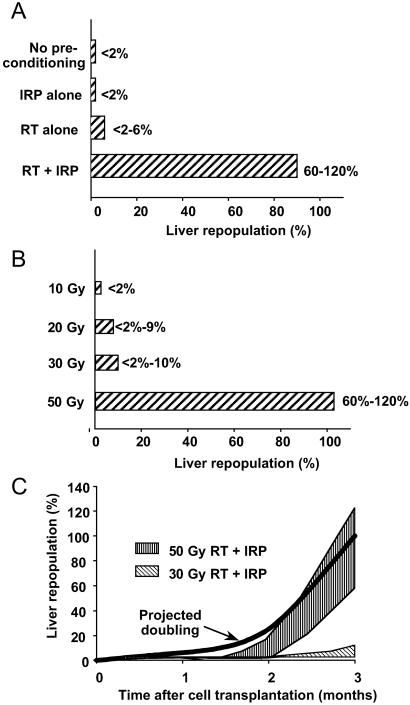
Mixtures of F344 and DPPIV− rat liver homogenates established that our biochemical assay of DPPIV activity successfully showed liver repopulation in the 2–100% range. This assay indicated that liver repopulation was <2% after 3 months in unconditioned control rats (Fig. 3A). Liver repopulation did not increase after preconditioning with IRP alone, although after 50 Gy of RT alone, liver repopulation increased on average to 8% (P < 0.05, Mann–Whitney U test). In contrast, liver repopulation in animals subjected to 50 Gy of RT plus IRP was 105% on average, (P < 0.001, t test). However, liver repopulation after RT plus IRP required at least 20–30 Gy, because liver repopulation did not increase after 10 Gy (Fig. 3B). This result indicated that a threshold of DNA damage was required for transplanted cell proliferation. Liver repopulation after RT and IRP was gradual in the first 8 weeks but accelerated 8–12 weeks after cell transplantation (Fig. 3C and Table 1). To determine this kinetics, we modeled how transplanted cell mass should increase during complete liver repopulation over 12 weeks (Table 1). Transplantation of 5 × 106 cells constitutes ≈1% of the rat hepatocyte mass (6 × 108 cells) (27). However, as only 20–30% of transplanted cells engraft in the liver, this should repopulate 0.2–0.3% of the liver. According to the analysis shown in Table 1, the transplanted cell mass should have doubled 9 times during 12 weeks. If transplanted cells proliferated at a fixed rate, doublings of transplanted cell masses should have required ≈9 days. The proliferation rates of transplanted cells in animals with 50 Gy of RT plus IRP approached those predicted by the model (Fig. 3C, Table 1), although cell proliferation rates were lower in animals conditioned with 30 Gy of RT plus IRP.
Figure 3.
Hepatic DPPIV activity. (A) The effect of preconditioning on liver repopulation at 3 months (means and ranges). IRP and RT combination was the most effective, and IRP alone was ineffective. (B) The effect of varying doses of RT with IRP for 90 min on liver repopulation at 3 months. Liver repopulation depended on the dose of radiation, with 50 Gy being the most effective. Both ischemic and nonischemic liver lobes were repopulated. (C) The kinetics of liver repopulation in animals conditioned with RT and IRP. The thick black line depicts projected doubling in transplanted cell mass. The shaded area toward the top shows the range of liver repopulation observed in animals treated with 50 Gy of RT and IRP. The hatched area toward the bottom shows the range of liver repopulation observed in animals treated with 30 Gy of RT and IRP.
Table 1.
Analysis of the kinetics of transplanted cell proliferation
| Time after cell transplantation, days | Projected liver repopulation, doublings needed | Observed liver repopulation with hepatic preconditioning, doublings
|
|
|---|---|---|---|
| 30 Gy + IRP | 50 Gy + IRP | ||
| Initial liver repopulation | 0.18% | 0.2% | 0.2% |
| 9 | 0.39% (1) | — | — |
| 18 | 0.78% (2) | — | — |
| 27 | 1.56% (3) | <2% (<3) | <2% (<3) |
| 36 | 3.13% (4) | — | — |
| 45 | 6.25% (5) | — | — |
| 54 | 12.5% (6) | — | — |
| 63 | 25% (7) | <2% (<3) | 2–20% (3–7) |
| 72 | 50% (8) | — | — |
| 81 | 100% (9) | 2–10% (3–6) | 60–120% (8–9) |
Hepatic Consequences of RT and IRP.
