Abstract
The idea that memory is encoded by means of synaptic growth is not new. However, this idea has been difficult to demonstrate in the mammalian brain because of both the complexity of mammalian behavior and the neural circuitry by which it is supported. Here we examine how eyeblink classical conditioning affects synapse number within the cerebellum; the brain region essential for long-term retention of the conditioned response. Results showed eyeblink-conditioned rats to have significantly more synapses per neuron within the cerebellar interpositus nucleus than both explicitly unpaired and untrained controls. Further analysis showed that the increase was caused by the addition of excitatory rather than inhibitory synapses. Thus, development of the conditioned eyeblink response is associated with a strengthening of inputs from precerebellar nuclei rather than from cerebellar cortex. These results demonstrate that the modifications of specific neural pathways by means of synaptogenesis contributes to formation of a specific memory within the mammalian brain.
For every act of memory, every exercise of bodily aptitude, every habit, recollection, train of ideas, there is a specific neural grouping, or co-ordination, of sensations and movement, by virtue of specific growths in cell junctions (1).
The neural circuits critical for the acquisition and performance of the conditioned eyeblink response are localized to the cerebellum (2). Information regarding the unconditioned stimulus (US) and conditioned stimulus (CS) converge within both the cerebellar cortex and the interpositus nucleus. CS information is relayed via ponto-cerebellar projections, whereas US information is relayed via the olivo-cerebellar pathway (2, 3). Although the cerebellar cortex is involved in modulating some aspects of the conditioned response (CR) (4, 5), the interpositus nucleus is the critical brain structure supporting long-term retention of the CR (2, 6, 7). Neuronal activity within the interpositus nucleus is highly correlated with development of the CR (5, 7), and inactivation of the interpositus prevents both CR acquisition and performance (8).
Although the locus of the memory trace is clear, the cellular mechanisms underlying the formation of the CS/US association are poorly understood. Several mechanisms have been proposed, including increases in the intrinsic excitability of interpositus neurons and reduced inhibition via depression of Purkinje cell activity (9). The fact that inhibition of specific synaptic enzymes (10) and neurotransmitter receptors (11) within the interpositus nucleus impair learning suggests that changes in synaptic function are involved. Transient changes in enzyme or receptor activity, however, would seem incapable of supporting the long-term encoding of the CS/US association. Recent work has shown that microinjections of a protein synthesis inhibitor into the interpositus nucleus impairs the acquisition but not the expression of the CR (12). This finding suggests that strengthening of the CS pathway may involve more permanent changes in cell structure. In the present experiment, we tested the hypothesis that development of the conditioned eyeblink response is associated with synapse formation within the interpositus nucleus.
Materials and Methods
Eyeblink Conditioning.
Adult rats were given i.p. injections of pentobarbital (1.6 ml/kg) and atropine sulfate (0.67 mg/kg) for anesthesia. The rat's head was positioned in a stereotaxic head holder and fitted with differential electromyography (EMG) electrodes that were implanted in the left eyelid muscle (orbicularis oculi) and a ground electrode was attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins in a plastic connector, which was secured to the skull with dental acrylic. A bipolar stimulating electrode (for delivering the shock US) was implanted subdermally, immediately caudal to the left eye. The bipolar electrode terminated in a plastic connector that was secured to the skull by dental acrylic.
