Abstract
The view seems to prevail that the frequency range of hearing is determined by the properties of the outer and middle ears. We argue that this view is an oversimplification, in part because the reactive component of cochlear input impedance, which affects the low-frequency sensitivity of the cochlea, is neglected. Further, we use comparisons of audiograms and transfer functions for stapes (or columella) velocity or pressure in scala vestibuli near the stapes footplate to show that the middle ear by itself is not responsible for limiting high-frequency hearing in the few species for which such comparisons are possible. Finally, we propose that the tonotopic organization of the cochlea plays a crucial role in setting the frequency limits of cochlear sensitivity and hence in determining the bandwidth of hearing.
This article has two purposes. The first is to argue that the often-stated notion that the shape of the audiogram is largely due to the frequency-filtering properties of the external and middle ears is an oversimplification. That notion neglects the fact that a reactive component of cochlear input impedance decreases the low-frequency sensitivity of the cochlea. More importantly, comparisons of behavioral thresholds and the magnitudes of stapes (or columella) velocity (V) or pressure in scala vestibuli near the stapes footplate (PSV) reveal that the middle ear does not limit high-frequency hearing in the few species for which such comparisons are possible. The second purpose is to offer the hypothesis that the tonotopic organization of the cochlea makes a crucial contribution to setting the frequency limits of inner-ear responses and hence the bandwidth of hearing thresholds.
Historical Overview
When it became evident that “high-frequency hearing is … a uniquely mammalian characteristic” (1), there was speculation on the physiological and phylogenetic origins of the frequency limitations of hearing. According to one view, high-frequency hearing in mammals “depends on the ossicular linkage in the middle ear and may have been one of the primary sources of selective pressure that resulted in the evolutionary transformation of reptilian jaw bones into mammalian auditory ossicles” (1). A more inclusive perspective was that “the ossicular chain of the mammalian ear is not the only factor involved in the high-frequency sensitivity of mammals” (2), and it was suggested that the high-frequency limit of hearing across species is well correlated with an index derived from the length and width of the basilar membrane (BM; ref. 3).
On the basis of theoretical considerations, it was postulated long ago that cochlear input impedance is resistive at most frequencies and that reactive components affect cochlear responses only at very low (e.g., <3–10 Hz) and very high (4, 5) frequencies, thus playing a very minor role in determining the bandwidth of hearing. However, a significant role for the cochlea in setting the low-frequency limit of hearing became evident when microphonics data suggested that cochlear input impedance includes a reactive component that acts as a high-pass filter below 100–200 Hz (refs. 6 and 7; see also refs. 8–10). The existence of this reactive component of cochlear input impedance subsequently was confirmed by direct measurements (11, 12). Nevertheless, the fact that “cochlear input impedance (is one of) the primary determiners of … behavioral sensitivity” (ref. 9, p. 126) has been neglected often in the recent literature, which contains statements to the effect that “the external and middle ears jointly … largely determine the frequency response properties (audiogram) of a given species” (ref. 13, pp. 10–11). Similarly, “to a large extent, the frequency range (of hearing) is determined by the properties of the outer and middle ears” (ref. 14, p. 25); “the human threshold of audibility as a function of sound frequency is determined to a large extent by the combined transfer functions of the outer and middle ear” (ref. 10, p. 83); “the shape of the auditory function is completely determined by external and middle ear function” (ref. 15, p. 236); and “the properties of the middle ear, combined with those of the ear canal and external ear, can for the most part account for the frequency dependence of the threshold of audibility for pure tones” (ref. 16, p. 122).†
The Low-Frequency Limit of Hearing Is Determined in Part by the Reactive Component of Cochlear Input Impedance
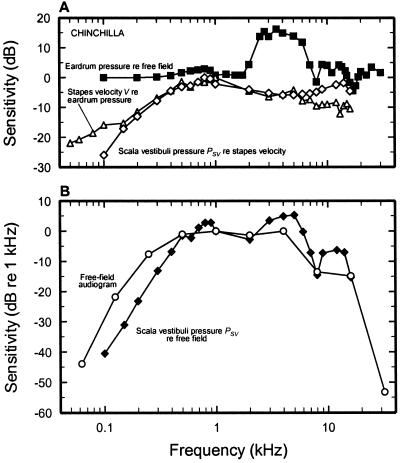
Fig. 1 illustrates how the external and middle ears, as well as cochlear input impedance, jointly contribute to determining the audiogram of chinchilla at low and middle frequencies. Fig. 1A represents three stages of transformation of the free-field acoustic signal into PSV fluctuations: the magnitude changes that sound undergoes as it approaches the eardrum (squares), the magnitude of V relative to pressure in the ear canal next to the eardrum (triangles), and the magnitude of the cochlear input impedance, i.e., PSV relative to V (diamonds). The decibel sum of the three magnitude functions yields the magnitude of PSV (closed diamonds, Fig. 1B), which resembles the audiogram (circles, Fig. 1B). Behavioral thresholds for low- and middle-frequency tones in cat, human, and guinea pig also are reasonably well predicted by combining cochlear input impedance and the transfer functions of the external and middle ears (9, 10, 17, 18).
