Abstract
It has been shown that mice deficient in the gene coding for endothelial nitric-oxide synthase (eNOS) have increased pulmonary arterial pressure and pulmonary vascular resistance. In the present study, the effect of transfer to the lung of an adenoviral vector encoding the eNOS gene (AdCMVeNOS) on pulmonary arterial pressure and pulmonary vascular resistance was investigated in eNOS-deficient mice. One day after intratracheal administration of AdCMVeNOS to eNOS−/− mice, there was an increase in eNOS protein, cGMP levels, and calcium-dependent conversion of l-arginine to l-citrulline in the lung. The increase in eNOS protein and activity in eNOS−/− mice was associated with a reduction in mean pulmonary arterial pressure and pulmonary vascular resistance when compared with values in eNOS-deficient mice treated with vehicle or a control adenoviral vector coding for β-galactosidase, AdCMVβgal. These data suggest that in vivo gene transfer of eNOS to the lung in eNOS−/− mice can increase eNOS staining, eNOS protein, calcium-dependent NOS activity, and cGMP levels and partially restore pulmonary arterial pressure and pulmonary vascular resistance to near levels measured in eNOS+/+ mice. Thus, the major finding in this study is that in vivo gene transfer of eNOS to the lung in large part corrects a genetic deficiency resulting from eNOS deletion and may be a useful therapeutic intervention for the treatment of pulmonary hypertensive disorders in which eNOS activity is reduced.
The formation of nitric oxide (NO) by the endothelium is believed to play an important role in maintaining pulmonary arterial pressure and vascular resistance at normal physiologic levels (1–4). The role of endothelial NO synthase (eNOS) in the development of pulmonary hypertension also has been investigated in genetic mice models, and it has been shown that targeted deletion of the eNOS gene results in elevated pulmonary arterial pressure and in increases in right ventricular pressure and mass after chronic hypoxic exposure (3–5). It is generally believed that pathophysiologic conditions that reduce NO formation in the lung can result in the development of pulmonary hypertension, and pulmonary arterial pressure is increased by NOS inhibitors in most laboratory species (6–8).
Inhaled NO is used in the management of pulmonary hypertension, and aerosolized type V phosphodiesterase inhibitors selectively reduce pulmonary arterial pressure by increasing cGMP levels in the lung (9, 10). Gene transfer to the pulmonary vascular bed has been used to deliver adenoviral vectors to the pulmonary artery and pulmonary airway epithelium and alters pulmonary vascular function (11–17). In addition, adenovirally mediated transfer of the gene coding for prepro calcitonin gene-related peptide (CGRP) has been shown to have a beneficial effect in chronic hypoxia-induced pulmonary hypertension in the mouse (13). Adenoviral transfer of the eNOS gene to the lung of the mouse and rat reduces hypoxic pulmonary vasoconstriction (12, 17). It is possible therefore that transfer of the eNOS gene to the lung may correct the genetic deficiency that results in pulmonary hypertension in eNOS−/− mice.
The present study was undertaken to investigate the effects of adenoviral transfer of the gene coding for eNOS to the lung in eNOS−/− mice using a right-heart catheterization technique to investigate changes in pulmonary hemodynamics in the intact-chest, spontaneously breathing animal. The goal of this study was to determine whether adenoviral transfer of the eNOS gene to the lung can correct genetic eNOS deficiency in eNOS−/− mice and restore pulmonary vascular resistance to values measured in eNOS+/+ mice.
Methods
Adenovirus Vectors.
Replication-deficient recombinant adenoviruses serotype 5-encoding nuclear-targeted β-galactosidase (AdCMVβgal), a vector used as a reporter gene that has no effect on pulmonary hemodynamics, was used as control (12) and eNOS (AdCMVeNOS), both driven by a cytomegalovirus (CMV) promoter, were prepared as described (18). Recombinant adenoviruses were plaque-purified and virus titer was determined by plaque assay on cells in culture. After purification the virus was suspended in PBS solution (pH 7.4) with 3% sucrose and kept at −80°C until use. Amplification and purification were done by the University of Iowa Vector Core Lab (Iowa City).
In Vivo Gene Delivery to the Pulmonary Vascular Bed.
eNOS+/+ and eNOS−/− mice (The Jackson Laboratory) weighing 22–29 g were anesthetized with thiopentobarbital (85–95 μg/kg i.p.) and ketamine (3 μg/kg i.p.) and placed in a supine position on a thermo-regulated surgical table. Body temperature was maintained at 37°C with a water-jacketed heating blanket. By using sterile technique, the trachea was approached via a midline neck incision and isolated by blunt dissection. Using a 27-gauge needle attached to a microliter syringe, 50 μl of vehicle (3% sucrose in phosphate-buffered 0.9% saline solution), AdCMVeNOS or AdCMVβgal (1 × 1012 parts per ml) were instilled into the trachea. Immediately before instillation a forced expiration was achieved by compression of the thorax. The chest compression was released after endotracheal instillation of 50 μl of the vehicle or virus followed by 200 μl of air as described (11–13), which resulted in a deep inspiration that facilitated adenoviral dispersion to distal air spaces. The neck incision was closed with suture, and the animals were studied 1 day later.
eNOS Expression, Activity, and Localization.
