Abstract
Delayed gastric ulcer healing is a well recognized problem associated with the use of cyclooxygenase (COX) inhibitors. In contrast, NO-releasing COX inhibitors do not interfere with ulcer healing. These divergent effects may in part be due to differences in their effects on platelets, which are known to influence ulcer healing. Therefore, we compared the effects of a nonselective COX inhibitor (flurbiprofen), a nitric oxide-releasing COX inhibitor (HCT-1026), and a selective COX-2 inhibitor (celecoxib) on gastric ulcer healing, angiogenesis, and platelet/serum levels of vascular endothelial growth factor (VEGF) and endostatin. Gastric ulcers were induced in rats by serosal application of acetic acid. Daily treatment with the test drugs was started 3 days later and continued for 1 week. Celecoxib and flurbiprofen impaired angiogenesis and delayed ulcer healing, as well as increasing serum endostatin levels relative to those of VEGF. HCT-1026 did not delay ulcer healing nor impair angiogenesis, and also did not change the ratio of serum endostatin to VEGF. Incubation of human umbilical vein endothelial cells with serum from celecoxib- or flurbiprofen-treated rats resulted in suppressed proliferation and increased apoptosis, effects that were reversed by an antiendostatin antibody. These results demonstrate a previously unrecognized mechanism through which nonsteroidal antiinflammatory drugs can delay ulcer healing, namely, through altering the balance of anti- and proangiogenic factors in the serum. The absence of a delaying effect of HCT-1026 on ulcer healing may be related to the maintenance of a more favorable balance in serum levels of pro- and antiangiogenic growth factors.
Keywords: nitric oxide‖angiogenesis‖nonsteroidal antiinflammatory drug‖endothelium‖growth factors
As well as causing the formation of gastric and duodenal ulcers, cyclooxygenase (COX) inhibitors are known to delay the healing of gastroduodenal ulcers. Although the mechanism underlying this effect is not completely understood, it has been suggested that inhibition of prostaglandin synthesis by these agents results in an impairment of the process of new blood vessel growth (angiogenesis), which is essential in ulcer repair (1, 2). Ulcer healing is a complex process that seems to be modulated by several growth factors, including epidermal growth factor (3), hepatocyte growth factor (4), and basic fibroblast growth factor (5). Platelets also play a key role in ulcer healing, in part by acting as a “delivery system” for several potent growth factors (6). We demonstrated that rats made thrombocytopenic with an antiplatelet serum exhibited impaired ulcer healing, whereas transfusion of platelets from a healthy donor restored ulcer-healing rates to normal (6). Moreover, we found that treatment with the antiplatelet drug, ticlopidine, impaired gastric ulcer healing through a mechanism that involved alteration of the platelet and serum levels of pro- and antiangiogenic growth factors (6). In particular, ticlopidine markedly increased platelet and serum levels of the antiangiogenic factor, endostatin.
Angiogenesis is a critical component of the ulcer-healing process, and is regulated by proangiogenic factors, including vascular endothelial cell growth factor (VEGF), and by antiangiogenic factors, such as endostatin. An imbalance in the production of antiangiogenic versus proangiogenic factors could result in impaired angiogenesis and wound healing, as has been suggested to occur in rheumatoid arthritis (7) and in experimental ulcer healing (6). On the other hand, a shift in the production of angiogenic factors in favor of those that promote angiogenesis could result in accelerated ulcer healing.
In recent years, several approaches have been taken to develop nonsteroidal antiinflammatory drugs (NSAIDs) that do not cause damage in the gastrointestinal tract. The best known of these new NSAIDs are the selective inhibitors of COX-2. These compounds exhibit a more reduced capacity to cause severe ulceration than is seen with conventional NSAIDs (8), but in experimental models, have exhibited a capacity similar to conventional NSAIDs to delay ulcer healing (9–11). These effects have been suggested to be due to inhibition of angiogenesis (12). NO-releasing COX inhibitors, on the other hand, exhibit gastric safety similar to the selective COX-2 inhibitors (13–15), but have been reported to accelerate gastric ulcer healing (16) or to abolish the delay of ulcer healing induced by a conventional COX inhibitors (17). It is possible that some of the differences in the effects of these newer COX inhibitors on ulcer healing could be attributable to divergent effects on angiogenesis. Moreover, such effects may be due to alterations in serum and/or platelet levels of pro- and antiangiogenic factors, such as VEGF and endostatin, respectively.