Figure 1.
The determination of the chinchilla audiogram by the external, middle, and inner ears. (A) Filled squares, the gain of the ear canal, expressed as the pressure magnitude near the tympanic membrane relative to (re) that of a free-field sound source situated directly ahead and equidistant from the two ears (57); open triangles, the magnitude of V normalized to sound-pressure level near the eardrum is given relative to its maximum (at 1 kHz) (from figure 10 of ref. 12); open diamonds, the magnitude of PSV normalized to V, i.e., the cochlear input impedance, is given relative to its maximum (at 800 Hz) (data were obtained from figure 15 of ref. 12), computed from velocity data of ref. 12 and pressure data of ref. 58. (B) Open circles, free-field audiogram, relative to the 1-kHz threshold (26); closed diamonds, predicted magnitude of PSV, relative to 1 kHz, stimulated by constant-level tones from a source directly in front of the head. The curve is the decibel summation of the gains indicated by the three curves of A. All data are for ears with a closed bulla.
Of greatest interest for the present discussion is that the reactive component of cochlear-input impedance evident in Fig. 1A contributes significantly to defining the low-frequency limit of PSV in the chinchilla cochlea and thus, probably, in determining the shape of the audiogram (Fig. 1B). The low-frequency cochlear reactance, which is at least as important as the elastic reactance of the middle-ear cavity (because of the cushion of air trapped in the bulla) in attenuating pressure magnitude as a function of decreasing frequency, may arise from the combined effects of the elasticity of the round window membrane, the annular ligament of the oval window, and perhaps the helicotrema (6, 11).
The External and Middle Ears Are Not the Only Determiners of the High-Frequency Limit of the Audiogram
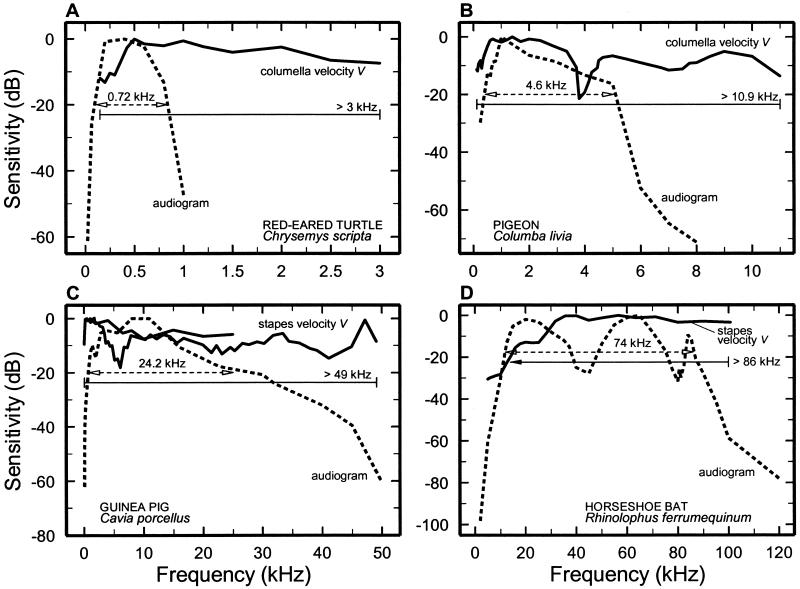
There is a widespread belief that “middle ears determine the upper frequency of hearing” (ref. 19, p. 1790). However, the relative contributions of the external, middle, and inner ears in the determination of the upper limit of hearing (and hence its bandwidth, defined here as the frequency range encompassed by the 20-dB low- and high-frequency cutoffs of the audiograms, ‡) have not been established rigorously in part because V has seldom been recorded at frequencies sufficiently high to be comparable to the upper frequency limit of the audiogram (surveyed in ref. 20).§ Although audiograms exist for many mammals and other amniotic species (e.g., see ref. 21), V has been measured in only two mammals, guinea pig and the horseshoe bat, at frequencies corresponding to the high-frequency limits of the audiograms. Fig. 2 compares audiograms for those two species as well as for pigeon and turtle, with magnitude-versus-frequency curves of stapes (or columella) velocity, V.
Figure 2.