The conditioning apparatus consisted of four small-animal sound attenuation chambers (BRS/LVE, Laurel, MD). Within each sound attenuation chamber was a small-animal operant chamber (BRS/LVE), where the rats were kept during conditioning. One wall of the operant chamber was fitted with two speakers. The electrode leads from the rat's headstage were connected to peripheral equipment and a desktop computer. Custom computer software controlled the delivery of stimuli and the recording of eyelid EMG activity. EMG activity was recorded differentially, filtered, and amplified. Animals in the Paired condition (n = 8) were given paired training sessions, which consisted of 100 paired presentations of a tone CS (300 msec, 85 dB SPL, 2.0 kHz) and a periorbital shock US (25 msec, 2.5 mA). The CS coterminated with the US, yielding an interstimulus interval of 275 msec. Animals in the Unpaired condition (n = 8) were given explicitly unpaired presentations of the CS and US. A Sit control group (n = 8) was placed in the chamber for 50 min per day (the same duration as the paired and unpaired training sessions), but was not presented with the CS or US. CRs were defined as responses that crossed a threshold of 0.4 units (amplified and integrated units) above baseline during the CS period after 80 msec. Behavioral data were examined from computer records of EMG responses.
Tissue Preparation.
Animals were deeply anesthetized with pentobarbital (120 mg/kg) and transcardially perfused with 2% paraformaldehyde/2.5% glutaraldehyde in 0.10 M phosphate buffer. The cerebellum was then serially sectioned (300 μm) in the horizontal plane on a vibratome. Each section was then examined under a dissection microscope, and samples of tissue containing the deep cerebellar nuclei were removed (approximately −5.90 mm dorsal ventral bregma). These tissue blocks were then washed in 0.10 M cacodylate, postfixed in 2% osmium tetroxide/1.5% potassium ferrocyanide in 0.10 M cacodylate buffer for 2 h, and en bloc stained with 2% uranyl acetate for 45 min. Samples were then dehydrated through a series of alcohols before being transferred into propylene oxide and gradually embedded in Eponate resin. One-micrometer sections were taken from prospective tissue blocks and examined under a light microscope. Only those tissue blocks where the anterior interpositus could be clearly distinguished from the other nuclei were then analyzed. All tissue samples were coded with respect to treatment condition before stereological analysis.
Estimating Changes in Synapse Number.
Behavioral training can lead to an increase in neuropil volume resulting from dendritic (13, 14) and glial (15) hypertrophy. Because of this potential volume increase, measures of synapse density alone may not reveal changes in synapse number. In conditions of stable neuron number, however, changes in neuropil volume can be accounted for by estimating neuron density. By obtaining the density of neurons and the density of synapses per unit volume, the number of synapses per neuron can be calculated and used to measure changes in synapse number (16–18).
Neuron Density.
Approximately 80, serial 1-μm sections were taken through the horizontal plane of the anterior interpositus nucleus and stained with Toluidine blue. Using a computer-assisted microscope, the physical disector (19) was used to obtain estimates of neuron density. Pairs of sections within each series were compared in succession. The first section in the series was considered the Reference section and the second the Lookup section, then the second section became the Reference section for the third section and so on. Neuronal nuclei were identified by the presence of a central nucleolus within a pale nucleus and frequently by the oval to pyramidal shape of the surrounding cell soma. Irregularly shaped, smaller nuclei with extensive chromatin characteristic of glial cells were not counted. Within an unbiased counting frame (410 μm × 272 μm), the number of neuronal nuclei that were present in the Reference section but not the Lookup section (Q−) were counted. The disector volume of tissue through which the cells were counted (Vdis) was given by
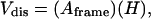 |
where Aframe was the area of the counting frame and H was the section thickness (1 μm) multiplied by the number of sections. Neuron density (NvNeuron) was then determined by
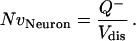 |
Neurons were sampled through ≈8.92 × 106 μm3 of neuropil (Vdis) within each animal.
Synapse Density.
The density of synapses per cubic μm (Nvsyn) was also obtained by using the physical disector from a series of approximately 20 serial, silver-gray (70 nm) sections taken through the interpositus by using a diamond knife and ultramicrotome. Serial electron micrograph four picture montages (print magnification = ×22,000) were taken from the same position in each section. The number of synapses present in the Reference section but not in the Lookup section (Q−) were counted. The volume through which the synapses were counted (Vdis) was determined by multiplying the area of the counting frame (72 μm2) by the number of sections (each being 70-nm thick). Nvsyn was then given by Q−/Vdis. The number of synapses/neuron was then given by (Nvsyn)/(Nvneur). Synapses were sampled through approximately 403 μm3 of neuropil (Vdis) within each animal.
