Abstract
Changes in hippocampal function seem critical for cognitive impairment in Alzheimer's disease (AD). Although there is eventual loss of synapses in both AD and animal models of AD, deficits in spatial memory and inhibition of long-term potentiation (LTP) precede morphological alterations in the models, suggesting earlier biochemical changes in the disease. In the studies reported here we demonstrate that amyloid β-peptide (Aβ) treatment of cultured hippocampal neurons leads to the inactivation of protein kinase A (PKA) and persistence of its regulatory subunit PKAIIα. Consistent with this, CREB phosphorylation in response to glutamate is decreased, and the decrease is reversed by rolipram, a phosphodiesterase inhibitor that raises cAMP and leads to the dissociation of the PKA catalytic and regulatory subunits. It is likely that a similar mechanism underlies Αβ inhibition of LTP, because rolipram and forskolin, agents that enhance the cAMP-signaling pathway, can reverse this inhibition. This reversal is blocked by H89, an inhibitor of PKA. These observations suggest that Αβ acts directly on the pathways involved in the formation of late LTP and agents that enhance the cAMP/PKA/CREB-signaling pathway have potential for the treatment of AD.
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is characterized by mild cognitive impairment at its onset and deficits in multiple cortical functions in later stages. To date, the vast majority of its symptoms have been attributed to the loss of synapses and the death of neurons that occur in the course of the disease. The overproduction and accumulation of the amyloid β-peptide (Aβ) and particularly its 42-aa form (Aβ1–42) have been shown to play a crucial role in both of these processes in animal models of AD (1, 2). Although these phenomena can account for the late debilitating stages of the disease, the mechanisms by which Aβ causes early cognitive and behavioral changes remain a matter of conjecture. Recent studies on animal models of AD have highlighted the discrepancy between behavioral deficits and neuropathological findings. Electrophysiological studies on mice that overexpress Aβ show impairment of long-term potentiation (LTP) that does not correlate with the extent of synaptic loss, amyloid deposition, or cell death (3–5). In addition, animals without detectable accumulation of Aβ have been reported to have behavioral deficits (6, 7). While examining gene expression in nerve growth factor-primed PC12 cells that had been exposed to Aβ1–42 for 3 h, we observed that a group of genes including CREB2 (ATF4) and ubiquitin C-terminal hydrolase, which have been implicated in the switch from early to late LTP, were regulated in a manner consistent with an Αβ-mediated inhibition of the cAMP-mediated signaling pathway for the consolidation of LTP.‖ The details of the biochemical pathway mediating the switch from early to late LTP have been worked out in aplysia and mice (8) and depend on the activation of the transcription factor CREB by phosphorylation by protein kinase A (PKA). This pathway is well preserved across species, functioning in olfactory memory in Drosophila as well as hippocampus-dependent memory in mice (9, 10). The gene-expression findings led us to look directly at the effects of Αβ treatment on PKA activity in cultured neurons. In the studies reported here, we examined the effects of Aβ1–42 on the function of the elements of the PKA/CREB pathway in cultured hippocampal neurons and correlated the changes observed with electrophysiological responses in hippocampal slice cultures.
Materials and Methods
Hippocampal Neuron Cultures.
Hippocampal cell cultures were prepared according to the method described previously (11). Briefly, fetuses at embryonic day 18 from timed pregnant Sprague–Dawley rats (Taconic Farms) were killed, and the hippocampi were removed. Neurons then were dissociated, plated on six-well plates coated with poly-L-lysine, and maintained in a defined serum-free medium. The obtained cultures resulted in a population enriched in large pyramidal neurons that constitute the main initial target in AD pathogenesis. After 5–6 days in vitro, cells were used for the experiments.
PKA Assay.