Comparison between the frequency dependence of behavioral thresholds and stapes or columella vibrations in the turtle (A), pigeon (B), guinea pig (C), and horseshoe bat (R. ferrumequinum) (D). The magnitude of stapes or columella velocity responses to tones (solid curves) and audiometric thresholds (dashed curves) were normalized to their minima and plotted as a function of frequency by using a decibel scale. The horizontal lines indicate bandwidths at −20 dB. (A) Turtle audiogram (59). V, ref. 60. (B) Pigeon audiogram (61). V, ref. 62. (C) Guinea pig audiogram. Median values, refs. 63 and 64; V, refs. 53 and 65. (D) Horseshoe bat audiogram. Median values, ref. 66; V, ref. 67.
Fig. 2 C and D compare the magnitudes of V with the behavioral thresholds for guinea pig and the horseshoe bat, Rhinolophus ferrumequinum. In both cases the 20-dB bandwidth of the magnitude of V amply exceeds that of the behavioral-threshold curve. Audiometric and middle-ear data are available also for red-eared turtles and pigeons (Fig. 2 A and B). In both species, V is fairly flat up to frequencies far exceeding the high-frequency cutoff of the audiograms. It is striking to consider that the pigeon middle ear could provide an adequately wide-band stimulus for cochlear analysis even for the barn owl, one of the avian species with the greatest hearing bandwidth, with a high-frequency audiometric cutoff of 10–12 kHz (22, 23).
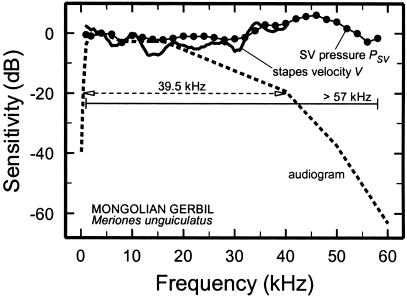
In the Mongolian gerbil (Fig. 3), the magnitude of V is fairly flat up to 40 kHz (20), a frequency at which behavioral threshold exceeds the minimum by 20 dB (24). More importantly, PSV (25) is flat at least up to 58 kHz, a frequency at which the behavioral threshold exceeds its minimum by more than 60 dB.
Figure 3.
Frequency dependence of behavioral thresholds, stapes vibrations, and PSV in the gerbil. The magnitudes of V (solid curve) and PSV (symbols) and audiometric thresholds (dashed curve) were normalized to 4 kHz and plotted as a function of frequency by using a decibel scale. The horizontal lines indicate bandwidths at −20 dB. Audiogram, ref. 24; PSV, ref. 25; V, ref. 20.
Finally, in chinchilla, a species with relatively low-frequency hearing (Figs. 1, 4, and 5), the magnitudes of V “exhibit a relatively flat frequency spectrum … up to at least 26–31 kHz,”¶ i.e., exceeding the high-frequency cutoff of its audiogram (Fig. 1; ref. 26).
Figure 4.
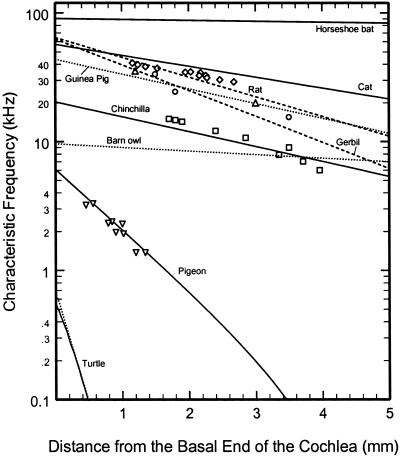
Tonotopic organization at the base of the cochlea in barn owl, cat, chinchilla, gerbil, guinea pig, horseshoe bat (R. rouxii), pigeon, rat, and turtle. Cochlear maps (lines) and BM CFs (symbols) for the basal region of the cochlea in several species. The maps for barn owl (40), cat (37), gerbil (38), horseshoe bat (41), pigeon (36), and rat (39) were derived from auditory-nerve fibers of known CF that were labeled and traced. The map for the guinea pig is based on recordings from spiral ganglion neurons (42). The map for turtle is based on identified sites of microelectrode recordings in the basilar papilla (figure 3 of ref. 45 and figure 2 of ref. 46). The map for chinchilla was derived by Greenwood (44) from correlations between hair-cell loss and audiometric data (43). BM CFs: cat, diamonds (68); chinchilla, squares [1.7 mm (69), 3.5 mm (12), and others (70)]; gerbil, up triangles [1.2 mm (71) and 3 mm (72)]; guinea pig, circles (73); pigeon, down triangles (74). Only relative distances are available for some of the chinchilla BM data (70).
