Abstract
The Xenopus oocyte is a very powerful tool for studies of the structure and function of membrane proteins, e.g., messenger RNA extracted from the brain and injected into oocytes leads to the synthesis and membrane incorporation of many types of functional receptors and ion channels, and membrane vesicles from Torpedo electroplaques injected into oocytes fuse with the oocyte membrane and cause the appearance of functional Torpedo acetylcholine receptors and Cl− channels. This approach was developed further to transplant already assembled neurotransmitter receptors from human brain cells to the plasma membrane of Xenopus oocytes. Membranes isolated from the temporal neocortex of a patient, operated for intractable epilepsy, were injected into oocytes and, within a few hours, the oocyte membrane acquired functional neurotransmitter receptors to γ-aminobutyric acid, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, kainate, and glycine. These receptors were also expressed in the plasma membrane of oocytes injected with mRNA extracted from the temporal neocortex of the same patient. All of this makes the Xenopus oocyte a more useful model than it already is for studies of the structure and function of many human membrane proteins and opens the way to novel pathophysiological investigations of some human brain disorders.
Keywords: γ-aminobutyric acid receptors‖kainate receptors
About two decades ago it was found that injecting mRNA isolated from the electric organ of Torpedo into Xenopus oocytes led to the heterologous expression of functional acetylcholine receptors (AcChoRs) in the oocyte plasma membrane (1). This process offered a new and useful approach to the study of many neurotransmitter receptors and ionic channels from vertebrate brains by us and others (2–5). With this procedure the oocytes translate the heterologous mRNA, process the products, and incorporate them into their plasma membrane where they form functional proteins. However, with DNA or RNA injections (mRNA or cRNA), the foreign receptors are inserted into a membrane that already contains other native proteins coupled to various signaling cascades. On the other hand, receptors in their native cells may have a different cohort of associated proteins and lipids that could confer to the receptors properties different from those seen in oocytes. To address this question, and to bypass the oocyte's protein processing machinery as well as the host oocyte membrane and its associated proteins, a few years ago a novel method was developed to incorporate into the oocyte membrane foreign AcChoRs and Cl− channels, already assembled in the membrane of electrocytes of the electric organ of Torpedo. Significantly, many steps between foreign mRNA injection into the oocyte and the insertion of AcChoRs into the oocyte's plasma membrane were avoided by injecting the oocytes with membrane vesicles from the electroplaques. With this method the oocyte membrane efficiently acquired functional AcChoRs and Cl− channels, still embedded in their native membrane (6, 7).
Using similar, but simplified, procedures, we describe here the transplantation of human neurotransmitter receptors, assembled in membranes of cells from the temporal neocortex, into the plasma membrane of Xenopus oocytes. This approach will help to elucidate the properties of many neurotransmitter receptors as they occur in the human brain, because they are incorporated into the host oocyte membrane while still in their native cell membrane. Thus, these receptors presumably retain their original subunit stoichiometry, structural features, and complement of associated proteins and lipids. Furthermore, because in the present case the membranes were obtained postoperatively, from a patient with intractable epilepsy, this approach may become a formidable tool for diagnostic and clinical investigations.
Materials and Methods
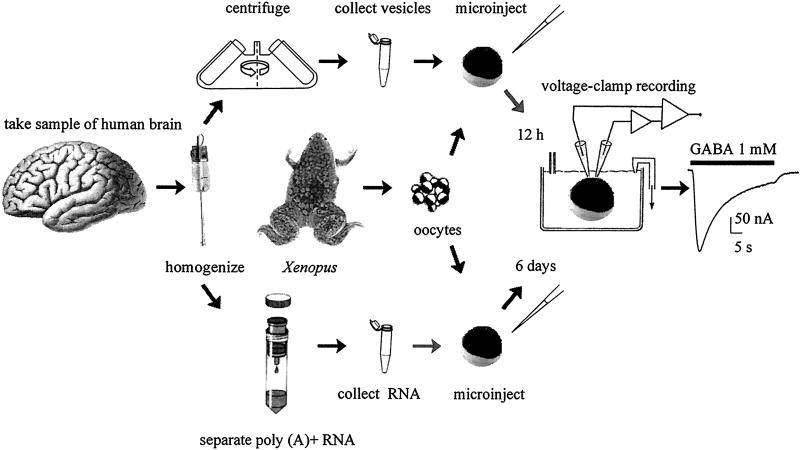
The methods used are shown in Fig. 1.
Figure 1.