To establish the overall safety of RT and IRP, we analyzed liver tests and histology. RT alone did not affect liver tests significantly. IRP, either alone or with RT, resulted in increased serum alanine aminotransferase levels after 6 hr, with normal levels after 48 hr, whereas serum bilirubin, alkaline phosphatase, and albumin levels were unchanged (Table 2).
Table 2.
Changes in liver tests
| Time after treatment | Condition | Serum ALT, units/liter | Total serum bilirubin, mg/dl | Serum alkaline phosphatase, units/liter |
|---|---|---|---|---|
| 6 hr | RT | 35 ± 8 | 1 ± 0.1 | 242 ± 17 |
| IRP | 1,670 ± 1,300* | 0.9 ± 0.1 | 321 ± 21 | |
| IRP + RT | 2,947 ± 2,360* | 1 ± 0.4 | 358 ± 55 | |
| 24 hr | RT | 28 ± 24 | 1 ± 0.3 | 235 ± 26 |
| IRP | 725 ± 515* | 0.6 ± 0.04 | 260 ± 99 | |
| IRP + RT | 287 ± 303* | 0.6 ± 0.1 | 307 ± 76 | |
| 48 hr | RT | 19 ± 16 | 1 ± 0.07 | 194 ± 19 |
| IRP | 36 ± 23 | 0.6 ± 0.060 | 302 ± 57 | |
| IRP + RT | 36 ± 42 | 0.6 ± 0.1 | 236 ± 48 | |
| 7 days | RT | 73 ± 40 | 0.9 ± 0 | 140 ± 57 |
| IRP | 22 ± 7 | 0.6 ± 0.07 | 281 ± 204 | |
| IRP + RT | 16 ± 17 | 0.7 ± 0.1 | 182 ± 22 |
Reference values in control unmanipulated rats (n = 10): serum alanine aminotransferase (ALT), 41 ± 6 units/liter; total bilirubin, 0.3 ± 0.06 mg/dl; and alkaline phosphatase, 318 ± 94 units/liter RT was to 50 Gy; IRP was for 90 min.
, P < 0.05, t tests.
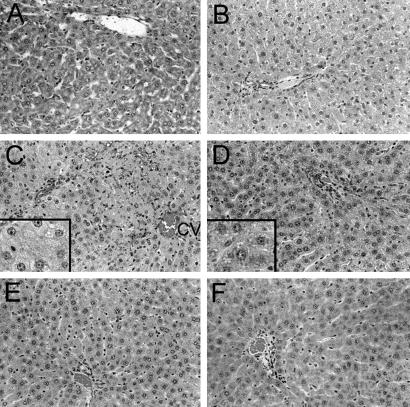
After RT alone, liver histology was normal both in the short term, and in the long term (Fig. 4). After IRP for 90 min, 30–50% of the liver lobules in ischemic liver lobes showed venous congestion and necrosis at 6 and 24 hr, although these findings were not observed in nonischemic liver lobes. On the other hand, steatosis was observed in both ischemic and nonischemic liver lobes. However, liver histology was normal seven days after IRP alone. Six and 24 hr after RT plus IRP, sinusoidal congestion, hepatocyte necrosis, and apoptosis were obvious in ischemic, but not in nonischemic liver lobes (Fig. 5). Hepatic steatosis was accelerated in both ischemic and nonischemic liver, with its onset in perivenous areas after 6 hr. Steatosis was observed throughout the liver after 24 hr and became more pronounced 48 hr and 7 days after RT and IRP, along with interspersed areas of cell apoptosis. One and 3 months after RT and IRP, polyploid hepatocytes were observed frequently in the liver, which was different from untreated control rats, and rats treated with RT or IRP alone. Hepatic polyploidy was observed in ischemic and nonischemic liver, again indicating that despite restriction of IRP to specific liver lobes, hepatic changes were global.
Figure 4.
Liver histology in F344 rats after either IRP or RT alone. (A) The normal liver from an untreated rat with a portal area (toward top). (B) The liver 1 month after 50 Gy of RT with no abnormalities. (C and D) The effects of IRP in ischemic liver lobe (C) and nonischemic liver lobe (D) after 24 hr, with areas of sinusoidal congestion and hepatocyte necrosis in the ischemic liver only (C), although widespread steatosis was present in both conditions (Insets). (E and F) Liver histology was normal after 7 days (not shown), 1 month (E, ischemic liver lobe; F, nonischemic liver lobe), and 3 months (not shown) after IRP.
Figure 5.