In the present study, we have examined the effects of a conventional NSAID (flurbiprofen), a NO-releasing derivative of flurbiprofen (HCT-1026) and a selective inhibitor of COX-2 (celecoxib) on gastric ulcer healing, angiogenesis, and platelet/serum levels of two key angiogenesis-modulating growth factors (VEGF and endostatin).
Materials and Methods
Ulcer Induction.
All experiments were approved by the University of Calgary Animal Care Committee and performed in accordance with the guidelines of the Canadian Council on Animal Care. Male Wistar rats (175–200 g) were fed standard laboratory chow and tap water and were kept in a room with controlled temperature (22 ± 1°C), humidity (65–70%), and light cycle (12 h light/12 h dark). The rats were fasted for 18 h. Gastric ulcers were induced by serosal application of acetic acid (0.5 ml, 80%) under halothane anesthesia, as described (18).
Assessment of Ulcer Healing.
One group of rats (n = 6) was killed 3 days after ulcer induction to allow for determination of ulcer size at the time of initiation of drug treatment. Beginning on day 3 and continuing for 7 days, the rats were treated orally each day with vehicle (0.5% carboxymethylcellulose; 2 ml/kg), celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), or HCT-1026 (6.5 mg/kg). The doses of test drugs were selected on the basis of equivalent antiinflammatory effects in the carrageenan-airpouch model (unpublished data). Moreover, the dose of HCT-1026 is equimolar to that of flurbiprofen. On day 10 after ulcer induction, the rats were anesthetized with halothane, and a blood sample was drawn from the descending aorta for measurement of serum VEGF and endostatin. The stomach was then removed and the ulcer area was measured planimetrically in a blind manner (16). A longitudinal section of tissue that included the ulcer base and both sides of ulcer margins was fixed in 4% neutral buffered formalin (4°C) and then embedded in paraffin and sectioned. A subset of rats (n = 5) from each group was killed and the stomach was removed for assessment of prostaglandin E2 (PGE2) synthesis, as described (19). In brief, a sample of tissue from the ulcer margin was taken from each rat and placed in 1 ml of sodium phosphate buffer (pH 7.4). After being finely minced with scissors, the sample was incubated at 37°C for 20 min. PGE2 levels in the supernatant were measured by ELISA.
Assessments of Angiogenesis.
Angiogenesis was assessed by counting the number of neomicrovessels with immunostaining for von Willebrand's factor (20). Three randomly selected areas of the granulation tissue on each slide were counted in a blind manner and the data were averaged. Any positive-staining endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels was considered an angiogenic microvessel (21).
Platelet Aggregation and Release of VEGF and Endostatin.
Rats (without ulcers) were given vehicle, celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), or HCT-1026 (6.5 mg/kg) intragastrically once daily for 7 days. Three hours after the final dose, blood was collected under halothane anesthesia and platelet-rich plasma was prepared (22). Platelet aggregation induced by thrombin (1 unit/ml) was monitored by using a platelet aggregometer, as described (22). The samples were then centrifuged (9,000 × g) and the supernatants stored at −70°C until the concentrations of VEGF and endostatin were measured by ELISA.
Endothelial Cell Proliferation and Apoptosis.