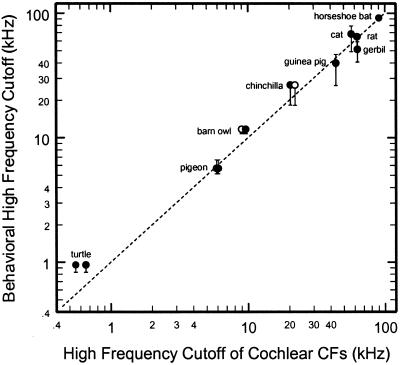
Figure 5.
The high-frequency cutoffs of audiograms and the highest CFs predicted by cochlear place-frequency maps. Filled symbols, CFs at the extreme base of the cochlea derived by extrapolation from cochlear place-frequency maps (Fig. 4); open symbols, the highest CFs of auditory-nerve fibers reported in the literature for pigeon (75), chinchilla (A.N.T., N. C. Rich, and M.A.R., unpublished observations), and barn owl (76). The ordinate indicates the frequencies at which audiometric thresholds exceed the minimum threshold by 20 (lower bracket), 40 (symbol), and 60 dB (upper bracket) (unavailable for chinchilla): barn owl (22, 23), cat (77), chinchilla (26), gerbil (24), guinea pig (64), horseshoe bat (R. rouxii) (78), pigeon (61), rat (79), and turtle (59).
Thus, far from supporting the idea that middle ears generally determine the upper frequency of hearing, the available data indicate that the bandwidths of V and PSV actually exceed the bandwidth of the audiogram in the cases of chinchilla, gerbil, guinea pig, horseshoe bat, pigeon, and turtle, which implies that in these species the high-frequency limit of cochlear and hearing thresholds is set internally in the cochlea. Below we present a hypothesis of how this is accomplished in these and perhaps other species.
The Tonotopic Organization of the Cochlea Contributes to Setting the Limits of Hearing
The cochlea is organized tonotopically: stimulus frequencies are mapped on cochlear distance. First described by von Békésy in his pioneering experiments on BM vibrations (27–29), the tonotopic organization of the cochlea is universally accepted (reviewed in ref. 30). However, it is seldom recognized (see Historical Overview) that tonotopicity, in combination with the finite length of the cochlear partition, must impose intrinsic frequency limits on cochlear bandwidth. In mammals, tonotopicity originates in the spatially graded BM stiffness, which directly determines the passive frequency tuning of local BM vibrations (29) and, less directly, the active frequency tuning of the BM and auditory-nerve fibers innervating adjacent inner hair cells (31, 32, ‖). In turtles, tonotopicity results largely from the electrical properties of hair cells, which also are spatially graded (33, 34). Whatever its origin, tonotopicity confines cochlear responses to a well defined frequency range determined by the characteristic frequencies (CFs) of the auditory-nerve fibers innervating the extreme apical and basal sites of the cochlea.
Specifically, our hypothesis posits that the shape of the audiogram resembles the envelope of the thresholds of auditory-nerve fibers. Thus, the low-frequency limit of hearing reflects the low-frequency arms of the frequency-threshold tuning curves of the auditory-nerve fibers with the lowest CFs. Similarly, the high-frequency limit of hearing corresponds to the high-frequency arms of the tuning curves of the auditory-nerve fibers with the highest CFs. For intermediate frequencies of the audiogram (spanning most of its range), behavioral thresholds parallel the envelope of the CF thresholds of auditory-nerve fibers.
We suspect that many hearing experts (especially those who study nonmammalian species) will find our hypothesis unsurprising if not unoriginal. However, to our knowledge the hypothesis has never been stated fully and explicitly before now. It may have a precedent in a suggestion (11) that the rising behavioral thresholds at low frequencies in cat could be due to the absence of auditory-nerve fibers with CFs lower than 100 Hz (35).
The Tonotopic Organization of the Cochlea Makes a Crucial Contribution to the Determination of the Upper-Frequency Limit of Hearing
Fig. 4 presents place-CF maps (lines) for the basal end of the cochleae of barn owl, cat, chinchilla, gerbil, guinea pig, horseshoe bat (Rhinolophus rouxii), pigeon, rat, and red-eared turtle (Chrysemys scripta). The maps for barn owl, cat, gerbil, horseshoe bat, pigeon, and rat were derived from auditory-nerve fibers with innervation sites ascertained by using cellular markers (36–41). For guinea pig, the map is based on recordings from somata in the spiral ganglion (42). For chinchilla, the map is based on correlations of audiometric and hair-cell loss data (43, 44). For turtle, the maps indicate the sites of microelectrode penetrations into the basilar papilla (45, 46, **).
