Abstract
A technique for genetic modification of hair follicles was developed which results in efficient alteration of the hair shaft phenotype. High-level in vivo transgene expression was maintained in hair follicles such that growing hair shafts were phenotypically altered. Mouse anagen skin fragments, maintained in histoculture, were genetically modified at high efficiency with adenoviral-GFP. The histocultured skin fragments were treated with collagenase which made hair follicles accessible to the adenoviral GFP gene, allowing high-efficiency transduction. These skin fragments were subsequently grafted on to nude mice where GFP was readily visualized in as many as 75% of hair follicles. Most follicles produced GFP-fluorescent growing hair shafts. This technique has produced efficient genetic modification of the hair shaft.
Keywords: gene transfer‖histoculture‖collagenase‖green fluorescent protein
During growth, hair follicles cycle through three major stages: anagen, the growth phase; catagen, the involution phase; and telogen, the resting phase (1). These phases are regulated by specific molecular signals (2–4) whose effects include stimulating epithelial stem cells to regenerate the follicles (5, 6). At the onset of each new anagen, stem cells (largely in the “bulge area” of the hair follicle) proliferate and give rise to progenitor matrix cells. In turn, these generate the hair shaft and its surrounding layers (4). The matrix cells determine the characteristics of the new hair (7) and are, therefore, an attractive target for genetic modification (7–11). However, previous attempts to modify the hair follicle have had very limited success.
In a previous study, we reported that topical liposomes could selectively deliver actively expressing DNA to the hair follicle (8). Since our report in 1995, a few publications reported genetic modification of hair follicles. Cotsarelis et al. (7) demonstrated that topical application of gene-containing liposomes at the onset of anagen was critical for successful transfection of mouse and human hair follicles. Alexeev et al. (11, 12) demonstrated that an RNA-DNA oligonucleotide (ROD) corrected a point mutation in the mouse tyrosinase gene in follicles, restoring melanin synthesis in vivo, albeit with very low efficiency. Increasing the efficiency of genetic alteration of the growing hair shaft requires new techniques.
In the present study, we describe a highly efficient genetic modification technique for hair follicles. It enables high transgene expression in growing hair shafts in genetically modified mouse skin grafted onto nude mice.
Materials and Methods
Adenovirus GFP Vector.
The GFP-containing adenovirus vector, pQBI-AdCMV5GFP, was purchased from Quantum (Durham, NC).
Mice.
C57BL/10 and C57BL/6-Tyr<c-2J>/+ mice (8 weeks old) were used as skin donors. CD-1 nude mice (7 weeks old) were used as skin graft recipients.
Induction of Anagen.
Telogen mice (judged by their pink skin color) were anesthetized [50 mg of ketamine (Fort Dodge Animal Health, Fort Dodge, IA)/kg of body weight]. The dorsal area (3 × 5 cm) of the mice was depilated with hair-removal wax to induce a synchronized anagen phase.
Skin Histoculture and Collagenase Treatment.
Animals were killed by cervical dislocation on the 6th day after depilation. The back skin was dissected at the level of the subcuitis. Tissue (s.c.) was removed, and the skin was rinsed in calcium- and magnesium-free PBS (CMF-PBS, pH 7.4). The skin samples were cut into small pieces (1 mm × 1 mm ≈ 2 cm × 2 cm). A fraction of the specimens were used directly as untreated controls cultured in RPMI medium 1640 containing 10% (vol/vol) FBS. The remainder of the specimens were incubated in a 2 mg/ml type I collagenase (Sigma) solution in medium from 45 min to 3 hr 45 min at 37°C and rinsed in CMF-PBS.
GFP Transduction of Hair Follicles in Histocultured Skin.
The collagenase-treated skin histocultures were infected with pQBI-AdCMV5GFP at 2.4 × 106 to 5.0 × 109 plaque forming units (pfu)/ml culture medium and then incubated in fresh RPMI medium 1640 (10% FBS) at 37°C for 1–6 h.
Grafting of GFP-Transduced Skin Histocultures.
To visualize the expression of the transgene in vivo, histocultured skin was grafted to nude mice or C57BL/10 mice after viral GFP transduction. Histocultured specimens were grafted within 24 h of harvest. Grafting surgery was performed in a laminar-flow hood using sterile procedures. Mice were anesthetized with Ketamine. Pieces of skin (1 × 1 cm) were grafted to a bed of similar size that was prepared by removing recipient mouse skin down to the fascia. Skin grafts were fixed in place with 6–0 nonabsorbable monofilament sutures.
Fluorescence Microscopy of GFP-Transduced Hair Follicles and Shafts.