Human umbilical vein endothelial cells (HUVEC) were obtained from the American Type Culture Collection and maintained in modified F12K medium supplemented with 0.1 mg/ml heparin, 0.03 mg/ml endothelial cell growth supplement, and 10% FBS. They were used at passages 38–44 (21). Cell proliferation was determined by using the 3-(4,5-dimethylthiaxol-2-yl)-2,5-diphenyltetrazolium bromide assay (23). HUVEC (2 × 104 per well) were incubated with F12K medium in 24-well plates. Beginning 4 h after plating, the cells were incubated for 24 h with serum from rats that had been treated once daily for the previous week with vehicle, celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), or HCT-1026 (6.5 mg/kg). These experiments were performed in either the presence or absence of an antiendostatin antibody (16 μg/ml). The medium was aspirated and 3-(4,5-dimethylthiaxol-2-yl)-2,5-diphenyltetrazolium bromide was added to each well (0.25 mg/ml). The cells were incubated for a further 3 h at 37°C. The medium was then aspirated and the cells were lysed with dimethyl sulfoxide. An aliquot of the lysate was transferred to a 96-well plate, and absorbance at 540 nm was measured. This method has been shown to yield data that are consistent with direct cell counting (6) or measurement of cell proliferation through monitoring of [3H]thymidine incorporation (24).
Apoptosis was measured with a commercially available ELISA kit (6) that quantifies DNA fragmentation, with the results expressed as a percentage of medium control values.
Statistical Analysis.
Data are expressed as mean ± SEM of at least five samples in each group. Comparisons of data among groups were performed with one-way analysis of variance followed by the Student–Newman–Keuls test. An associated probability (P value) of less than 5% was considered significant.
Materials.
Reagents were obtained from the following sources: flurbiprofen, heparin, 3-(4,5-dimethylthiaxol-2-yl)-2,5-diphenyltetrazolium bromide, and endothelial cell growth supplement were from Sigma; celecoxib and HCT-1026 were from NicOx S.A. (Sophia Antipolis, France); antibodies and ELISA kits for measurement of VEGF and endostatin were from Chemicon; the apoptosis ELISA kit was from Roche Diagnostic; the PGE2 ELISA kit was from Cayman Chemicals (Ann Arbor, MI); F12K medium was from American Type Culture Collection; and thrombin was from Calbiochem.
Results
Gastric Ulcer Healing and Angiogenesis.
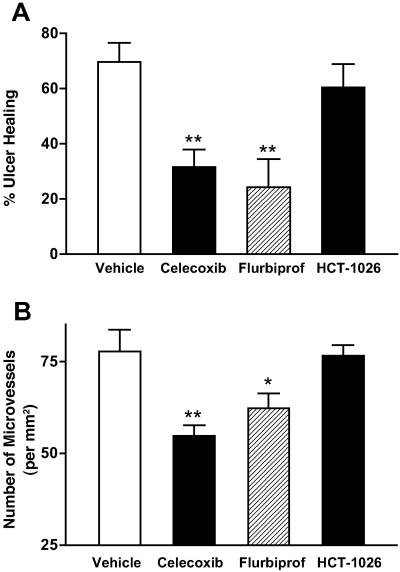
Gastric ulcers were well established 3 days after serosal application of acetic acid, with a mean area of 86 ± 8 mm2. The damage spanned the full thickness of the mucosa and penetrated through the muscularis mucosae. In some cases, ulceration extended to the muscularis propria, but perforations were not observed during the course of study. In rats treated with vehicle for 1 week thereafter, the ulcers healed considerably, the mean ulcer area being reduced by more than 70% (Fig. 1). In contrast, daily treatment for one week with celecoxib or flurbiprofen resulted in a significant delay in ulcer healing (Fig. 1), which was accompanied by a significant reduction in the number of angiogenic vessels in the ulcer bed. Treatment with HCT-1026 did not significantly affect the rate of ulcer healing or the extent of angiogenesis in the ulcer bed (Fig. 1).
Figure 1.
Effects of COX inhibitors on (A) gastric ulcer healing and (B) angiogenesis in the ulcer bed. Oral treatment with celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), HCT-1026 (6.5 mg/kg), or vehicle was started 3 days after ulcer induction and continued, once daily, for a week. Ulcer healing is expressed as a percent reduction in ulcer size from that on day 3 . *, P < 0.05; **, P < 0.01 (vs. the vehicle-treated group).