Diagram of the procedures used to transplant neurotransmitter receptors from the human brain to the oocyte plasma membrane by injecting either brain cell membranes (Upper) or brain mRNA (Lower) into Xenopus oocytes.
Membrane Preparation.
Membranes were prepared as described (6) with slight modifications. All procedures with human tissue (temporal lobe neocortex) were performed with the informed consent of the patient and were approved by the Ethics Committee of the University of Rome “La Sapienza,” First Faculty of Medicine. Using a Teflon glass homogenizer, about 0.5 g of previously frozen tissue was homogenized in 2 ml of glycine buffer (200 mM glycine/150 mM NaCl/50 mM EGTA/50 mM EDTA/300 mM sucrose), plus 20 μl of protease inhibitors (Sigma P2714), pH 9 with NaOH. The filtrate was centrifuged for 15 min at 9,500 × g in a Beckmann centrifuge (C1015 rotor). The supernatant was then centrifuged for 2 h at 100,000 × g in a SW40 rotor at 4°C. The pellet was washed, resuspended in 5 mM glycine, used directly (Fig. 1) or aliquoted, and kept at −80°C for use later.
mRNA Preparation.
For comparative studies, poly(A)+ RNA was extracted from 0.5 g of the same frozen tissue as was used for the membrane preparation by using Fast Track (Invitrogen) according to the manufacturer's instructions. The poly(A)+ RNA was dissolved in water (≈1 ng/nl) and used directly (Fig. 1) or stored at −80°C in 2-μl aliquots.
Oocyte Injection of Membrane Vesicles and mRNA.
Preparation of Xenopus laevis oocytes and mRNA injection procedures were as described (8). Oocytes were injected with membranes (≈100 nl; 1–2 mg protein per ml) dissolved in 5 mM glycine at 1:1, 1:2, and 1:10 ratio or 50–100 ng of poly(A)+ RNA and maintained at 16°C in Barth's solution plus antibiotics until the electrophysiological recordings were performed. As controls, some oocytes from the same batch were injected with only 100 nl of water or 5 mM glycine.
Electrophysiology.
From a few hours after membrane injection, and 5–7 days after mRNA injection, membrane currents were recorded from voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl (9). The oocytes were placed in a recording chamber (approximately 0.1 ml) and perfused continuously, 10–11 ml/min, with oocyte Ringer's solution (82.5 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/1 mM MgCl2/5 mM Hepes, adjusted to pH 7.4 with NaOH) at room temperature (20–22°C). To obtain γ-aminobutyric acid (GABA) dose/current response relations, GABA was applied to the oocytes at 3-min intervals. The half dissociation constant (EC50) of GABA was estimated by fitting the data to Hill equations, using least-square routines (cf. ref. 8). Solution exchange was achieved by using computer-controlled electromagnetic valves and, unless otherwise indicated, the oocyte membrane potential was held at −80 mV and current–voltage relationships were constructed by stepping the membrane potential for 2–5 min to a desired value before applying GABA. Drugs were applied by adding them to the superfusing fluid. All drugs were purchased from Sigma, except α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainic acid (KAI), bicuculline (Bic), 6-cyano-7-nitroquinoxaline-2,3-dione, and cyclothiazide (all from Tocris, Bristol, U.K.).
Results
Incorporation of Functional Human GABA Type A Receptors.