Also shown in Fig. 4 are the CFs of several BM sites in five of the same species (symbols). The BM data for chinchilla, gerbil, guinea pig, and pigeon were obtained in relatively healthy cochleae, whereas the data for cat came from passive (i.e., linear) cochleae, thus probably underestimating the “true” CFs by ≈0.5 octave. Perusal of this figure reveals that, in general, there is an excellent correspondence between the BM data and the maps derived from neural data, consistent with the consensus that the CFs of auditory-nerve fibers directly reflect the CFs of BM sites near their point of innervation of inner hair cells (e.g., see refs. 31 and 32).
Fig. 5 compares the high-frequency cutoffs of the cochlear maps (i.e., the y intercepts of Fig. 4) with the cutoffs of the audiograms, measured as the frequencies at which thresholds exceed the minimum of each audiogram by 20 (lower brackets), 40 (symbols) and 60 dB (upper brackets). The data points are scattered close to a 45° line, indicating a good match between the upper-frequency cutoffs of the audiogram and the cutoffs of the cochlear maps. Fig. 5 is consistent with our hypothesis that the high-frequency limit of the audiogram in great part is determined internally in the cochlea by the high-frequency arms of the frequency-threshold tuning curves of auditory-nerve fibers with the highest CFs in each species. Specifically, the data of Fig. 5 provide an explanation for the finding that the magnitudes of V or PSV do not limit the bandwidth of hearing in chinchilla, gerbil, guinea pig, horseshoe bat, pigeon, or turtle.
Conclusions and Speculations on the Evolution of Sensory Systems
The evidence presented here contradicts the notion that the shape of the auditory function is determined largely by external and middle-ear function. Rather, it is consistent with the view that the middle ear is a wide-band pressure transformer with a flat velocity-response function spanning most of (if not exceeding) the frequency range of hearing except for the lowest frequencies (ref. 20, p. 269). It seems reasonable that instead of frequency-limiting the acoustic stimulus, the middle ear permissively transmits to the cochlea a sufficiently wide-band signal suitable for frequency analysis.
It remains to be seen whether the finding that the bandwidth of middle-ear vibrations exceeds that of the audiogram in chinchilla, gerbil, guinea pig, horseshoe bat, pigeon, and turtle will be confirmed and, if so, whether this is the general rule for amniotic tetrapods (mammals, birds, and reptiles). It may turn out that the bandwidth of V generally exceeds the CF cutoff of inner-ear responses in avian and reptilian species (except for birds with unusually extended high-frequency hearing such as the barn owl), whereas the bandwidth of V approximately coincides with that of the audiogram in most mammals.
A coincidence between the frequency limits of middle-ear transmission and cochlear analysis could have resulted from the operation of a principle of “optimization” during phylogenetic evolution (47). Optimization seems to have informed the evolution of the eye, in which both the ocular media (cornea, aqueous humor, lens, and vitreous humor) and the density of photoreceptors in the retinal fovea approach the diffraction limit of an ideal lens with similar aperture. In other words, “the density of receptors is … appropriate to utilize fully the optical performance of the front of the eye” (ref. 47, p. 284). Similarly, in birds “the primary influence on … retinal design appears to be the range of wavelengths available … regardless of whether that range is determined by the spectral distribution of the natural illumination or the spectral transmittance of the ocular media ” (ref. 48, p. 676). Thus, for example, the narrow spectral bandwidth of illumination of the Humboldt penguin's aqueous habitat “appears to be reflected in the spectral characteristics of its photoreceptors” (ref. 48, p. 697), whereas “the ocular media … of (avian) species which have a UVS (UV) visual pigment generally transmit more short wavelengths” (ref. 48, p. 687). Although widespread in the retinas of other vertebrates, mammalian retinas lack receptors of UV light apparently because they “are incompatible with lenses that absorb UV light” (47) such as those in mammalian eyes.
In the case of the mammalian ear and regardless of whether three-ossicle middle-ear systems in mammal-like reptiles predated the appearance of high-frequency mammalian cochleae (e.g., see refs. 1, 49, and 50; also contrast ref. 51 with 52, pp. 34–35), the bandwidths of middle-ear transmission and cochlear analysis may have been subsequently optimized by iterative accommodation to each other's performance to meet behavioral requirements while avoiding extending either one unnecessarily (and perhaps wastefully). It will be of special interest to ascertain whether the relatively low high-frequency cutoff of hearing in chinchillas and humans was brought about by parallel evolutionary reductions in the bandwidths of both the cochlea and middle-ear transmission, which allowed ultrasonic hearing in their ancestors (1).
Acknowledgments
Special thanks to Mary Ann Cheatham, Peter Dallos, Luis Robles, Mikhail Vorobiev, Beverly Wright, and Joe Zwislocki for reading and criticizing previous drafts of the article and to Claus-Peter Richter and Jon Siegel for helpful discussions. The writing of this article was supported by National Institutes of Health Grant DC-00419 from the National Institute on Deafness and Other Communication Disorders.