Treatment with each of the antiinflammatory drugs resulted in a marked suppression of PGE2 by tissue taken from the margin of the gastric ulcers. In rats treated with vehicle, the mean level of PGE2 synthesis was 223 ± 28 pg/mg. Treatment with celecoxib, flurbiprofen, or HCT-1026 reduced the PGE2 synthesis to 96 ± 15, 53 ± 5, and 58 ± 6 pg/mg, respectively (all P < 0.05 vs. the vehicle-treated group).
Serum VEGF and Endostatin Levels.
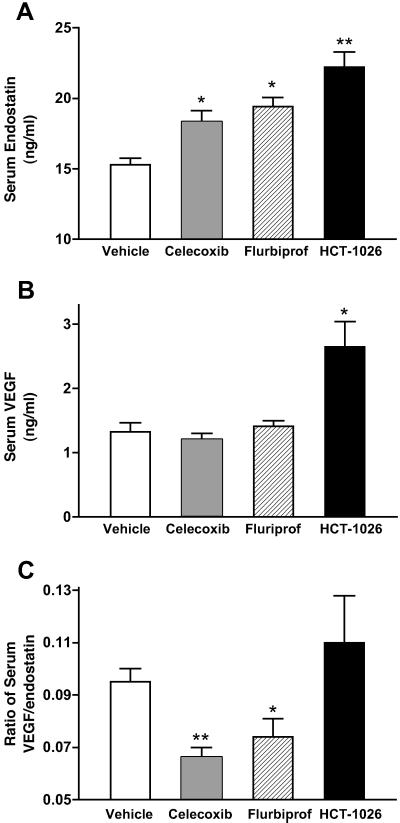
All three of the test drugs used in this study significantly increased serum endostatin concentrations (by ≈50%; Fig. 2A). However, only HCT-1026 significantly affected serum VEGF levels, causing a near-doubling over those in samples from vehicle-treated rats (Fig. 2B). The mean ratio of serum VEGF to endostatin was not significantly changed by HCT-1026, but in the rats treated with celecoxib or flurbiprofen, this ratio was significantly reduced (by 30–40%; Fig. 2C).
Figure 2.
Effects of COX inhibitors on (A) serum endostatin, (B) serum VEGF, and (C) the ratio of serum VEGF to endostatin. Oral treatment with celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), HCT-1026 (6.5 mg/kg), or vehicle was started 3 days after ulcer induction and continued, once daily, for a week. *, P < 0.05; **, P < 0.01 (vs. the vehicle-treated group).
Effects of Serum from COX Inhibitor-Treated Rats on HUVEC Proliferation and Apoptosis.
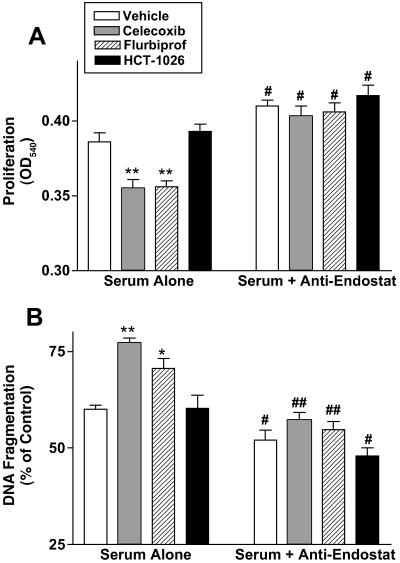
Incubation of HUVEC with serum from celecoxib- or flurbiprofen-treated rats resulted in a significant reduction of the rate of proliferation and an increase in the rate of apoptosis in comparison with HUVEC exposed to serum from vehicle-treated rats. In contrast, serum from HCT-1026-treated rats did not produce effects different from what was observed with serum from vehicle-treated rats. The decreased proliferation and increased apoptosis observed when HUVEC were exposed to serum from rats treated with flurbiprofen, HCT-1026, or celecoxib were completely reversed if the cells were coincubated with an antibody directed against endostatin (Fig. 3 A and B).
Figure 3.