Hippocampal neurons (11), cultured for 5 days, were exposed to 1 μM Aβ for 3, 6, 12, and 24 h. Cells were harvested in 1× modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris⋅HCl, pH 7.4/1% Nonidet P-40/0.25% sodium deoxycholate/150 mM NaCl/1 mM EDTA/1 mM PMSF/1 μM aprotinin/1 μM leupeptin/1 μM pepstatin/1 μM NaF/1 μM sodium vanadate) containing 80 mM β-glycerophosphate after two washes in 1× PBS, and the lysate was transferred to a tube and centrifuged for 10 min at 10,000 × g at 4°C. The supernatants then were used to measure basal PKA activity by using the heptapeptide Leu-Arg-Arg-Ala-Ser-Leu-Gly (kemptide, Sigma) as substrate. Protein extracts were incubated in the presence of 10 μg/μl kemptide, 0.1 μCi/μl [γ-32P]ATP (1 Ci = 37 GBq), and 50 μM ATP with or without the PKA inhibitor amide fragment 6–22 (PKI, Sigma) for 20 min at 30°C. The reaction mixtures then were spotted onto phosphocellulose filters (Whatman). After a 5-min incubation the filters were washed three times in 20% orthophosphoric acid and counted. The results of the difference between the total and PKA-specific phosphorylation cpm are reported as percentages of the untreated controls. Aβ1–42 was obtained from David Teplow, Harvard University (Boston).
PKA Regulatory Subunit II α (RIIα) Determination.
After 5 days in vitro, dissociated hippocampal neurons were treated with 1 μM Aβ for 3, 6, and 12 h. Cells were harvested in 1× RIPA buffer, and the lysates were centrifuged for 20 min at 85,000 × g at 4°C. We used the supernatants for immunoblotting on a 12% PAGE with an antibody that recognizes an epitope at the C terminus of isoform IIα of PKA regulatory subunit (Santa Cruz Biotechnology). All samples were normalized against unphosphorylated extracellular response kinase (ERK). We used NIH IMAGE software to analyze the scanned blots and quantify the intensity of the protein signals.
Phosphorylated CREB (pCREB) Determination.
Levels of pCREB were measured after a 15-min exposure to 50 μM glutamate of hippocampal cultures that were preincubated with either Aβ alone or Aβ with 3 μM rolipram (Sigma). After the preincubation step, the culture medium was replaced by a defined salt medium containing 119 mM NaCl, 5 mM KCl, 2 mM CaCl2 20 mM Hepes, 1 μM glycine, 300 mM glucose, and 50 μM glutamate (Aβ and rolipram were added freshly to the culture medium at this point to maintain their concentration throughout the experiments). The osmolarity of the medium was adjusted to 325 milliosmol by using sucrose, and the pH was raised to 7.3 with 10 N NaOH. After 15 min, cells were harvested in 1× modified RIPA buffer with 80 mM glycerophosphate, and whole-cell extracts were made. The lysates were centrifuged at 10,000 × g for 10 min at 4°C. Protein was separated on 12% PAGE, and the immunoblotting was performed as described above. pCREB was detected with an antibody for CREB phosphorylated at Ser-133 (1:1,000 dilution, Upstate Biotechnology, Lake Placid, NY).
Measurement of LTP.
Hippocampal slice preparations were performed as described (12). Briefly, mice (C57/Bl6) were decapitated, and their hippocampi were removed. Transverse hippocampal slices of a thickness of 400 μm were made on a tissue chopper and transferred to an interface chamber, where they were maintained at 29°C. They were perfused (1–3 ml/min) with saline solution (124.0 mM NaCl/4.4 mM KCl/1.0 mM Na2HPO4/25.0 mM NaHCO3/2.0 CaCL2/2.0 mM MgSO4/10 mM glucose) continuously bubbled with 95% O2 and 5% CO2. Slices were permitted to recover for at least 90 min before recording. A concentric bipolar platinum-iridium stimulation electrode and a low-resistance glass recording microelectrode filled with saline solution (5 mΩ resistance) were placed in CA1 stratum radiatum to record the extracellular field excitatory postsynaptic potential (fEPSP). An input–output curve was used to set the baseline fEPSP at ≈35% of maximal slope. Baseline stimulation was delivered every minute (0.01-ms duration pulses) for 15 min before beginning the experiment to assure stability of the response. Aβ, forskolin, rolipram, H89, or vehicle was added (in 0.1% DMSO) to perfused slices for 20 min in interleaved experiments. LTP was induced by using θ-burst stimulation (4 pulses at 100 Hz, with the bursts repeated at 5 Hz and each tetanus including three 10-burst trains separated by 15 s). Responses were recorded for 1 h after tetanization. Data analysis was performed with a factorial ANOVA with post hoc correction. The results were expressed as mean ± SEM.
Results
When we measured the basal PKA phosphorylation activity in whole-cell extracts of hippocampal cells treated with 1 μM Aβ1–42 for 3, 6, 12, and 24 h and compared them to untreated cells, we observed that PKA activity fell rapidly in the treated cells, reaching 50% of control values in 3 h and 15% in 24 h (Fig. 1).