Synapse Classification.
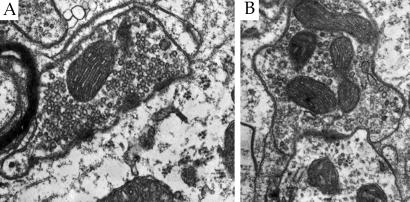
To provide a more detailed description of how training might affect anterior interpositus circuitry, presynaptic elements were classified as either excitatory or inhibitory. This classification was made on the basis of synaptic vesicle and postsynaptic density morphology. Excitatory synapses were identified by the presence of round synaptic vesicles within the presynaptic element (Fig. 1A). Inhibitory synapses were identified by the presence of at least three oval or flattened synaptic vesicles (20–22) (Fig. 1B).
Figure 1.
Electron micrographs (×32,000) illustrating an excitatory and inhibitory synapse within the anterior interpositus nucleus. Excitatory synapses (A) were identified by the presence of round synaptic vesicles. Inhibitory synapses (B) were identifed by the presence of at least three flattened synaptic vesicles.
Results
Behavioral.
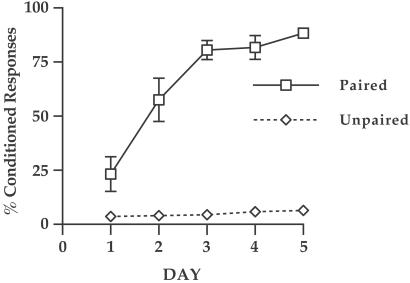
A within-subjects ANOVA with CONDITION as a between-subject factor revealed a significant CONDITION × DAY interaction [F(1,4) = 22.64; P < 0.001)]. Paired animals (n = 8) exhibited a significant increase in the percentage of trials with CRs as training progressed (Fig. 2). Unpaired animals (n = 8) did not show any significant increase in the percentage of trials with continued training.
Figure 2.
Development of CRs across the 5 days of training. Paired animals (n = 8) exhibited a significant increase in the percentage of trials with CRs as training progressed. Unpaired animals (n = 8) did not show any significant increase in the percentage of trials with continued training.
Anatomical.
ANOVA with CONDITION as a between-subject factor was conducted on all anatomical data. This analysis revealed a significant effect of CONDITION on the density of neurons within the anterior interpositus [F(2,21) = 8.53, P < 0.05)]. Paired animals had a lower neuron density than both the Unpaired and Sit animals [Fisher's least significant difference (LSD); P < 0.05)] (Table 1). No significant differences in the overall density of synapses [F(2,21) = 0.21; P >0.05] the density of excitatory [F(2,21) = 1.12; P > 0.05] or inhibitory synapses [F(2,21) = 0.04; P > 0.05)] were found (Table 1).
Table 1.
Neuron and synapse density
| Condition | Neurons per mm3 | Synapses per mm3 | E-Synapses per mm3 | I-Synapses per mm3 |
|---|---|---|---|---|
| Paired | 8,851 (473)* | 3.80 (0.44) × 107 | 2.73 (0.24) × 107 | 1.07 (0.31) × 107 |
| Unpaired | 11,687 (536) | 3.43 (0.22) × 107 | 2.29 (0.11) × 107 | 1.14 (0.17) × 107 |
| Sit | 11,008 (512) | 3.51 (0.49) × 107 | 2.34 (0.34) × 107 | 1.17 (0.32) × 107 |
Mean density of neurons, total synapses (Synapses), excitatory synapses (E-Synapses), and inhibitory synapses (I-Synapses) per cubic millimeter (± SEM) within the anterior interpositus. Animals in the Paired condition exhibited a significant decrease in neuron density in comparison to animals in both the Unpaired and Sit conditions (*, P < 0.05; Fisher's LSD). No significant differences in synapse density were observed.