Liver changes after RT and IRP. A, C, and E are from ischemic liver and B, D, and F are from nonischemic liver. (A) The liver at 6 hr after treatments, showing sinusoidal congestion and hepatic necrosis in periportal area (Pa), as well as apoptotic nuclei (arrows) at the periphery of the ischemic area. (B) The nonischemic liver at 6 hr without sinusoidal congestion or necrosis. Insets in A and B show perivenous areas with mild fatty change. (C and D) Changes shown 24 hr after RT and IRP. Fatty change was widespread and became more pronounced after 48 hr and 7 days (Insets). (E and F) The livers from animals 3 months after RT and IRP with polyploid hepatocytes containing megalonuclei (arrows) in both conditions. Arrowheads indicate normal hepatocyte nuclei for size comparison. Fatty change was still evident in some hepatocytes.
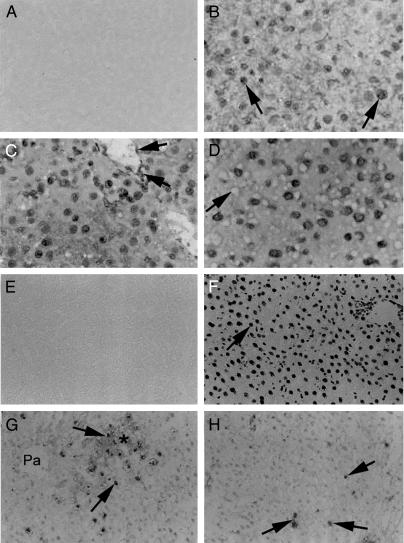
To verify that these changes were related to the onset of oxidative injury in the liver, additional studies were conducted. Hepatic catalase activity decreased after RT alone and IRP alone, in ischemic as well as nonischemic liver (mean 50% of controls, showing 18 ± 0.4 units per mg of protein per min), but became normal after 7 days. Catalase activity was depleted more after 50 Gy of RT plus IRP and remained low after 7 days (mean 30% of controls), which was in agreement with the onset of oxidative events in the liver. 8-Hydroxyguanine adducts were observed within 6 hr after RT or IRP (Fig. 6 A–D), with 70–100% of hepatocyte nuclei staining positive in all conditions. The hepatocyte fraction showing 8-hydroxyguanine adducts was similar in ischemic and nonischemic liver areas. In situ TUNEL showed activation of apoptosis after IRP and IRP plus RT (Fig. 6 E–H), whereas in the normal liver and after RT alone, apoptosis was infrequent (<0.01% of hepatocytes). Finally, the prevalence of polyploid cells was altered in the liver, such that considerably more polyploid cells accumulated 30 days after RT and IRP (data not shown).
Figure 6.
(A–D) Immunohistochemistry showing 8-hydroxyguanine adducts in liver 7 days after manipulations. (A) No staining in the liver when primary antibody was omitted. (B) Positive nuclear staining (arrow) in liver subjected to RT alone. (C) The effect of IRP alone in ischemic lobe. Nuclear staining was observed in hepatocytes, or vascular and nonparenchymal cells (arrows). (D) The effects of RT and IRP on an ischemic live lobe with nuclear staining. The fatty change is apparent as empty spaces (arrow). The nonischemic liver lobes from animals in C and D exhibited identical nuclear staining patterns. (E and F) TUNEL assays for hepatic apoptosis. (E) Negative control tissue with the omission of terminal deoxynucleotidyltransferase from the reaction. (F) DNase-treated positive control liver with extensive nuclear staining (arrow). (G) Shows effect of RT and IRP in ischemic liver after 6 hr. The asterisk indicates an area with ischemic necrosis. TUNEL-positive nuclei (arrows) were in proximity with ischemic areas. (H) Apoptosis 7 days after RT and IRP in ischemic liver with TUNEL-positive nuclei (arrows). Similar changes were observed in nonischemic liver areas.
Discussion
These studies established that hepatic preconditioning with the combination of RT and IRP was effective for inducing extensive proliferation of transplanted cells. The overall hepatic injury produced by RT plus IRP was modest and was well tolerated by animals. Acute liver injury was elicited by elevated serum alanine transferase levels, as well as by histological findings, although evidence for long-term perturbations in the liver, such as accumulation of polyploid cells, was much more subtle.