Figure 1.
Measurement of PKA basal activity. After 3 h of treatment with 1 μM Aβ, the kinase activity was decreased to 50.5% of the control (CTRL) ± 12.9 (n = 4) and remained stable near this value until 6 h (56.3 ± 24.2%, n = 4), dropping to 32.2 ± 16% (n = 4) at 12 h. After 24 h the residual kinase activity was 14.8 ± 12.3% (n = 4). No significant changes were detected in the control samples after 24 h.
The PKA complex is composed of two catalytic and two regulatory subunits, the interaction of which is controlled by cAMP. Only the dissociated catalytic subunits are active and capable of activating CREB by phosphorylating it at Ser-133. cAMP also regulates the proteosomal degradation of the regulatory subunits of PKA (13). To determine whether the loss of PKA activity in response to Aβ treatment was due to a failure to degrade the regulatory subunit, we examined the levels of isoform IIα of the PKA regulatory subunit, the predominant neuronal isoform, as a function of exposure to Aβ1–42. We exposed dissociated hippocampal neurons from embryonic day-18 rat brain that had been cultured for 5 days (11) to 1 μM Aβ1–42 for 3, 6, and 12 h and subjected the protein extracts to Western blotting by using an antibody against isoform IIα of the PKA regulatory subunit. When cells were treated with 1 μM Aβ1–42 there was a marked increase in RIIα at 6 h that was sustained at 12 h (Fig. 2). Only minimal cell death is seen within 48 h at 1 μM Aβ1–42. Pretreating the cultures with 0.1 mg/ml cycloheximide did not result in a fall in the RIIα (data not shown). These data suggest that persistence of the regulatory subunit does not play a role in diminishing PKA activity at times earlier than 6 h, although it may do so at later times.
Figure 2.
Aβ modulation of PKA RIIα levels: Western blot analysis of protein extracts from hippocampal cells showing time course and dose-response expressions. After 5 days in vitro, dissociated hippocampal neurons were treated with 1 μM Aβ for 3, 6, and 12 h. Cells were harvested in 1× RIPA buffer, and the lysates were centrifuged for 20 min at 85,000 × g at 4°C. We used the supernatants for immunoblotting with an antibody that recognizes an epitope at the C terminus of isoform IIα of the PKA regulatory subunit (Santa Cruz Biotechnology; n = 4). All samples are normalized against unphosphorylated ERK. Ctrl, control.
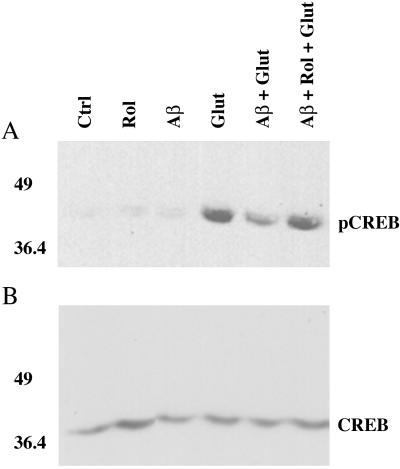
Because CREB phosphorylation depends on PKA activity, we extended our observations by measuring pCREB levels in response to a glutamate stimulus (50 μM). Glutamate is the major excitatory neurotransmitter in the hippocampus and mediates the neuronal response through CREB activation and LTP formation, eventually leading to synapse development and strengthening. In vitro studies on cultured hippocampal neurons (14, 15) have used brief glutamate stimuli to investigate the biochemical changes that underlie synaptic strength modification. We found that cultures pretreated for 2 h with 5 μM Aβ showed a 40–45% decrease in CREB phosphorylation in response to treatment with 50 μM glutamate for 15 min (Fig. 3). These results are consistent with those reported previously by using higher Aβ levels (16). To determine the role of cAMP in regulating this response, we added 3 μM rolipram, a type IV-specific phosphodiesterase inhibitor that has been reported to lead to increased cAMP levels during the preincubation with Aβ. This pretreatment totally blocked the effect of Aβ on CREB phosphorylation (Fig. 3), which is consistent with reports that rolipram treatment leads to increased cAMP levels, dissociation of the regulatory and catalytic subunits of PKA, and reinforcement of LTP (17).
Figure 3.