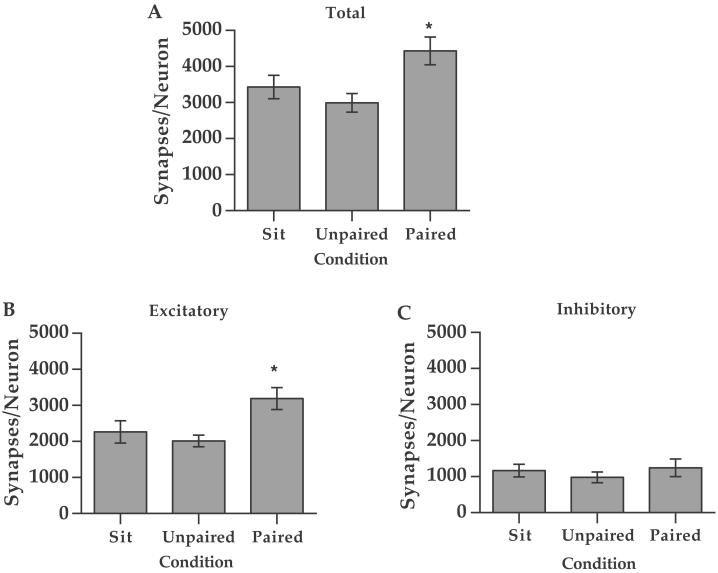
A significant effect of CONDITION was found on the total number of synapses/neuron within the anterior interpositus [F(2,21) = 4.43, P < 0.05]. Paired animals had a significantly greater number of synapses than both the Unpaired and Sit animals (Fisher's LSD; P < 0.05) (Fig. 3A). Further, a significant effect of CONDITION was also found on the number of excitatory synapses/neuron [F(2,21) = 3.35; P < 0.05]. Paired animals had a significantly greater number of excitatory synapses than both the Unpaired and Sit animals (Fisher's LSD; P < 0.05) (Fig. 3B). However, no significant difference in the number of inhibitory synapses was found between any two groups [F(2,21) = 0.238; P > 0.05].
Figure 3.
(A) The mean total number of synapses per neuron (±SEM) within the interpositus nucleus in the Paired (n = 8), Unpaired (n = 8), and Sit (n = 8) animals. Paired animals had a significantly greater number of synapses than both the Unpaired and Sit animals (*, Fisher's LSD; P < 0.5). (B and C) Mean number of excitatory (B) and inhibitory (C) synapses per neuron (±SEM) within the interpositus nucleus. Paired animals had a significantly greater number of excitatory synapses than both the Unpaired and Sit animals (*, Fisher's LSD; P < 0.05). No significant difference in the number of inhibitory synapses was found between any two groups.
Discussion
Although increases in synapse number have been reported within the mammalian brain after behavioral training (23), there is no evidence that the additional synapses are involved in the encoding of specific information resulting from the training experience. This is because of the global nature of the training paradigms, the complexity of the resulting behavioral changes, and the fact that increases in synapse number are often observed across multiple brain areas. The present experiment avoids these problems by examining a simple learned behavior that is supported by well defined neural circuitry. The results show an increase in synapse number that is consistent with both computational models of cerebellar learning (24) and physiological changes observed during learning (7, 8).