These findings significantly extend our ability to induce proliferation in transplanted liver cells, especially for clinical applications. Strategies to induce proliferation in transplanted cells for use in people are lacking, with a significant impact on cell therapy. For instance, patients with genetically determined abnormalities in serum lipid familial hypercholesterolemia (FH) or bilirubin levels (Crigler–Najjar syndrome) are amenable to liver cell therapy (13, 14). However, transplanted cells do not proliferate in these situations because the liver itself is not injured. Therefore, because of limited liver repopulation, cell therapy failed to cure patients with FH or Crigler–Najjar syndrome (13, 14). On the other hand, if transplanted cells would possess selective proliferation advantages, as shown here, far superior results could be achieved. In animals with chronic liver disease, loss of diseased native hepatocytes promotes survival and proliferation of healthy transplanted cells (8–11, 28). In some situations, as shown by studies in LEC rats and Pgy-2-knockout mice, proliferation of transplanted cells requires several months (12, 28), whereas the use of preconditioning regimens enormously accelerates liver repopulation with obvious improvements in cell therapy results. Early liver repopulation should be especially desirable in life-threatening situations, as exemplified by Crigler–Najjar syndrome and Wilson's disease, where irreversible brain damage can occur. Such findings should be especially applicable to parallel situations in patients, including for treating chronic viral hepatitis, although the latter will require imparting resistance to transplanted cells against specific viral infection (29). In this context, it is noteworthy that RT can be restricted to portions of the liver by conformal targeting, such that accidental damage to adjacent tissues is avoided (30). Similarly, hepatic IRP has been used safely for patients with chronic liver disease (31).
RT is well known to induce free radical-mediated oxidative DNA damage. Warm IRP too induces oxidative injury (24). Within 2 hr after IRP, reactive oxygen species are produced in activated Kupffer cells, followed within 6 hr by release of cytokines, e.g., interleukins and tumor necrosis factor α (TNF-α). Whether soluble signals, such as these, regulated induction of oxidative damage in nonischemic liver areas in our animals requires further study. The findings of early hepatotoxicity after IRP were in agreement with previous animal studies (25). However, the delayed hepatic consequences of RT and IRP over several months have not previously been established. Our data demonstrated that oxidative DNA damage led to significant perturbations in the liver with the eventual accumulation of polyploid cells. These findings were in agreement with the hypothesis that oxidative DNA injury is associated with attenuation of cellular proliferation and survival capacity (19). Therefore, development of this RT/IRP rat model should also facilitate study of molecular mechanisms regulating hepatic oxidative injury and testing of therapies for acute as well as chronic oxidative liver injury. Finally, as oxidative DNA damage is ubiquitously encountered in tissues, our findings will be applicable to epithelial renewal in additional organs.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK46952, P30-DK-41296, P30-CA13330, and MO1 RR12248.
Abbreviations
- IRP
ischemia–reperfusion
- PH
two-thirds partial hepatectomy
- DPPIV
dipeptidyl peptidase IV
- RT
whole liver radiation
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Malhi H, Gupta S. J Hepatobiliary Pancreat Surg. 2001;8:40–50. doi: 10.1007/s005340170049. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Rajvanshi P, Lee C-D. Proc Natl Acad Sci USA. 1995;92:5860–5864. doi: 10.1073/pnas.92.13.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Rajvanshi P, Sokhi R, Vaidya S, Irani A N, Gorla G R. J Biol Chem. 1999;274:2157–2165. doi: 10.1074/jbc.274.4.2157. [DOI] [PubMed] [Google Scholar]