Effects of serum from rats treated with various COX inhibitors on (A) proliferation and (B) apoptosis of HUVEC in the absence and presence of an antibody against endostatin. HUVEC were incubated with serum from rats treated daily for 1 week with vehicle, celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), or HCT-1026 (6.5 mg/kg). *, P < 0.05; **, P < 0.01 (vs. the vehicle-treated group). #, P < 0.05; ##, P < 0.01 (vs. corresponding group without antiendostatin).
Platelet Endostatin and VEGF Content and Release.
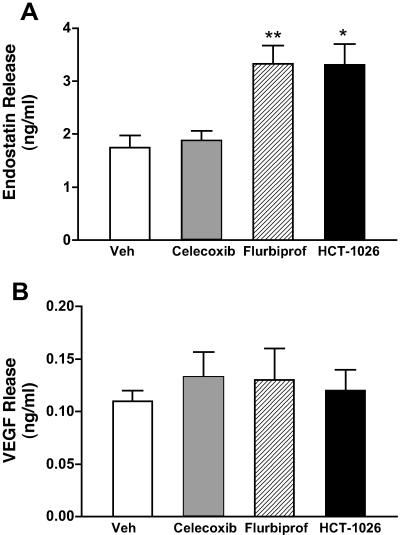
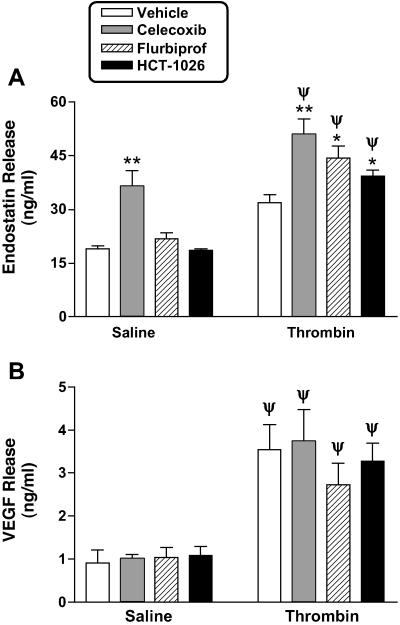
Daily treatment with flurbiprofen or HCT-1026 for 1 week significantly increased platelet endostatin content, whereas celecoxib had no effect (Fig. 4A). Treatment with celecoxib increased the basal levels of release of endostatin from platelets (Fig. 5A), whereas flurbiprofen and HCT-1026 had no effect. Thus, platelets from celecoxib-treated rats contained less endostatin than platelets from the flurbiprofen- or HCT-1026-treated rats, but released more of the endostatin under basal conditions. This increase in basal endostatin release from platelets from celecoxib-treated rats was not observed in rats that had been treated with only a single dose of celecoxib 3 h before harvesting the platelets. In response to stimulation with thrombin, endostatin release was significantly increased in all groups (Fig. 5A).
Figure 4.
Effects of COX inhibitors on platelet content of (A) endostatin and (B) VEGF. Rats were treated once daily for 7 days with vehicle (Veh), celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), or HCT-1026 (6.5 mg/kg). Three hours after the final dose, washed platelets (2 × 108 per ml) were prepared and endostatin levels were determined in platelet lysates. *, P < 0.05; **, P < 0.01 (vs. the vehicle-treated group).
Figure 5.
Effects of COX inhibitors on basal and thrombin (1 unit/ml)-induced release of (A) endostatin and (B) VEGF from platelets. Rats were treated with vehicle, celecoxib (10 mg/kg), flurbiprofen (5 mg/kg), or HCT-1026 (6.5 mg/kg) once daily for 7 days. Three hours after the final dose, the platelets were harvested and challenged with saline or thrombin in a platelet aggregometer. Supernatants of the aggregates were collected and concentrations of endostatin and VEGF were measured by ELISA. *, P < 0.05; **, P < 0.01 (vs. vehicle-treated group). Ψ, P < 0.05 vs. the corresponding saline-treated group.
Platelet VEGF content was not significantly changed by treatment with any of the three test drugs. Basal levels of VEGF release and release of VEGF in response to stimulation with thrombin were similar in all groups (Fig. 5B).