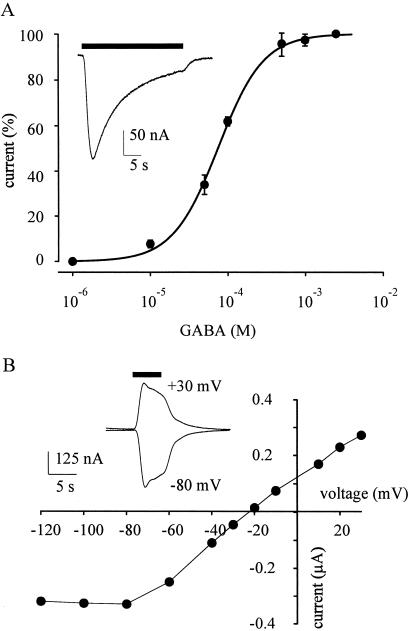
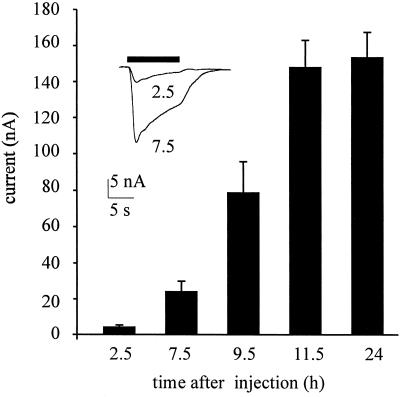
Oocytes injected with membranes from the temporal neocortex of the operated epileptic patient acquired GABA receptors that gated inward membrane currents (Figs. 1–3). These GABA currents varied in amplitude, not only among oocytes from different frogs, but also with the amount of membranes injected. For instance, 24 h after being injected with the membrane preparation diluted to 1/2 the oocytes responded to GABA (1 mM) by generating currents of 180 ± 42 nA (mean ± SEM; n = 5); whereas in oocytes from other frogs the GABA currents were much smaller even without diluting the membranes (69 ± 20 nA; n = 5) or when they were diluted 1/10 (54 ± 25 nA; n = 5). The membrane preparation, kept frozen, retained its potency to express functional GABA receptors for many weeks. When compared with the expression of GABA receptors by brain mRNA, the incorporation of receptors after membrane injections was much faster (see also ref. 6). Human GABA receptors were already detectable in the oocyte membrane 2 h after injection, and thereafter the amplitude of the responses to GABA continued to increase for a couple of days, probably because of the continued incorporation of human membranes bearing GABA receptors (Fig. 2). The responses to GABA were dose-dependent (Fig. 3A), and the GABA currents desensitized rapidly when they were generated by concentrations of GABA above approximately 50 μM (Figs. 1–4). Furthermore, the GABA currents reversed at −20 to −25 mV membrane potential (e.g., Fig. 3B) indicating an increase in Cl− permeability (cf. ref. 10).
Figure 3.
Properties of GABA currents in oocytes injected with human brain membranes. (A) GABA dose/current response relation from five oocytes recorded 36–48 h after injection (one donor frog). GABA currents were normalized to their individual maxima: mean = 164 nA; EC50 = 75 ± 3 μM; nH = 1.5 ± 0.1. (Inset) Sample of current response to GABA (1 mM). (B) GABA current/membrane voltage relation from an oocyte 36–48 h after membrane injection. (Inset) Superimposed GABA currents (200 μM) obtained at the indicated potentials, 24 h after membrane injection. Reversal potential, −22 mV. Note outward rectification.
Figure 2.
Time course of incorporation of GABA receptors after injecting cell membranes from the human temporal neocortex. Each column represents the mean value (± SEM; n = 6; one frog) of the current elicited by GABA (1 mM) at the times indicated. (Inset) Superimposed currents elicited by GABA (1 mM) at the indicated times (h) after injection. In this and subsequent figures the horizontal bars above the records show the timing of drug applications.
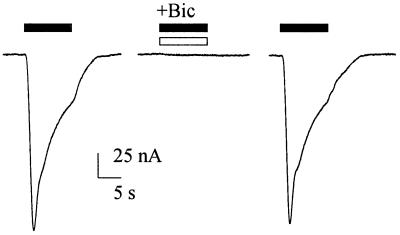
Figure 4.
Antagonism of the GABA current by Bic. Response to GABA (100 μM; Left), fully blocked by Bic (150 μM; Center), and its recovery (Right) 2 min after withdrawal of Bic, in an oocyte injected with human brain membranes. Representative of four experiments.
Bic is used routinely as an specific and competitive antagonist of GABA type A receptors. As illustrated in Fig. 4, Bic (150 μM) abolished completely, and reversibly, the currents elicited by GABA (100 μM) in oocytes injected with human brain membranes; and when the GABA concentration was raised to 1 mM, the GABA current was reduced to about half by the same dose of Bic (not shown). All of these findings are in agreement with a competitive action of Bic on the human GABA receptors incorporated into the oocyte plasma membrane. These findings indicate that the GABA currents reported here are caused by activation of GABA type A receptors.
Modulation of Human GABA Receptors.
It is well known that in native nerve cells, as well as in various cell expression systems, the responses to GABA are potently modulated by benzodiazepines and barbiturates (11–16). It was therefore of interest to determine whether the human receptors incorporated by the oocytes were similarly modulated. The GABA currents elicited in the membrane injected oocytes were potentiated by flunitrazepam (FLU, 0.3 μM), applied 30–60 s before 100 μM GABA, and also potentiated by pentobarbital (PB) (10 μM) applied simultaneously with GABA (3–100 μM, e.g., Fig. 5).