- 4.Sokhi R P, Rajvanshi P, Gupta S. Am J Physiol. 2000;279:G631–G640. doi: 10.1152/ajpgi.2000.279.3.G631. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Rajvanshi P, Aragona E, Yerneni P R, Lee C-D, Burk R D. Am J Physiol. 1999;276:G629–G638. doi: 10.1152/ajpgi.1999.276.3.G629. [DOI] [PubMed] [Google Scholar]
- 6.Rhim J A, Sandgren E P, Degen J L, Palmiter R D, Brinster R L. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 7.Mignon A, Guidotti J E, Mitchell C, Fabre M, Wernet A, De La Coste A, Soubrane O, Gilgenkrantz H, Kahn A. Nat Med. 1998;4:1185–1188. doi: 10.1038/2681. [DOI] [PubMed] [Google Scholar]
- 8.Gagandeep S, Sokhi R, Slehria S, Gorla G R, Furgiuele J, DePinho R A, Gupta S. Mol Ther. 2000;1:358–365. doi: 10.1006/mthe.2000.0051. [DOI] [PubMed] [Google Scholar]
- 9.Braun K M, Degen J L, Sandgren E P. Nat Med. 2000;6:320–326. doi: 10.1038/73179. [DOI] [PubMed] [Google Scholar]
- 10.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou C N, Finegold M, Grompe M. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 11.Irani A N, Malhi H, Slehria S, Gorla G R, Volenberg I, Schilsky M L, Gupta S. Mol Ther. 2001;3:302–309. doi: 10.1006/mthe.2001.0271. [DOI] [PubMed] [Google Scholar]
- 12.De Vree J M, Ottenhoff R, Bosma P J, Smith A J, Aten J, Oude Elferink R P. Gastroenterology. 2000;119:1720–1730. doi: 10.1053/gast.2000.20222. [DOI] [PubMed] [Google Scholar]
- 13.Grossman M, Rader D J, Muller D W M, Kolansky D M, Kozarsky K, Clark B J, III, Stein E A, Lupien P J, Brewer H B, Jr, Raper S E, Wilson J M. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 14.Fox I J, Chowdhury J R, Kaufman S S, Goertzen T C, Chowdhury N R, Warkentin P I, Dorko K, Sauter B V, Strom S C. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 15.Laconi E, Oren R, Mukhopadhyay D K, Hurston H, Laconi S, Pani P, Dabeva M, Shafritz D A. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guha C, Sharma A, Gupta S, Alfieri A, Gorla G R, Gagandeep S, Sokhi R, Roy-Chowdhury N, Tanaka K E, Vikram B, Roy-Chowdhury J. Cancer Res. 1999;59:5871–5874. [PubMed] [Google Scholar]
- 17.Michalopoulos G K, DeFrances M C. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 18.Sigal S H, Rajvanshi P, Gorla G R, Saxena R, Sokhi R P, Gebhardt D F, Jr, Reid L M, Gupta S. Am J Physiol. 1999;276:G1260–G1272. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- 19.Gorla G R, Malhi H, Gupta S. J Cell Sci. 2001;114:2943–2951. doi: 10.1242/jcs.114.16.2943. [DOI] [PubMed] [Google Scholar]
- 20.Gordon G J, Coleman W B, Grisham J W. Hepatology. 2000;32:312–320. doi: 10.1053/jhep.2000.9144. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S. Semin Cancer Biol. 2000;10:161–171. doi: 10.1006/scbi.2000.0317. [DOI] [PubMed] [Google Scholar]
- 22.Oren R, Dabeva M D, Karnezis A N, Petkov P M, Rosencrantz R, Sandhu J P, Moss S F, Wang S, Hurston E, Laconi E, et al. Hepatology. 1999;30:903–913. doi: 10.1002/hep.510300418. [DOI] [PubMed] [Google Scholar]
- 23.Torres S, Diaz B P, Cabrera J J, Diaz-Chico J C, Diaz-Chico B N, Lopez-Guerra A. Am J Physiol. 1999;276:G155–G163. doi: 10.1152/ajpgi.1999.276.1.G155. [DOI] [PubMed] [Google Scholar]
- 24.Lentsch A B, Kato A, Yoshidome H, McMasters K M, Edwards J M. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 25.Frederiks W M, James J, Bosch K S, Schroder M J R, Schuyt H C. Exp Pathol (Jena) 1982;22:245–252. doi: 10.1016/s0232-1513(82)80015-7. [DOI] [PubMed] [Google Scholar]
- 26.Gorla G R, Malhi H, Gupta S. J Cell Sci. 2001;114:2943–2951. doi: 10.1242/jcs.114.16.2943. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Rajvanshi P, Sokhi R P, Slehria S, Yam A, Kerr A, Novikoff P M. Hepatology. 1999;29:509–519. doi: 10.1002/hep.510290213. [DOI] [PubMed] [Google Scholar]
- 28.Malhi H, Irani A N, Volenberg I, Schilsky M L, Gupta S. Gastroenterology. 2002;122:438–447. doi: 10.1053/gast.2002.31086. [DOI] [PubMed] [Google Scholar]
- 29.Das S, Ott M, Yamane A, Tsai W, Gromeier M, Lahser F, Gupta S, Dasgupta A. J Virol. 1998;72:5638–5647. doi: 10.1128/jvi.72.7.5638-5647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunderson L L, Haddock M G, Foo M L, Todoroki T, Nagorney D. Ann Oncol. 1999;10, Suppl. 4:221–225. [PubMed] [Google Scholar]
- 31.Nagasue N, Uchida M, Kubota H, Hayashi T, Kohno H, Nakamura T. Eur J Surg. 1995;161:181–186. [PubMed] [Google Scholar]