Thrombin (1 unit/ml) caused a similar degree of aggregation of platelets from rats treated with vehicle, celecoxib, or flurbiprofen (≈55–75% of maximal; no significant difference among the groups). Platelets from rats treated with HCT-1026, however, exhibited a reduced degree of aggregation in response to thrombin (≈35% of maximal; P < 0.05 vs. the other groups).
Discussion
COX, the key enzyme for synthesis of prostaglandins, exists in at least two isoforms. COX-1 is constitutively expressed in the gastrointestinal tract and has been suggested to be of critical importance in the maintenance of mucosal integrity (25). COX-2 is expressed at low levels in the gastrointestinal tract, but can be rapidly induced in response to a variety of stimuli, including ischemia and topical irritation (26–28). Selective COX-2 inhibitors were developed on the premise that, by sparing COX-1 activity, they would spare the gastrointestinal tract of injury (25). However, evidence that COX-2 plays several physiological roles in addition to mediating pain and inflammation is increasing (29). The ability of NSAIDs to induce gastrointestinal injury depends on the inhibition of both COX-1 and COX-2 (30). Selective COX-2 inhibitors delay the healing of experimental gastric ulcers to the same extent as conventional NSAIDs (9, 10), and exacerbate experimental colitis and human inflammatory bowel disease (11, 31).
The present study confirms the ability of a selective COX-2 inhibitor (celecoxib) and a nonselective COX inhibitor (flurbiprofen) to delay experimental ulcer healing. Moreover, we provide evidence of a previously unrecognized mechanism that could explain, at least in part, the underlying mechanism for this effect. We have previously observed that induction of an ulcer in the rat results in an increase in the ratio of serum VEGF to endostatin, representing a shift that would favor angiogenesis (6). VEGF is the most potent stimulus for angiogenesis (32), whereas endostatin is a very potent inhibitor of angiogenesis. Platelets play a significant role in wound healing by releasing growth factors, including VEGF and endostatin, at sites of vascular injury (33). Thus, alterations in the release of pro- and antiangiogenic factors from platelets, and the relative levels of pro- versus antiangiogenic factors in serum, can determine whether angiogenesis will proceed. We have observed that the antiplatelet drug, ticlopidine, inhibited ulcer healing by preventing the shift in the ratio of pro- and antiangiogenic factors in serum that usually occurs after induction of an ulcer (6). Ticlopidine caused an increase in the serum levels of endostatin, whereas it reduced serum levels of VEGF. Like ticlopidine, both celecoxib and flurbiprofen significantly increased serum levels of endostatin, and increased the ratio of serum endostatin to VEGF. On the other hand, the NO-releasing COX inhibitor, HCT-1026, increased endostatin levels in serum, but also caused a parallel increase in serum levels of VEGF. Thus, with HCT-1026 treatment the ratio of serum endostatin to VEGF was unchanged from what is seen in rats with ulcers that were not treated with a COX inhibitor. HCT-1026 did not interfere with ulcer healing, nor did it cause the reduction of angiogenesis in the ulcer bed that was seen with the other two COX inhibitors.
Consistent with the alteration in the balance between pro- and antiangiogenic factors in serum, treatment with celecoxib or flurbiprofen altered the ability of the serum to influence endothelial cell proliferation and apoptosis. Addition of rat serum to cultured HUVEC resulted in an increase in proliferation and a decrease in apoptosis. However, when the serum was from rats treated with celecoxib or flurbiprofen, the extent of proliferation was significantly reduced, but the extent of apoptosis was significantly increased. These in vitro effects are consistent with an antiangiogenic effect of the serum, which is in turn consistent with the detrimental effect on ulcer healing. The fact that the reduction of HUVEC proliferation and increase in apoptosis was completely blocked by an antiendostatin antibody strongly suggests that the increases in serum endostatin levels elicited by treatment with flurbiprofen or celecoxib could have accounted for the delay in ulcer healing in rats treated with those drugs. Endostatin has been shown to inhibit endothelial cell proliferation (34) and migration (35), but to promote endothelial apoptosis (36).