A Nikon fluorescent microscope and a Leica fluorescence stereo microscope model LZ12 (Leica, Deerfield, IL) equipped with a mercury 50W lamp power supply were used. Emitted fluorescence was collected through a long-pass filter GG475 (Chroma Technology, Brattleboro, VT) on a Hamamatsu C5810 three-chip cooled color charge-coupled device camera (Hamamatsu Photonics Systems, Hamamatsu City, Japan).
Quantification of GFP-Transduced Hair Follicles.
The number of hair follicles and GFP-positive hair follicles was determined under bright-field microscopy and fluorescence microscopy. The calculations were based on an average number of hairs from five randomly chosen microscopic fields covering an area of 0.581 mm2 (one field at ×200 magnification). At least 500 hairs per group were counted to generate the percentage of GFP-positive hair follicles. Hair follicles in which GFP was visualized anywhere in the hair bulb or shaft were scored as GFP-positive.
Isolation of Hair Follicles and Dermal Papillae from GFP-Transduced Histocultured and Grafted Skin.
After viral GFP transduction, hair follicles were isolated from histocultured skin and grafted skin at several time points to determine the location of GFP expression. Pieces of histocultured skin or skin grafts were incubated in a 2 mg/ml type I collagenase solution in culture medium for 2 h at 37°C and rinsed in culture medium to release hair follicles.
Preparation of Skin Samples for Histological and Cytological Studies.
After viral GFP transduction, skin pieces were taken at several time points for histological study to determine the location of GFP expression. Pieces of histocultured skin or skin grafts were stored at −80°C. Frozen specimens were sectioned on a cryostat (Hacker Instruments, Fairfield, NJ) and collected onto glass slides (Fisher Scientific).
Isolation of RNA from Histocultured Skin and RT-PCR.
Skin samples (100 mg) were homogenized in 1 ml of Tri reagent (Sigma) to extract RNA (13, 14). For RT-PCR, approximately 10 μg of RNA was reverse-transcribed to first cDNA chains. Reverse transcription was carried out in 20 μl of first-strand buffer, 500 μM of each dNTP, and 20 units of avian myeloblastosis virus reverse transcriptase (Strategene, San Diego). The primer for the first strand was pGFP antisense. Incubation was at 42°C for 50 min. The products of the reverse transcription were then amplified by the PCR. Mouse β-actin mRNA was used as the standard. The sequence of the GFP upstream primer was 5′-ATG GCT AGC AAA GGA GAA GAA CT-3′. The downstream primer was 5′-TCA GTT GTA CAG TTC ATC CAT G-3′. The PCR conditions for both GFP and β-actin were as follows: first denaturation at 97°C for 30 s; annealing at 55°C for 30 s; extension at 72°C for 45 s; then, a final extension at 72°C for 10 min.
Results
GFP Fluorescence in Genetically Transduced Hair Follicles in Histocultured Skin.
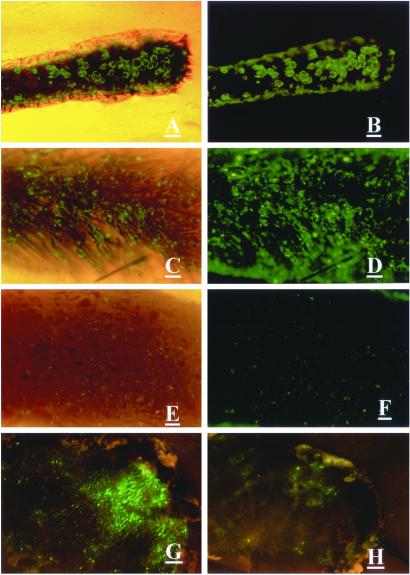
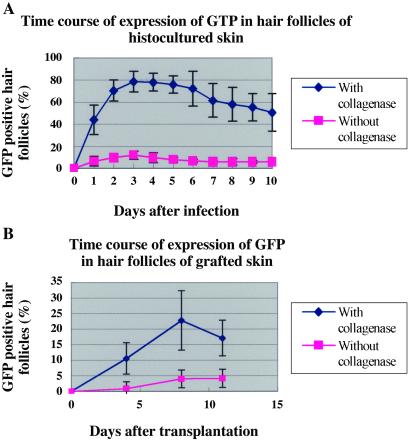
Three days after ex-vivo adenoviral GFP gene treatment, GFP fluorescence appeared in 79% of the hair follicles in collagenase-treated histocultured skin. In contrast, untreated histocultured skin showed only 12% of hair follicles expressed fluorescing GFP (Figs. 1 A–F and 2A). Strong GFP fluorescence was expressed in histocultured hair follicles for at least 35 days (data not shown).