The magnitude of the learning-specific increase in excitatory synapses is substantial. If every cerebellar memory produced such a large-scale change in synapses, the cerebellum would quickly reach its maximum capacity. However, it is unlikely that there is a simple linear relationship between learning and increases in synapse number. A more plausible explanation would be that the increased synapse number represents a strengthening of neural pathways involved in learning a specific class of behaviors. Thus, increases in synapse number could both support newly acquired behavior and facilitate further learning involving the same pathway. Furthermore, measuring synapse number does not reveal changes in the pattern of synaptic connectivity. The development of additional CS/US associations might involve synaptic remodeling within the interpositus whereby some synaptic contacts are lost and others added without significantly altering total synapse number. An alternative view is that eyeblink conditioning is an unusually powerful source of sensory stimulation, activating many afferent fibers simultaneously. The collective effect of large-scale afferent activation might be large-scale changes in synaptic connections. This hypothesis could be evaluated by examining the relationship between the magnitude of the stimulus inputs, the magnitude of learning, and the magnitude of synaptogenesis in the interpositus nucleus.
Although excitatory inputs to the interpositus nucleus originate from both the pontine nuclei and the inferior olive, several lines of evidence suggest that the increase in excitatory synapses involve mossy fibers (the conditioned stimulus pathway). First, the eyeblink CR can be elicited by presentations of just the CS (25) and the onset of the CR precedes the onset of the US. These observations suggest that the memory trace is activated by stimulating the CS pathway (mossy fibers). Second, the training-dependent increases in neuronal activity observed within the interpositus nucleus could be supported by increases in the efficacy of mossy fiber input. Third, there is a dramatic decrease in the threshold to elicit a CR via stimulation of the mossy fiber inputs after conditioning (26). The decreased threshold could be mediated by an increase in the number of mossy fiber synapses within the interpositus nucleus. Finally, the production of the CR is associated with a decrease in activity within the inferior olive (27). Thus, conditioning is associated with reduced rather than increased input from the inferior olive to the interpositus nucleus.
The stability of inhibitory synapse number within the interpositus nucleus is also of interest. Purkinje cells within the cerebellar cortex are the primary source of inhibitory input to the deep cerebellar nuclei. The failure to find any significant difference in the number of inhibitory synapses/neuron suggests that the inputs to the interpositus nucleus from the cerebellar cortex are not affected by conditioning. It is has been hypothesized that parameters of the CR such as timing and amplitude are mediated by plasticity within the Purkinje cells (5, 6), which then influence the CR via output to the interpositus nucleus. This hypothesis is consistent with computational models of cerebellar learning that predict plasticity within cerebellar circuitry to be localized to parallel fiber to Purkinje cell and mossy fiber to interpositus synapses (24). The result is also consistent with recent work showing that inhibitory inputs from Purkinje cells are involved in the timing of the CR but not its expression (28)
Although synaptogenesis is an attractive mechanism for encoding the association between the CS and US within the interpositus nucleus, other forms of plasticity may also be involved. For example, increases in the intrinsic excitability of interpositus neurons (29) and reduced inhibition via depression of Purkinje cell activity have also been proposed (30). Furthermore, the behavioral demands associated with a given training experience may dictate the nature and locus of plasticity. For example, contrary to conditioning of the eyeblink response, the development of more complex motor skills involving coordinated limb movements is not associated with changes in synapse number within deep cerebellar nuclei (20). In addition, trace eyeblink conditioning was associated with an increase in the area of synaptic contacts within the hippocampus but not an overall increase in synapse number (31). Thus, memory may be encoded via several mechanisms that coalesce in different ways across multiple brain regions (32). The use of simple learning paradigms, such as eyeblink conditioning, provides the opportunity to determine which cellular changes support specific types of memory. The present results demonstrate that classical conditioning of the eyeblink response is associated with an increase in excitatory synapses within the interpositus nucleus. Synapse formation then represents a neural mechanism by which long-term memory is encoded within the cerebellum.
Acknowledgments
We thank Bryan Kolb and Sergio Pellis for their thoughtful comments on the manuscript. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes for Health Research, Alberta Heritage Foundation for Medical Research and Canada Foundation for Neuroscience (to J.A.K.), and National Institute of Neurological Disorders and Stroke Grant NS-38890 (to J.H.F.).