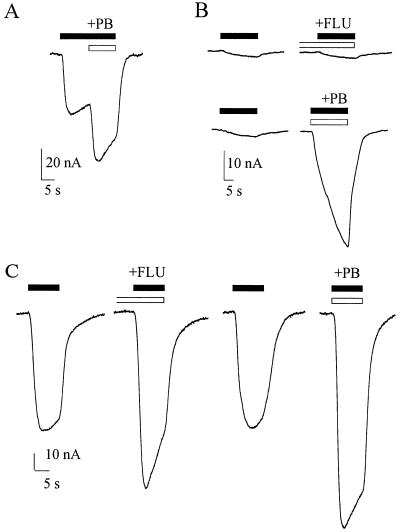
Figure 5.
Potentiation of GABA currents in oocytes 24–48 h after membrane injection. (A) Current response to GABA (100 μM) potentiated by PB (10 μM). (B) Current response to GABA (3 μM), in a different oocyte from the same batch, and greatly potentiated by PB (10 μM) but not by FLU (0.3 μM) applied 30 s before GABA. Traces were recorded at 2-min intervals (from top to bottom). (C) Current response to GABA (100 μM) in another oocyte (same frog), potentiated by FLU (0.3 μM) applied for 30 s before GABA, and even more by PB (10 μM). Records were obtained at 3-min intervals.
Incorporation of Other Human Neurotransmitter Receptors.
In addition to GABA, most oocytes were exposed to several other neurotransmitters. It was found that all of the oocytes that were sensitive to GABA were also responsive to KAI and AMPA plus cyclothiazide (15 oocytes from four donors, e.g., Fig. 6 Upper). AMPA, applied alone, generated little or no current, and glycine induced only a small current, part of which was caused by activation of a native glycine transporter. So far, neither nicotine (50–500 μM) nor 5-hydroxytryptamine (serotonin; 30–500 μM) was able to generate substantial membrane currents. The inward currents activated by AMPA and KAI, like the GABA currents, appeared within 2 h after membrane injection and increased in amplitude over time, reaching a maximum 2–3 days after membrane injection. Moreover, the AMPA currents elicited in the presence of cyclothiazide (Fig. 6 Upper) were fairly well maintained, in agreement with the slowing of AMPA receptor desensitization caused by cyclothiazide (17); and both AMPA and KAI currents were fully blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (20 μM; not shown).
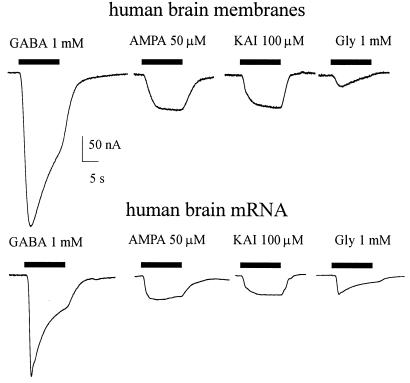
Figure 6.
Transmitter-activated currents in oocytes injected with membrane vesicles or mRNA derived from the human brain temporal neocortex. (Upper) Currents elicited by indicated concentrations of transmitters, recorded in an oocyte 24 h after membrane injection. (Lower) Transmitter-activated currents elicited in another oocyte from the same frog, 6 days after mRNA injection. All AMPA currents were elicited after a 30-s pretreatment with, and in the presence of, cyclothiazide (20 μM; not shown).
To examine briefly whether the receptors expressed after injection of human brain membranes are similar to the neurotransmitter receptors expressed after the injection of human brain mRNA, some oocytes were injected with poly(A)+ RNA extracted from a different sample of the same tissue used to prepare the membranes. The mRNA-injected oocytes expressed receptors to the same neurotransmitters as the oocytes injected with the brain membranes, and the currents generated appear to have the same gross characteristics (e.g., Fig. 6).
Discussion
The properties of ligand-gated ion channels are known to be regulated by many factors, including the composition of the host lipid membrane (18) and posttranslational processes such as glycolsylation and phosphorylation (19–21). Therefore, when transplanting a neurotransmitter receptor from its native membrane to a host cell membrane, it is important not only to transplant the proteins that make up the receptor, but it is important to also retain its structure and lipid environment. In this work we describe a rapid and efficient method for studying human neurotransmitter receptors, possessing their native structure and still embedded in their natural lipid membrane, but transplanted to a very convenient host cell system as is the Xenopus oocyte. Using this simple method we were able to transplant GABA-, glutamate-, and glycine-gated receptors from human brain cells into the oocytes and record their respective transmitter-activated currents. Our preliminary study shows that the main features of these currents are similar to those of currents activated by the same transmitters in oocytes injected with foreign mRNA extracted from the same brain tissue. However, a more detailed characterization is necessary to determine whether there are any differences in the properties of the receptors expressed by the two procedures. Nevertheless, it is fair to assume that the properties of the receptors incorporated by the oocyte after injection of the human brain membranes reflect more faithfully the properties of the receptors in their native cells.