Figure 1.
Effect of collagenase treatment on adenoviral GFP transduction of hair follicles in skin. (A–D) Histocultured mouse skin treated with collagenase and transduced with adenoviral GFP. (A and C) Bright field and fluorescence. (B and D) Fluorescence. (E and F) Untreated histocultured skin transduced with adenoviral GFP. (Magnification = ×40; bar = 0.2 mm.) (G) Grafted skin, transduced with adenoviral GFP after collagenase treatment ex vivo. (H) Untreated grafted skin, transduced with adenoviral GFP. (Bar = 1 mm.) (E) Bright field and fluorescence. (F) Fluorescence. (A–F) The view from the undersurface. (G and H) The view from the surface.
Localization of Expressed GFP Within the Hair Follicle.
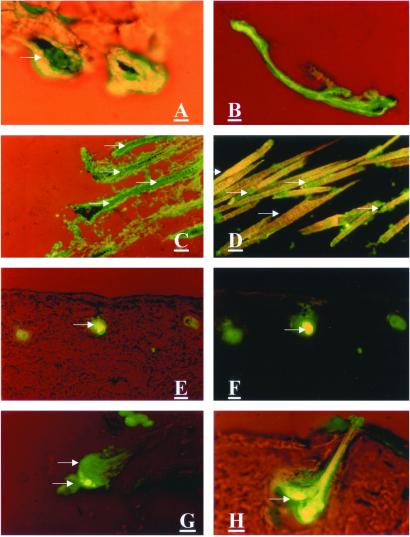
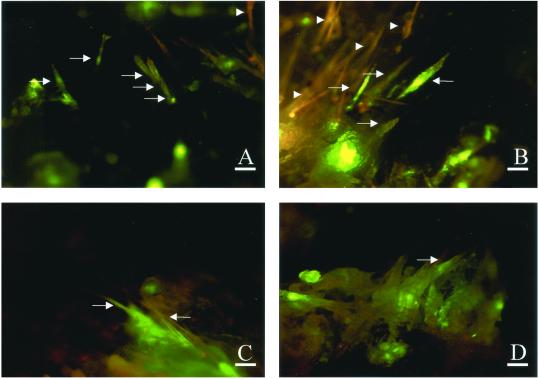
Adenovirus-transduced GFP expression could be detected in the hair matrix cells as early as day 1. (Fig. 3A). On day 3, hair follicles were isolated from histocultured skin to localize more accurately the GFP fluorescence. GFP appeared in the majority of the hair bulbs and dermal papillae (Fig. 4 A and B). On day 7, GFP also appeared in the hair shafts (Fig. 3 B–D). The presence or absence of GFP in the hair shafts was clearly and unambiguously distinguished by its characteristic fluorescence (Fig. 3 C and D).
Figure 3.
GFP visualization in hair matrix cells, dermal papillae, and hair shafts of adenoviral GFP-transduced histocultured and grafted skin. Specimens of histocultured skin and grafted skin were sectioned on a cryostat. (A–D) Histocultured skin treated with collagenase and transduced with adenoviral GFP. (A) One day after adenoviral GFP treatment. Arrow indicates GFP-transduced matrix cells of hair bulb. (Magnification = ×200; bar = 40 μm.) (B–D) Seven days after adenoviral GFP treatment. (B: magnification = ×100; bar = 80 μm.) (C and D: magnification = ×200; bar = 40 μm.) (E–H) Grafted skin, transduced with adenoviral GFP after collagenase treatment. (E–G) Six days after grafting. (H) Thirteen days after grafting. (E, F, and H: magnification, ×400; bar = 20 μm.) (G: magnification = ×200; bar = 40 μm.) Arrows indicate GFP-positive areas. Arrowheads indicate GFP-negative (autofluorescent) areas.
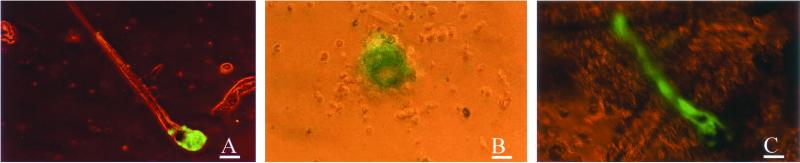
Figure 4.
GFP visualization in the hair bulb, dermal papillae, and hair shaft isolated from adenoviral GFP-transduced histocultured and grafted skin. (A) Isolated hair follicle from collagenase-treated histocultured skin. (Magnification = ×100; bar = 60 μm.) (B) Isolated dermal papillae from collagenase-treated histocultured skin. (Magnification = ×400; bar = 20 μm.) (C) GFP-transduced hair follicle and shaft isolated from collagenase-treated grafted skin. (Magnification = ×100; bar = 60 μm.)