Abbreviations
- V
stapes or columella velocity
- PSV
pressure in scala vestibuli near the stapes
- BM
basilar membrane
- CF
characteristic frequency
Footnotes
Some texts that disregard the role of the cochlea in setting the bandwidth of hearing were written by investigators who elsewhere acknowledge the role of cochlear input impedance in shaping the audiogram. For example, contrast the 1973 and 1996 quotations from Dallos (ref. 9, p. 126, and ref. 13, p. 11). Similarly, contrast the quote from Zwislocki (ref. 10, p. 83) with a question posed elsewhere in the same publication: “What is the physical significance of the decreased cochlear input impedance at low sound frequencies and of its departure from pure resistance toward inertance?” (ref. 10, p. 141). The inconsistency between statements by the same authors reflects an implicit (and probably unwitting) conflation of inner-ear properties into those of the middle ear. Specifically, interpreting cochlear-input impedance as “merely” a load to middle-ear transmission apparently led to dismissing its cochlear origin.
More restrictive cutoffs (e.g., 3 or 6 dB) such as those commonly used in other contexts (e.g., specifications of audio hardware) are impractical, because the pass bands of audiograms are typically not completely flat and often contain irregularities (Fig. 2).
Malleus or incus vibrations are not considered here because the shapes of their transfer functions may differ substantially from those of the stapes (see figures 6 and 7 of ref. 53 and figure 8 of ref. 12) and thus may not reflect faithfully the characteristics of the input to the cochlea.
Temchin, A. N., Robles, L. & Ruggero, M. A. (2002) Assoc. Res. Otolaryngol. Mid-Winter Meeting Abstr. 25, 154 (abstr.).
It is still an open question whether the sharp frequency tuning of BM vibrations in the healthy mammalian cochlea results in part from mechanisms other than the passive tuning of the BM. Additional frequency tuning could be provided, for example, by micromechanical interactions between the stereocilia and the tectorial membrane (e.g., see ref. 54) or an intrinsically tuned outer hair cell “stereociliar amplifier” analogous to the one present in sacculus hair cells (55).
References
- 1.Masterton R B, Heffner H E, Ravizza R. J Acoust Soc Am. 1969;45:966–985. doi: 10.1121/1.1911574. [DOI] [PubMed] [Google Scholar]
- 2.Manley G A. Nature (London) 1971;230:506–509. doi: 10.1038/230506a0. [DOI] [PubMed] [Google Scholar]
- 3.Manley G A. Evolution (Lawrence, Kans) 1972;26:608–621. doi: 10.1111/j.1558-5646.1972.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 4.Zwislocki J J. Acta Otolaryngol Suppl. 1948;72:1–76. [Google Scholar]
- 5.Zwislocki J J. In: Handbook of Mathematical Psychology. Luce R C, Bush R R, Galanter E, editors. New York: Wiley; 1965. pp. 1–97. [Google Scholar]
- 6.Dallos P. J Acoust Soc Am. 1970;48:489–499. doi: 10.1121/1.1912163. [DOI] [PubMed] [Google Scholar]
- 7.Weiss T F, Peake W T, Sohmer H S. J Acoust Soc Am. 1971;50:602–615. doi: 10.1121/1.1912676. [DOI] [PubMed] [Google Scholar]
- 8.Franke R, Dancer A. Arch Otorhinolaryngol. 1982;234:213–218. doi: 10.1007/BF00453634. [DOI] [PubMed] [Google Scholar]
- 9.Dallos P. The Auditory Periphery—Biophysics and Physiology. New York: Academic; 1973. [Google Scholar]
- 10.Zwislocki J J. Auditory Sound Transmission—An Autobiographical Perspective. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- 11.Lynch T J, III, Nedzelnitsky V, Peake W T. J Acoust Soc Am. 1982;72:108–130. doi: 10.1121/1.387995. [DOI] [PubMed] [Google Scholar]
- 12.Ruggero M A, Rich N C, Robles L, Shivapuja B G. J Acoust Soc Am. 1990;87:1612–1629. doi: 10.1121/1.399409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallos P. In: The Cochlea. Dallos P, Popper A N, Fay R R, editors. New York: Springer; 1996. pp. 1–43. [Google Scholar]