Abbreviations
- CR
conditioned response
- CS
conditioned stimuli
- US
unconditioned stimuli
- LSD
least significant difference
- EMG
electromyography
References
- 1.Bain A. Mind and Body: The Theories of Their Relation. London: Henry King; 1873. [Google Scholar]
- 2.Thompson R F. Science. 1986;223:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz J E. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 4.Lavond D G, Steinmetz J E. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- 5.Perrett S P, Ruiz B P, Mauk M D. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick D A, Thompson R F. J Neurosci. 1991;4:2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman J H, Nicholson D A. Brain Res. 1999;833:225–233. doi: 10.1016/s0006-8993(99)01547-4. [DOI] [PubMed] [Google Scholar]
- 8.Krupa D J, Thompson J K, Thompson R F. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 9.Lavond D G, Hembree T L, Thompson R F. Brain Res. 1985;326:179–182. doi: 10.1016/0006-8993(85)91400-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Steinmetz J E. Brain Res. 2000;856:193–201. doi: 10.1016/s0006-8993(99)02429-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Steinmetz J E. Brain Res. 2000;887:144–156. doi: 10.1016/s0006-8993(00)03005-5. [DOI] [PubMed] [Google Scholar]
- 12.Bracha V, Irwin K B, Webster M L, Wunderlich D A, Stachowiak M K, Bloedel J R. Brain Res. 1998;788:169–178. doi: 10.1016/s0006-8993(97)01535-7. [DOI] [PubMed] [Google Scholar]
- 13.Greenough W T, Larson J R, Withers G S. Behav Neural Biol. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 14.Kleim J A, Swain R A, Czerlanis C M, Kelly J, Pipitone M A, Greenough W T. Neurobiol Learn Mem. 1997;67:29–33. doi: 10.1006/nlme.1996.3742. [DOI] [PubMed] [Google Scholar]
- 15.Anderson B J, Li X, Alcantara A, Isaacs K R, Black J E, Greenough W T. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- 16.Black J E, Isaacs K, Anderson B J, Alcantara A A, Greenough W T. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleim J A, Lussnig E, Schwarz E R, Greenough W T. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleim J A, Swain R A, Armstrong K E, Napper R M A, Jones T A, Greenough W T. Neurobiol Learn Mem. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- 19.Sterio D C. J Microsc (Oxford) 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 20.Kleim J A, Pipitone M A, Czerlanis C, Greenough W T. Neurobiol Learn Mem. 1998;69:290–306. doi: 10.1006/nlme.1998.3828. [DOI] [PubMed] [Google Scholar]
- 21.Palay S L, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. New York: Springer; 1974. [Google Scholar]
- 22.Uchizono K. Nature (London) 1965;207:642–643. doi: 10.1038/207642a0. [DOI] [PubMed] [Google Scholar]
- 23.Bailey C H, Kandel E R. Annu Rev Physiol. 1993;16:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 24.Mauk M D, Donegan N H. Learn Mem. 1997;4:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz J E, Lavond D G, Thompson R F. Synapse. 1989;3:225. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- 26.Tracy J A, Thompson J K, Krupa D J, Thompson R F. Behav Neurosci. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- 27.Sears L L, Steinmetz J E. Brain Res. 1991;545:114–122. doi: 10.1016/0006-8993(91)91276-7. [DOI] [PubMed] [Google Scholar]
- 28.Bao S, Chen L, Kim J J, Thompson R F. Proc Natl Acad Sci USA. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizenman C D, Linden D J. Nat Neurosci. 1999;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- 30.Hansel C, Linden D J, D'Angelo E. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 31.Geinisman Y, Disterhoft J F, Gundersen H J, Mcechron M D, Persina I S, Power J M, van der Zee E A, West M J. J Comp Neurol. 2000;417:49–59. [PubMed] [Google Scholar]
- 32.Chapman P F. Nat Neurosci. 2001;4:556–558. doi: 10.1038/88367. [DOI] [PubMed] [Google Scholar]