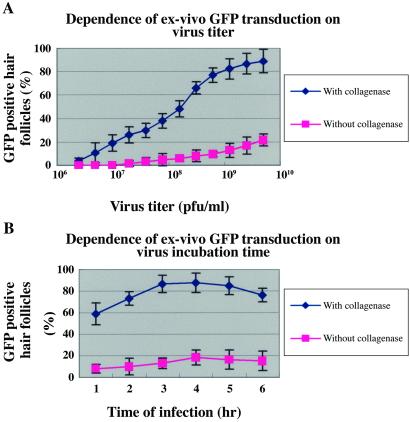
Dependence of GFP Transduction on Virus Titer and Length of Incubation.
Both collagenase-treated and untreated skin histocultures were incubated with adenoviral GFP at concentrations of 2.4 × 106 to 5.0 × 109 pfu/ml for 2.5 h at 37°C. On day 3, after GFP transduction, the fraction of GFP-positive hair follicles in collagenase-treated histocultures was as high as 80% at the maximum virus titer. In untreated histocultures, the number of GFP-positive hair follicles was 4× smaller and increased only slightly at the maximum virus titer used (Fig. 5A).
Figure 5.
Dependence of adenoviral GFP-transduction on virus titer and incubation time. (A) Skin histocultures were treated with various titers of adenoviral GFP for 2.5 h at 37°C. (B) Skin histocultures were incubated with adenoviral GFP at 6.8 × 108 pfu/ml at 37°C for various time periods. Hair follicles were counted as in Fig. 2.
The kinetics of uptake of the viral GFP was examined next. Both collagenase-treated and untreated histocultures were incubated with adenoviral GFP at 3.4 × 108 pfu per ml for 1–6 h at 37°C. The fraction of GFP-positive hair follicles in collagenase-treated histocultures increased up to 80% with the length of virus incubation for up to 4 h, after which it plateaued. By contrast, the number of GFP-positive hair follicles in untreated histocultures was very small and increased only slightly with time (Fig. 5B).
Dependence of GFP Transduction Efficiency on Time of Collagenase Treatment.
The fraction of GFP-positive hair follicles increased with the length of collagenase treatment of skin histocultures for up to 1 h 30 min, after which the fraction declined.
In Vivo GFP Expression in Hair Follicles and Hair Shafts.
To go beyond the limited GFP expression achieved in vitro, the genetically modified histocultured skin was grafted onto nude mice. More extensive GFP fluorescence of hair follicles resulted in the collagenase-treated histocultured skin after grafting, whereas untreated skin showed only a limited response (Fig. 1 G and H). On day 8 after grafting, GFP expression was maximum in the central area of the graft where it reached 75% of hair follicles. GFP fluorescence was visualized in the root sheath cells and matrix cells of the hair bulb on postgrafting day 6 (Fig. 3 E–H). GFP expression was similar in GFP-adenoviral-treated skin grafted to immune-competent C57BL10 mice (data not shown).
GFP Fluorescence in Growing Hair Shafts of Grafted Skin.
GFP fluorescence appeared in numerous growing hair shafts of grafted skin on postgrafting days 8 and 12 (Fig. 6), day 13 (Fig. 3H), and day 17 (Fig. 4C). The region of the growing hair shaft nearest to the GFP-positive hair bulbs expressed GFP at the highest levels (Fig. 3 G and H). GFP expression was predominantly in hair follicles and occurred to a much lesser extent in the epidermis and dermal fibroblasts (Fig. 3 G and H).
Figure 6.
GFP visualization in hair shafts of adenoviral GFP-transduced grafted skin. (A) Eight days after grafting. (Magnification, ×32; bar = 3 mm.) (B–D) Twelve days after grafting. (B and C: magnification = ×80; bar = 1 mm.) (D: magnification = ×63; bar = 1.5 mm.) (A–D) The view from the surface. Arrows indicate GFP-positive areas. Arrowheads indicate GFP-negative (autofluorescent) areas.
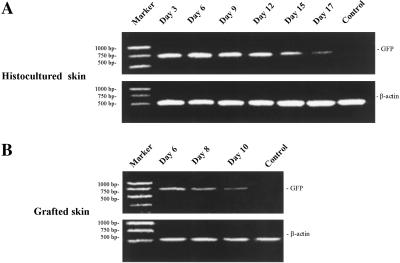
Extended Expression of GFP mRNA in Histocultured and Grafted Skin.