- 14.Long G R. In: Comparative Hearing: Mammals. Fay R R, Popper A N, editors. New York: Springer; 1994. pp. 18–56. [Google Scholar]
- 15.Rosowski J J. In: Comparative Hearing: Mammals. Fay R R, Popper A N, editors. New York: Springer; 1994. pp. 172–247. [Google Scholar]
- 16.Relkin E M. In: Physiology of the Ear. Jahn A F, Santos-Sacchi J, editors. New York: Raven; 1988. pp. 103–123. [Google Scholar]
- 17.Rosowski J J. J Acoust Soc Am. 1991;90:124–135. doi: 10.1121/1.401306. [DOI] [PubMed] [Google Scholar]
- 18.Zwislocki J J. In: The Nervous System. Tower D B, Eagles E L, editors. Vol. 3. New York: Raven; 1975. pp. 45–55. [Google Scholar]
- 19.Fay R R. In: Encyclopedia of Acoustics. Crocker M J, editor. Vol. 4. New York: Wiley; 1997. pp. 1789–1797. [Google Scholar]
- 20.Overstreet E H, Ruggero M A. J Acoust Soc Am. 2002;111:261–270. doi: 10.1121/1.1420382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay R R. Hearing in Vertebrates: A Psychophysics Handbook. Winnetka, IL: Hill-Fay Associates; 1988. [Google Scholar]
- 22.Konishi M. Am Sci. 1973;61:414–424. [Google Scholar]
- 23.Dyson M L, Klump G M, Gauger B. J Comp Physiol A. 1998;182:695–702. [Google Scholar]
- 24.Ryan A F. J Acoust Soc Am. 1976;59:1222–1226. doi: 10.1121/1.380961. [DOI] [PubMed] [Google Scholar]
- 25.Olson E S. J Acoust Soc Am. 2001;110:349–367. doi: 10.1121/1.1369098. [DOI] [PubMed] [Google Scholar]
- 26.Heffner R S, Heffner H E. Hear Res. 1991;52:13–16. doi: 10.1016/0378-5955(91)90183-a. [DOI] [PubMed] [Google Scholar]
- 27.von Békésy G. Akust Z. 1944;9:3–11. [Google Scholar]
- 28.von Békésy G. J Acoust Soc Am. 1947;19:452–460. [Google Scholar]
- 29.von Békésy G. J Acoust Soc Am. 1948;20:227–241. [Google Scholar]
- 30.Robles L, Ruggero M A. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayan S S, Temchin A N, Recio A, Ruggero M A. Science. 1998;282:1882–1884. doi: 10.1126/science.282.5395.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruggero M A, Narayan S S, Temchin A N, Recio A. Proc Natl Acad Sci USA. 2000;97:11744–11750. doi: 10.1073/pnas.97.22.11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fettiplace R, Crawford A C. Hear Res. 1980;2:447–454. doi: 10.1016/0378-5955(80)90081-7. [DOI] [PubMed] [Google Scholar]
- 34.Art J J, Fettiplace R. J Physiol (London) 1987;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberman M C. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- 36.Smolders J W, Ding-Pfennigdorff D, Klinke R. Hear Res. 1995;92:151–169. doi: 10.1016/0378-5955(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 37.Liberman M C. J Acoust Soc Am. 1982;72:1441–1449. doi: 10.1121/1.388677. [DOI] [PubMed] [Google Scholar]
- 38.Müller M. Hear Res. 1996;94:148–156. doi: 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
- 39.Müller M. Hear Res. 1991;51:247–254. doi: 10.1016/0378-5955(91)90041-7. [DOI] [PubMed] [Google Scholar]
- 40.Köppl C, Gleich O, Manley G A. J Comp Physiol A. 1993;171:695–704. [Google Scholar]
- 41.Vater M, Feng A S, Betz M. J Comp Physiol A. 1985;157:671–686. doi: 10.1007/BF01351361. [DOI] [PubMed] [Google Scholar]
- 42.Robertson D, Johnstone B M. J Acoust Soc Am. 1979;66:466–469. doi: 10.1121/1.383097. [DOI] [PubMed] [Google Scholar]
- 43.Eldredge D H, Miller J D, Bohne B A. J Acoust Soc Am. 1981;69:1091–1095. doi: 10.1121/1.385688. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood D D. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 45.Fettiplace R. Trends Neurosci. 1987;10:421–425. [Google Scholar]
- 46.Art J J, Crawford A C, Fettiplace R. Hear Res. 1986;22:31–36. doi: 10.1016/0378-5955(86)90073-0. [DOI] [PubMed] [Google Scholar]
- 47.Goldsmith T H. Q Rev Biol. 1990;65:281–322. doi: 10.1086/416840. [DOI] [PubMed] [Google Scholar]