Although endostatin seems to be the key factor mediating changes in HUVEC proliferation and apoptosis in response to exposure to serum from rats treated with flurbiprofen or celecoxib, the major differences between the effects of HCT-1026 and those of the other COX inhibitors was seen with the serum VEGF levels. All three of the COX inhibitors elevated serum endostatin, but only HCT-1026 significantly elevated serum VEGF. In addition to the platelet, VEGF is produced by endothelial and vascular smooth muscle cells. The synthesis of VEGF by these cells has been shown to be augmented by NO (37, 38). The most obvious feature that differentiates HCT-1026 from flurbiprofen is that HCT-1026 releases NO (13). The NO released from HCT-1026 may have stimulated increased expression and production of VEGF, resulting in the significant increase in serum VEGF levels. In addition to NO being able to stimulate the synthesis of VEGF, NO has been shown to make an important contribution to the proangiogenic actions of VEGF (39).
In conclusion, our study has demonstrated a previously unrecognized mechanism through which COX inhibitors delay gastric ulcer healing. Through effects likely mediated by inhibition of COX-2, these drugs increase serum levels of endostatin, a very potent antiangiogenic factor. The absence of a detrimental effect on ulcer healing of an NO-releasing COX inhibitor (HCT-1026) may be attributable to the ability of this drug to cause a parallel increase in serum levels of a potent proangiogenic factor, VEGF. NO-releasing COX inhibitors may therefore represent an attractive alternative to conventional and COX-2 selective NSAIDs, by virtue of their ability to permit angiogenesis, and thus healing, to occur.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research. J.L.W. is an Alberta Heritage Foundation for Medical Research Senior Scientist. L.M. is supported by a Canadian Association of Gastroenterology/AstraZeneca Fellowship.
Abbreviations
- PGE2
prostaglandin E2
- VEGF
vascular endothelial growth factor
- COX
cyclooxygenase
- NSAID
nonsteroidal antiinflammatory drug
- HUVEC
human umbilical vein endothelial cells
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Folkman J, Szabo S, Stovroff M, McNeil N, Li W, Shing Y. Ann Surg. 1991;241:414–425. doi: 10.1097/00000658-199110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmassmann A, Tarnawski A, Peskar B M, Varga L, Flogerzi B, Halter F. Am J Physiol. 1995;268:G276–G285. doi: 10.1152/ajpgi.1995.268.2.G276. [DOI] [PubMed] [Google Scholar]
- 3.Tarnawski A, Halter F. J Clin Gastroenterol. 1995;21:S93–S97. [PubMed] [Google Scholar]
- 4.Schmassmann A, Stettler C, Poulsom R, Tarasova N, Hirschi C, Flogerzi B, Matsumoto K, Nakamura T, Halter F. Gastroenterology. 1997;113:1858–1872. doi: 10.1016/s0016-5085(97)70005-2. [DOI] [PubMed] [Google Scholar]
- 5.Szabo S, Khomenko T, Gombos Z, Deng X M, Jadus M R, Yoshida M. Aliment Pharmacol Ther. 2000;14, Suppl. 1:33–43. doi: 10.1046/j.1365-2036.2000.014s1033.x. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Elliott S N, Cirino G, Buret A, Ignarro L J, Wallace J L. Proc Natl Acad Sci USA. 2001;98:6470–6475. doi: 10.1073/pnas.111150798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagashima M, Asano G, Yoshino S. J Rheumatol. 2002;27:2339–2342. [PubMed] [Google Scholar]