The transduced GFP gene continued expression in hair follicles that were maintained in histoculture and even more strongly in implanted skin. RT-PCR analysis detected GFP-specific mRNA in histocultured skin at least through day 17. Electrophoretic analysis (Fig. 7) showed that the amplified cDNA from the GFP-transduced histocultured skin had the appropriate size of 720 bp. In contrast, RT-PCR of total RNA from untransduced histocultured skin did not produce this band. As a control, mouse β-actin (514 bp) was amplified by the RT-PCR with equal yields from GFP-positive and -negative skin. Also, the transduced GFP gene continued expression in vivo after grafting the GFP-containing skin into nude mice. GFP-specific mRNA was detected at each time point as well (Fig. 7).
Figure 7.
RT-PCR expression analysis of GFP gene in adenoviral GFP-transduced skin. Total RNA was extracted from the skin, reverse-transcribed, and amplified by PCR, as described in Materials and Methods. Mouse β-actin was used as the standard. PCR DNA markers show the molecular weight. Amplified GFP cDNA had the predicted size of 720 bp. (A) Histocultured skin. (B) Grafted skin. No GFP cDNA was amplified from uninfected skin.
Discussion
In this study, we have developed an efficient technique for genetic modification of the growing hair shaft. The ability of collagenase to enhance gene transfer to the hair follicle may be caused by partial digestion of the dermis, which exposes the follicle more completely to GFP-adenovirus. The GFP fluorescence initially seen in follicles later migrated into growing keratinized hair shafts.
In previous attempts to genetically modify follicles, genes were administered via the epidermis or dermis (7–9, 11). The delivery system described in the present report used both the epidermis and dermis in the floating collagenase-treated skin histocultures. The availability of the epidermis as well as the dermis can explain, at least in part, the high efficiency of gene delivery achieved. The present report demonstrates expression of a delivered transgene in large numbers of growing hair shafts.
Cotsarelis et al. (7) reported that β-gal activity was detected for up to 4 days in hair follicles after topical application of the liposome-entrapped lac-Z genes. In the present study, GFP fluorescence was maintained for a least 35 days in histoculture and for at least 17 days after grafting onto nude mice. Similar results were seen by RT-PCR. These data suggest that GFP is a relatively stable and useful marker for hair-follicle genetic modification. GFP can be visualized in the hair shafts and followed noninvasively in the same mice, in the same skin area, and in the same hair follicles without killing the mice.
The approach to hair follicle genetic modification technique described in the present report gives rise to the possibility of a safe genetic modification technique in a very accessible and visible organ. Both cosmetic and therapeutic genes could be delivered to the hair follicles with this strategy.
Figure 2.
Time course of visualization of GFP in hair follicles in skin after adenoviral GFP treatment. (A) Histocultured skin. (B) Grafted skin. The number of hair follicles was counted under bright-field microscopy. The number of GFP-positive hair follicles was counted under fluorescence microscopy.
References
- 1.Kligman A M. J Invest Dermatol. 1959;33:307–316. doi: 10.1038/jid.1959.156. [DOI] [PubMed] [Google Scholar]
- 2.Paus R, Cotsarelis G. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 3.Stenn K S, Combates N J, Eilertsen K J, Gordon J S, Pardinas J R, Parimoo S, Prouty S M. Dermatol Clin. 1996;14:543–558. doi: 10.1016/s0733-8635(05)70383-1. [DOI] [PubMed] [Google Scholar]
- 4.Hardy M H. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G, Sun T-T, Lavker R M. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 6.Cotsarelis G, Miller S E. Trends Mol Med. 2001;7:293–301. doi: 10.1016/s1471-4914(01)02027-5. [DOI] [PubMed] [Google Scholar]
- 7.Domashenko A, Gupta S, Cotsarelis G. Nat Biotechnol. 2000;18:420–423. doi: 10.1038/74480. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Hoffman R M. Nat Med. 1995;1:705–706. doi: 10.1038/nm0795-705. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Leopold P L, Crystal R G. J Clin Invest. 1999;104:855–864. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman R M. Nat Biotechnol. 2000;18:20–21. doi: 10.1038/71866. [DOI] [PubMed] [Google Scholar]
- 11.Alexeev V, Igoucheva O, Domashenko A, Cotsarelis G, Yoon K. Nat Biotechnol. 2000;18:43–47. doi: 10.1038/71901. [DOI] [PubMed] [Google Scholar]
- 12.Alexeev V, Yoon K. Nat Biotechnol. 1998;16:1343–1346. doi: 10.1038/4322. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P. BioTechniques. 1993;15:532–534. , 536–537. [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]