Modulation of glutamate-stimulated CREB phosphorylation by Aβ. A shows the phosphorylation of CREB at Ser-133. Aβ alone has no consistent effect on basal pCREB levels. Phosphorylation is stimulated strongly by treatment with 50 μM glutamate (Glut) for 15 min. Pretreatment with 5 μM Aβ for 2 h results in a 40–45% decrease in pCREB (Aβ + Glut). This effect is completely opposed by the addition of 3 μM rolipram to the preincubation mixture (Aβ + Rol + Glut). Neither rolipram nor Aβ by themselves had a significant effect on basal pCREB levels. Ctrl, control. B shows the same membranes reprobed with a phosphorylation-insensitive antibody for CREB (1:1,000, Santa Cruz Biotechnology).
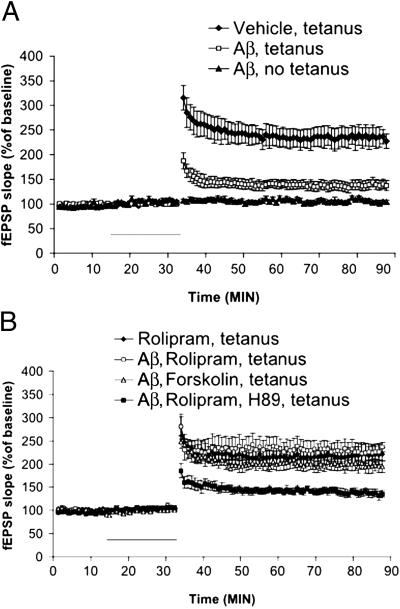
These results strongly suggest that inhibition of the PKA/CREB pathway underlies the effects of Aβ on LTP (18, 19) and that drugs that enhance PKA activity might be able to reverse this inhibition in the brain. To test this possibility, we studied LTP responses in acute mouse hippocampal slices. As expected from previous reports (18, 19) we found that Aβ strongly inhibited LTP generation in the CA1 hippocampal region when slices were exposed to 200 nM Aβ for 20 min before tetanic stimulation of the Schaeffer collateral pathway (137.54 ± 6.14% of baseline slope at 60 min after tetanus, n = 9; Fig. 4A). The amount of inhibition was statistically significant compared with control tetanized slices treated with vehicle alone (227.36 ± 14.53%, n = 10, P < 0.001; Fig. 4A). Aβ had no effect on basal synaptic responses both during (Fig. 4A) and 60 min after its application in experiments where no tetanic stimulation was applied (103.43 ± 1.88 versus 99.9 ± 9% of baseline slope at 60 min, n = 6; Fig. 4A). Addition of forskolin, a selective activator of adenylate cyclase, at 50 μM, showed a significant protection under the same conditions (194.27 ± 12.83% of baseline slope at 60 min after tetanus, n = 6, versus tetanized slices treated with Aβ, P < 0.01; Fig. 4B). The protection was not due to enhancement of LTP by forskolin that was maintained in the presence of Aβ, because forskolin alone without Aβ did not enhance tetanus-induced LTP (220 ± 14.15%, n = 4; data not shown), nor was is due to an action of forskolin on baseline transmission (104.34 ± 4.15 versus 99.9 ± 9% of baseline slope at 60 min, n = 4; data not shown). Consistent with these results, lower doses of forskolin (5 μM) also showed protection (165.14 ± 3.11% of baseline slope at 60 min after tetanus, n = 8; data not shown). Similarly, 1 μM rolipram, added to the incubation medium at the time of the stimulation, completely abolished the Aβ inhibition (236.43 ± 8.04% of baseline slope at 60 min after tetanus, n = 7, versus tetanized slices treated with Aβ, P < 0.01; Fig. 4B), whereas it had no effect on baseline transmission (103.86 ± 3.47 versus 99.9 ± 9% of baseline slope at 60 min, n = 4; data not shown). Moreover, as was the case with forskolin, rolipram alone did not produce LTP enhancement (221.15 ± 31.35%, n = 6; Fig. 4B), suggesting that the drug truly reverses the Aβ effect. To confirm that the detected changes of LTP response were due to modulation of PKA activity, 50 μM H89, a cell membrane-permeable PKA inhibitor, was added to the slices. This treatment completely abolished the protective effect of rolipram on Aβ-treated slices (135.23 ± 8.04% of baseline slope at 60 min after tetanus; Fig. 4B). H89 treatment in the absence of Αβ reduced LTP to levels identical to those obtained with Αβ (data not shown). These results indicate that Aβ depression of LTP generation in CA1 hippocampal region involves the cAMP/PKA/CREB pathway and are consistent with our biochemical observation that PKA is inhibited by Αβ. A recent study has demonstrated that naturally secreted oligomers of Aβ strongly inhibit LTP (20).