- 8.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz M B, Hawkey C J, Hochberg M C, et al. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- 10.Schmassmann A, Peskar B M, Stettler C, Netzer P, Stroff T, Flogerzi B, Halter F. Br J Pharmacol. 1998;123:795–804. doi: 10.1038/sj.bjp.0701672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter B K, Asfaha S, Buret A, Sharkey K A, Wallace J L. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones M K, Wang H, Peskar B M, Levin E, Itani R M, Sarfeh I J, Tarnawski A S. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 13.Wallace J L, Reuter B, Cicala C, McKnight W, Grisham M B, Cirino G. Gastroenterology. 1994;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 14.Wallace J L, Reuter B, Cicala C, McKnight W, Grisham M, Cirino G. Eur J Pharmacol. 1994;257:249–255. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 15.Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, Mannucci R, Del Soldato P, Morelli A. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 16.Elliott S N, McKnight W, Cirino G, Wallace J L. Gastroenterology. 1995;109:524–530. doi: 10.1016/0016-5085(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 17.Brzozowski T, Kwiecien S, Konturek P C, Konturek S J, Mitis-Musiol M, Duda A, Bielanski W, Hahn E G. Med Sci Monit. 2001;7:592–599. [PubMed] [Google Scholar]
- 18.Ma L, Wallace J L. Am J Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- 19.Wallace J L, Bak A, McKnight W, Asfaha S, Sharkey K A, MacNaughton W K. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- 20.Weidner N, Semple J P, Welch W R, Folkman J J. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Chow J Y, Cho C H. Am J Physiol. 1999;276:G238–G248. doi: 10.1152/ajpgi.1999.276.1.G238. [DOI] [PubMed] [Google Scholar]
- 22.Wallace J L, McKnight W, Del Soldato P, Baydoun A R, Cirino G. J Clin Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmichael J, DeGraff W G, Gazdar A F, Minna J D, Mitchell J B. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 24.Wagner U, Burkhardt E, Failing K. Vet Immunol Immunopathol. 1999;70:151–159. doi: 10.1016/s0165-2427(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 25.Masferrer J L, Isakson P C, Seibert K. Gastroenterol Clin N Am. 1996;25:363–372. doi: 10.1016/s0889-8553(05)70252-1. [DOI] [PubMed] [Google Scholar]
- 26.Davies N M, Sharkey K A, Asfaha S, MacNaughton W K, Wallace J L. Aliment Pharmacol Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 27.Maricic N, Ehrlich K, Gretzer B, Schuligoi R, Respondek M, Peskar B M. Br J Pharmacol. 1999;128:1659–1666. doi: 10.1038/sj.bjp.0702966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gretzer B, Ehrlich K, Maricic N, Lambrecht N, Respondek M, Peskar B M. Br J Pharmacol. 1998;123:927–935. doi: 10.1038/sj.bjp.0701673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace J L, Muscara M N. Dig Liver Dis. 2001;33:S21–S28. doi: 10.1016/s1590-8658(01)80155-9. [DOI] [PubMed] [Google Scholar]
- 30.Wallace J L, McKnight W, Reuter B K, Vergnolle N. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 31.Bonner G F. Am J Gastroenterol. 2001;96:1306–1308. doi: 10.1111/j.1572-0241.2001.03730.x. [DOI] [PubMed] [Google Scholar]
- 32.Szabo S, Vincze A. J Physiol (Paris) 2000;94:77–81. doi: 10.1016/s0928-4257(00)00146-7. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. Thromb Haemost Suppl. 1978;63:337–346. [PubMed] [Google Scholar]
- 34.O'Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E, Birkhead J R, Olsen B R, Folkman J. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 35.Dhanabal M, Ramchandran R, Volk R, Stillman I E, Lombardo M, Iruela-Arispe M L, Simons M, Sukhatme V P. Cancer Res. 1999;59:189–197. [PubMed] [Google Scholar]
- 36.Dhanabal M, Ramchandran R, Waterman M J, Lu H, Knebelmann B, Segal M, Sukhatme V P. J Biol Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 37.Jozkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke J P. Arterioscler Thromb Vasc Biol. 2000;20:659–666. doi: 10.1161/01.atv.20.3.659. [DOI] [PubMed] [Google Scholar]
- 38.Frank S, Stallmeyer B, Kampfer H, Schaffner C, Pfeilschifter J. Biochem J. 1999;338:367–374. [PMC free article] [PubMed] [Google Scholar]
- 39.Papapetropoulos A, Garcia-Cardena G, Madri J A, Sessa W C. J Clin Invest. 1997;100:945–946. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]