Perhaps the main outcome of this work is that it shows that the simple procedure described here can be used to study, in the oocyte, many processes related to human membrane proteins: processes such as the incorporation and internalization of receptors, channels, and other membrane proteins, as well as their mechanisms of activation and modulation. Moreover, this method also can be used to study in detail the biophysical and pharmacological properties of human neurotransmitter receptors to better understand many neurological disorders, such as the intractable temporal lobe epilepsy of the patient who so generously contributed to this work. Because we were able to express, almost immediately, several functional transmitter receptors from the human brain, using tissue obtained postoperatively, it seems likely that this method will extend the usefulness of the Xenopus oocyte. One may envisage using this model not only for developing new medications, but also for diagnosing and studying the pathogenesis of medically intractable neurological diseases of humans undergoing surgery and/or autopsy.
Acknowledgments
We are especially grateful to patient L.A. for donating the tissue that made this work possible. We thank Drs. Francesca Grassi and Sergio Fucile and Prof. Fabio Ruzzier for critical reading of the manuscript and Dr. V. Esposito for obtaining the brain tissue. This work had the financial support of the Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (to F.E.), the National Science Foundation (Neuronal and Glial Mechanisms, Grant 998285 to R.M.), and Consejo Nacional de Ciencia y Tecnología Grant 3717P-N9608.
Abbreviations
- GABA
γ-aminobutyric acid
- AMPA
α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid
- KAI
kainic acid
- Bic
bicuculline
- FLU
flunitrazepam
- PB
pentobarbital
References
- 1.Barnard E A, Miledi R, Sumikawa K. Proc R Soc London Ser B. 1982;215:241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- 2.Sumikawa K, Parker I, Miledi R. Proc Natl Acad Sci USA. 1984;81:7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gundersen C B, Miledi R, Parker I. Nature (London) 1984;308:421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- 4.Miledi R, Parker I, Sumikawa K. Fidia Research Foundation Neuroscience Lectures. Vol. 3. New York: Raven; 1989. pp. 57–90. [Google Scholar]
- 5.Sigel E. J Membr Biol. 1990;117:201–221. doi: 10.1007/BF01868451. [DOI] [PubMed] [Google Scholar]
- 6.Marsal J, Tigyi G, Miledi R. Proc Natl Acad Sci USA. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales A, Aleu J, Ivorra I, Ferragut J A, Gonzalez-Ros J M, Miledi R. Proc Natl Acad Sci USA. 1995;92:8468–8472. doi: 10.1073/pnas.92.18.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miledi R. Proc R Soc London Ser B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 10.Kusano K, Miledi R, Stinnakre J. J Physiol (London) 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker I, Gundersen C B, Miledi R. J Neurosci. 1986;6:2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker I, Sumikawa K, Miledi R. Proc R Soc London Ser B. 1988;233:201–216. doi: 10.1098/rspb.1988.0019. [DOI] [PubMed] [Google Scholar]
- 13.Woodward R M, Polenzani L, Miledi R. Mol Pharmacol. 1993;43:609–625. [PubMed] [Google Scholar]
- 14.Sigel E, Buhr A. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- 15.Demuro A, Martinez-Torres A, Francesconi W, Miledi R. Br J Pharmacol. 1999;127:57–64. doi: 10.1038/sj.bjp.0702504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters R J, Hadley S H, Morris K D W, Amin J. Nat Neurosci. 2000;12:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- 17.Patneau D K, Vyklicky L, Jr, Mayer M L. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrantes F J. FASEB J. 1993;7:1460–1467. doi: 10.1096/fasebj.7.15.8262330. [DOI] [PubMed] [Google Scholar]
- 19.Huganir L R. Curr Top Membr Transp. 1988;33:147–163. [Google Scholar]
- 20.Sumikawa K, Miledi R. Mol Brain Res. 1989;5:183–192. doi: 10.1016/0169-328x(89)90034-x. [DOI] [PubMed] [Google Scholar]
- 21.Giovannelli A, Grassi F, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1991;88:1808–1811. doi: 10.1073/pnas.88.5.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]