Figure 4.
Aβ-induced impairment of LTP is mediated through PKA activation. (A) LTP is reduced strongly by 200 nM Aβ for 20 min. Aβ did not affect baseline transmission. The horizontal bar indicates the period during which Aβ was added to the bath solution. fEPSP, extracellular field excitatory postsynaptic potential. (B) Application of 50 μM forskolin rescued the Aβ-induced impairment of LTP without affecting baseline transmission. Rolipram (1 μM) completely reversed the Aβ-induced impairment of LTP without affecting baseline transmission. However, H89 (50 μM) blocked the effect of rolipram. The horizontal bar indicates the period during which different drugs were added to the bath solution. Experiments in A and B were interleaved with each other.
Discussion
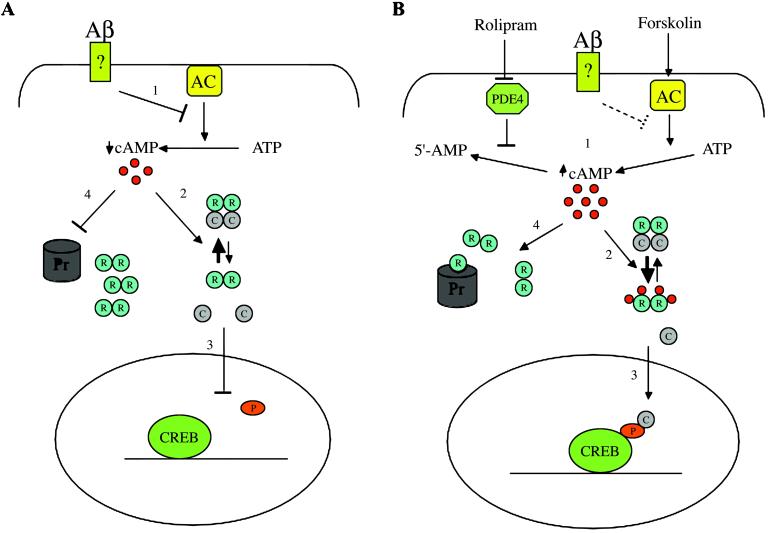
The data presented provide evidence that Aβ1–42, at concentrations well below those that induce cell death, causes a rapid and sustained decrease in the activity of PKA in cultured hippocampal neurons and a rapid inhibition of CREB phosphorylation in response to glutamate stimulation. Rolipram and forskolin, agents that increase the intracellular levels of cAMP, reverse this inhibition most probably by favoring the dissociation of regulatory and catalytic subunits of PKA and the restoration of PKA activity (Fig. 5). This loss of activity precedes the intracellular accumulation of the IIα isoform of the PKA regulatory subunit by several hours, suggesting that the dynamic balance between the free catalytic subunit and the tetrameric form of PKA is shifted initially in favor of the tetramer and that at later times there is an accumulation of regulatory subunit, possibly as a result of proteosomal inhibition (Fig. 5). Both of these would result from a decrease in cAMP levels in the cell.
Figure 5.
Aβ modulation of PKA/CREB pathway: a working hypothesis. (A) After binding with a putative membrane receptor Aβ inhibits adenylate cyclase (AC), leading to lower cAMP levels {1}, which shifts the equilibrium in the PKA complex toward the inactive tetramer {2}. As a consequence, the transcription factor CREB cannot be phosphorylated {3} and initiate transcription. Later, the lower cAMP levels lead to a decrease in proteosomal degradation of the RIIα subunit (R) {4}, resulting in its accumulation and a further shift of equilibrium toward the tetramer. (B) Rolipram and forskolin act to increase the intracellular levels of cAMP either decreasing its degradation by the phosphodiesterase PDE4 (rolipram) or increasing its synthesis by adenylate cyclase (forskolin) {1}. The increased cAMP levels can counteract Aβ effects on PKA. Four cAMP molecules cooperatively bind to one RIIα molecule, inducing a conformational change and reducing its affinity for the catalytic subunit (C) {2}. The freed catalytic subunit is activated and can phosphorylate CREB at Ser-133 {3}. The accumulation of cAMP could also drive the degradation of RIIα restoring the residual function of the proteasome (Pr) {4}, further shifting PKA to the active state. P, phosphoric group.