- 48.Hart N S. Prog Retin Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. [DOI] [PubMed] [Google Scholar]
- 49.Frost S B, Masterton R B. Hear Res. 1994;76:67–72. doi: 10.1016/0378-5955(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 50.Luo Z X, Crompton A W, Sun A L. Science. 2001;292:1535–1540. doi: 10.1126/science.1058476. [DOI] [PubMed] [Google Scholar]
- 51.Fleischer G. Adv Anat Embryol Cell Biol. 1978;55:3–70. doi: 10.1007/978-3-642-67143-2. [DOI] [PubMed] [Google Scholar]
- 52.Manley G A. Peripheral Hearing Mechanisms in Reptiles and Birds. Berlin: Springer; 1990. [Google Scholar]
- 53.Cooper N P, Rhode W S. Hear Res. 1992;63:163–190. doi: 10.1016/0378-5955(92)90083-y. [DOI] [PubMed] [Google Scholar]
- 54.Zwislocki J J, Kletsky E J. Science. 1979;204:639–641. doi: 10.1126/science.432671. [DOI] [PubMed] [Google Scholar]
- 55.Martin P, Hudspeth A J. Proc Natl Acad Sci USA. 1999;96:14306–14311. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawford A C, Fettiplace R. J Physiol (London) 1980;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Bismarck G. M.S. dissertation. Cambridge: Massachusetts Institute of Technology; 1967. [Google Scholar]
- 58.Décory L. Ph.D. dissertation. Bordeaux, France: Université Bordeaux 2; 1989. [Google Scholar]
- 59.Patterson W C. J Aud Res. 1966;6:453–464. [Google Scholar]
- 60.Moffat A J M, Capranica R R. J Comp Physiol A. 1978;127:97–107. [Google Scholar]
- 61.Goerdel-Leich A, Schwartzkopff J. Naturwissenschaften. 1984;71:98–99. doi: 10.1007/BF01156360. [DOI] [PubMed] [Google Scholar]
- 62.Gummer A W, Smolders J W, Klinke R. Hear Res. 1989;39:15–25. doi: 10.1016/0378-5955(89)90078-6. [DOI] [PubMed] [Google Scholar]
- 63.Heffner R, Heffner H, Masterton R B. J Acoust Soc Am. 1971;49:1888–1895. doi: 10.1121/1.1912596. [DOI] [PubMed] [Google Scholar]
- 64.Prosen C A, Petersen M R, Moody D B, Stebbins W C. J Acoust Soc Am. 1978;63:559–566. doi: 10.1121/1.381754. [DOI] [PubMed] [Google Scholar]
- 65.Johnstone B M, Taylor K J. J Otolaryngol Soc Aust. 1971;3:226–228. [PubMed] [Google Scholar]
- 66.Long G R, Schnitzler H U. J Comp Physiol A. 1975;100:211–219. [Google Scholar]
- 67.Wilson J P, Bruns V. Hear Res. 1983;10:1–13. doi: 10.1016/0378-5955(83)90015-1. [DOI] [PubMed] [Google Scholar]
- 68.Cooper N P, Rhode W S. Hear Res. 1992;63:191–196. doi: 10.1016/0378-5955(92)90084-z. [DOI] [PubMed] [Google Scholar]
- 69.Narayan S S, Ruggero M A. In: Recent Developments in Auditory Mechanics. Wada H, Takasaka T, Ikeda K, Ohyama K, Koike T, editors. Singapore: World Scientific; 2000. pp. 95–101. [Google Scholar]
- 70.Rhode W S, Recio A. J Acoust Soc Am. 2000;107:3317–3332. doi: 10.1121/1.429404. [DOI] [PubMed] [Google Scholar]
- 71. Overstreet, E. H., Temchin, A. N. & Ruggero, M. A. (2002) J. Assoc. Res. Otolaryngol., 10.1007/s101620020023. [DOI] [PMC free article] [PubMed]
- 72.Olson E S. Nature (London) 1999;402:526–529. doi: 10.1038/990092. [DOI] [PubMed] [Google Scholar]
- 73.Cooper N P. J Acoust Soc Am. 1999;106:L59–L64. doi: 10.1121/1.428147. [DOI] [PubMed] [Google Scholar]
- 74.Gummer A W, Smolders J W, Klinke R. Hear Res. 1987;29:63–92. doi: 10.1016/0378-5955(87)90206-1. [DOI] [PubMed] [Google Scholar]
- 75.Sachs M B, Young E D, Lewis R H. Brain Res. 1974;70:431–447. doi: 10.1016/0006-8993(74)90253-4. [DOI] [PubMed] [Google Scholar]
- 76.Köppl C. J Neurophysiol. 1997;77:364–377. doi: 10.1152/jn.1997.77.1.364. [DOI] [PubMed] [Google Scholar]
- 77.Heffner R S, Heffner H E. Hear Res. 1985;19:85–88. doi: 10.1016/0378-5955(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 78.Schuller G. J Comp Physiol A. 1980;139:349–356. [Google Scholar]
- 79.Kelly J B, Masterton B. J Comp Physiol Psychol. 1977;91:930–936. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]