There is increasing experimental evidence placing PKA at the crossroads in the regulation of the synaptic response both at the pre- and postsynaptic sites (21). Its critical substrates include the transcription factor CREB and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptor, which mediates the excitatory response to glutamate in the hippocampus. These substrates are likely to be expressed differentially during development (embryonic versus postnatal) and as a function of culture conditions (dissociated neuronal cultures versus acute slices), and this variation may account for the differences in sensitivity to Aβ1–42 in dissociated hippocampal neurons and acute hippocampal slices seen in our studies. Nonetheless two findings from our experiments strongly suggest that PKA inhibition by Aβ1–42 plays a key role in both experimental models. First, rolipram, a well known activator of PKA, is able to consistently rescue the phosphorylation of CREB at Ser-133 in hippocampal neuronal cultures exposed to Aβ1–42. Second, perfusion of acute hippocampal slices with either rolipram or forskolin, two agents used to trigger PKA kinase activity, promotes the recovery of LTP formation. Furthermore, the pretreatment of slices with the selective PKA inhibitor H89 abolishes the effects of the two drugs.
Studies on amyloid precursor protein transgenic animals carrying the Swedish mutation show decreased ERK2 phosphorylation and CREB phosphorylation at advanced ages (20 months) when compared with wild-type animals. This inhibition has been linked to chronic Aβ activation of the α-7-nicotinic receptor (22). However, this mechanism does not seem to explain acute Aβ-induced inhibition of LTP, because acute activation of the α-7-nicotinic receptor leads to increased ERK2 activation, which would be expected to activate CREB and facilitate LTP (22).
Our results do not exclude the possibility that Aβ exerts its effects by impairment of other pathways, such as the calmodulin kinase or mitogen-activated protein kinase pathways, at the same time or at later stages of exposure. However, they clearly show that reversal of PKA inhibition is sufficient to restore CREB phosphorylation and LTP.
The fact that the PKA/CREB pathway, widely recognized to be important for memory acquisition, is selectively impaired by Aβ at sublethal concentrations underscores the importance of the pathway itself and offers a molecular explanation of the discrepancy between behavioral deficits and pathological findings described in amyloid precursor protein transgenic mice. It may be that slightly to moderately elevated levels of Aβ could impair the excitatory response at single synapses acutely. When this happens for an extended period, it could lead to the progressive exclusion of those synapses that were not strengthened from the cellular network and to limited residual function of the hippocampus. More importantly these mechanisms would open up a temporal frame at which it still could be possible to intervene therapeutically before any irreversible damage has ensued. This possibility finds support in studies that describe either the correlation between early detectable cognitive changes and prediction of developing dementia late in life (23) or the importance of education as a protective factor (24, 25). We can hypothesize that the correlation between poorer performance in psycholinguistic tests and the probability of developing AD later in life might be explained by impaired neuronal plasticity throughout development due to higher Aβ levels in the brain. Because the PKA/CREB pathway has been shown to induce functional presynaptic boutons in the hippocampus (26), it is possible that profound inhibition by very high levels of Aβ, such as are found in Down's syndrome, might result in extensive alterations in synaptic development and mental retardation. Stimuli such as education would act to mitigate this effect, maybe involving alternative pathways.
These results also suggest that Aβ may function as a modulator of memory storage, perhaps in response to stress or at times when the brain is confronted with more information than it process effectively. If this were so, we would expect to see Aβ levels increase as a function of the induction of high levels of neuronal activity. This possibility is supported by the amnestic actions of infused amyloid peptides (27, 28).
Finally, the results presented here suggest a therapeutic avenue that might be effective in preventing or delaying the onset of AD and in ameliorating the memory defect in the early phases of the disease. It is possible that long-term treatment of patients at risk for AD with agents that elevate cAMP could reverse the risks associated with higher Aβ levels.
Acknowledgments
We acknowledge helpful discussions from Jaya Padmanabhan, Carol Troy, Asa Abeliovich, James Angelastro, Eric Kandel, and Lloyd Greene in this work. These studies were supported by grants from the National Institutes of Health and the Alzheimer's Association.
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid β-peptide
- LP
long-term potentiation
- PKA
protein kinase A
- RIIα
regulatory subunit II α
- ERK
extracellular response kinase
- pCREB
phosphorylated CREB
Footnotes
Vitolo, O. V., Angelastro, J. M., Greene, L. A. & Shelanski, M. L. (2001) Am. Soc. Cell Biol. Meeting Abstract, 2063 (abstr.).
References
- 1.Mucke L, Masliah E, Yu G Q, Mallory M, Rockenstein E M, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troy C M, Rabacchi S A, Friedman W J, Frappier T F, Brown K, Shelanski M L. J Neurosci. 2000;20:1386–1392. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman P F, White G L, Jones M W, Cooper-Blacketer D, Marshall V J, Irizarry M, Younkin L, Good M A, Bliss T V, Hyman B T, et al. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 4.Giacchino J, Criado J R, Games D, Henriksen S. Brain Res. 2000;876:185–190. doi: 10.1016/s0006-8993(00)02615-9. [DOI] [PubMed] [Google Scholar]
- 5.Larson J, Lynch G, Games D, Seubert P. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 6.D'Hooge R, Nagels G, Westland C E, Mucke L, De Deyn P P. NeuroReport. 1996;7:2807–2811. doi: 10.1097/00001756-199611040-00080. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi F, Richards S J, Beyreuther K, Salbaum M, Carlson G A, Dunnett S B. NeuroReport. 1991;2:781–784. doi: 10.1097/00001756-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Abel T, Nguyen P V, Barad M, Deuel T A, Kandel E R, Bourtchouladze R. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 9.Bach M E, Barad M, Son H, Zhuo M, Lu Y F, Shih R, Mansuy I, Hawkins R D, Kandel E R. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva A J, Kogan J H, Frankland P W, Kida S. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 11.Goslin K, Asmussen H, Banker G. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 12.Son H, Lu Y F, Zhuo M, Arancio O, Kandel E R, Hawkins R D. Learn Mem. 1998;5:231–245. [PMC free article] [PubMed] [Google Scholar]
- 13.Chain D G, Casadio A, Schacher S, Hegde A N, Valbrun M, Yamamoto N, Goldberg A L, Bartsch D, Kandel E R, Schwartz J H. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. [DOI] [PubMed] [Google Scholar]
- 14.Antonova I, Arancio O, Trillat A C, Wang H G, Zablow L, Udo H, Kandel E R, Hawkins R D. Science. 2001;294:1547–1550. doi: 10.1126/science.1066273. [DOI] [PubMed] [Google Scholar]
- 15.Malgaroli A, Tsien R W. Nature (London) 1992;357:134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- 16.Tong L, Thornton P L, Balazs R, Cotman C W. J Biol Chem. 2001;276:17301–17306. doi: 10.1074/jbc.M010450200. [DOI] [PubMed] [Google Scholar]
- 17.Barad M, Bourtchouladze R, Winder D G, Golan H, Kandel E. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullen W K, Suh Y H, Anwyl R, Rowan M J. NeuroReport. 1997;8:3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Itoh A, Akaike T, Sokabe M, Nitta A, Iida R, Olariu A, Yamada K, Nabeshima T. Eur J Pharmacol. 1999;382:167–175. doi: 10.1016/s0014-2999(99)00601-9. [DOI] [PubMed] [Google Scholar]
- 20.Walsh D M, Klyubin I, Fadeeva J V, Cullen W K, Anwyl R, Wolfe M S, Rowan M J, Selkoe D J. Nature (London) 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 21.Cowan M W, Sudhof T C, Stevens C F. Synapses. Baltimore: Johns Hopkins Univ. Press; 2001. [Google Scholar]
- 22.Dineley K T, Westerman M, Bui D, Bell K, Ashe K H, Sweatt J D. J Neurosci. 2001;27:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snowdon D A, Kemper S J, Mortimer J A, Greiner L H, Wekstein D R, Markesbery W R. J Am Med Assoc. 1996;275:528–532. [PubMed] [Google Scholar]
- 24.Stern Y, Gurland B, Tatemichi T K, Tang M X, Wilder D, Mayeux R. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 25.Zhang M Y, Katzman R, Salmon D, Jin H, Cai G J, Wang Z Y, Qu G Y, Grant I, Yu E, Levy P, et al. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, Zablow L, Kandel E R, Siegelbaum S A. Nat Neurosci. 1999;2:24–30. doi: 10.1038/4525. [DOI] [PubMed] [Google Scholar]
- 27.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1991;88:3363–3366. doi: 10.1073/pnas.88.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurice T, Lockhart B P, Privat A. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]