The expression of eNOS in the mouse lung was assessed 1 day after transfection. The animals were killed, and the lungs were excised and studied immediately or quick-frozen in liquid nitrogen. To extract total protein, the lungs were homogenized (Polytron, Brinkmann) in ice-cold buffer (5 mM Hepes, pH 7.9/26% glycerol/1.5 mM MgCl2/0.2 mMEDTA/0.5 mM DTT/0.5 mM phenylmethylsulfonyl fluoride) with NaCl (300 mM final) and incubated on ice for 30 min. After centrifugation twice at 15,000 × g at 4°C for 20 min, the supernatant was mixed with an equal volume of 2% SDS/1% β-mercaptoethanol and fractionated by using 8% SDS/PAGE (70 μg per lane). The proteins then were transferred to a nitrocellulose membrane (Hybond-ECL, Amersham Pharmacia Life Sciences, Ghent, Belgium) by semidry electroblotting for 1 h. The membranes were blocked for 1 h at room temperature with blotto-Tween (5% nonfat dry milk/0.1% Tween 20) and incubated with a primary monoclonal mouse anti-eNOS IgG antibody (Santa Cruz Biotechnology). Bound antibody was detected with labeled rabbit anti-mouse IgG secondary antibody (Santa Cruz Biotechnology) and visualized by using enhanced chemiluminescence.
For determination of constitutive NOS enzyme activity (Calbiochem-Novabiochem, La Jolla, CA), l-arginine-to-l-citrulline conversion was assayed in lung extracts. Briefly, lung samples were homogenized in 250 mM Tris⋅HCl, pH 7.4/10 nM EDTA/10 mM EGTA and centrifuged (Savant μSpeed SFR13K, Global Medical Instrumentation, Albertville, MN) at 12,000 rpm for 10 min at +4°C. The supernatant was incubated in 10 mM NADPH/1 μCi/liter (1 Ci = 37 GBq) l-[3H]arginine/6 mM CaCl/50 mM Tris⋅HCl, pH 7.4/6 μM tetrahydrobiopterin/2 μM FAD/2 μM FMN for 60 min at 24°C. The reaction was stopped with 50 μM Hepes, pH 5.5/5 mM EDTA. The radioactivity of the sample eluate was measured by liquid scintillation counting. Enzyme activity was expressed as citrulline production in pmol⋅mg protein−1⋅h−1. In experiments to determine the role of inducible NOS activity in the lung samples, l-[3H]arginine-to-l-[3H]citrulline conversion was studied under calcium-free conditions. For immunohistochemical localization of eNOS in eNOS−/− mice, the animals were killed, and the lungs were sectioned as described (12). The sections were incubated for 2 h in anti-eNOS IgG followed by a 30-min incubation in biotinylated secondary antibody. The sections then were incubated with horseradish peroxidase-streptovidin. The sections were rinsed and counterstained with hematoxylin (12).
Measurement of cGMP Levels.
One day after instillation of vehicle, AdCMVβgal, or AdCMVeNOS, lungs were quick-frozen in liquid nitrogen and stored at −70°C until cGMP levels were measured. Whole lung tissue was homogenized in 1 ml of ice-cold 6% trichloroacetic acid, pH 4.0. Each sample then was centrifuged at 1,500 × g for 10 min at 4°C, the supernatant was transferred to a 10-ml test tube, and the trichloroacetic acid was extracted with H2O-saturated diethyl ether. The samples were assayed for cGMP by using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI). Lung cGMP levels are expressed as picomoles of cGMP per mg protein.
Measurement of Pulmonary Hemodynamics.
One day after administration of vehicle or adenovirus, the mice were anesthetized with thiopentobarbital (85–95 μg/kg i.p.) and ketamine (3 μg/kg i.p.) and placed on a thermo-regulated surgical table. The trachea was cannulated [polyethylene (PE) 90 tubing], and the animals breathed room air enriched with 95% O2/5% CO2. A femoral artery was cannulated (PE 10 tubing heated and gently pulled over a 0.10-inch coronary angioplasty guide wire) for measurement of systemic arterial pressure. The left jugular vein was cannulated (PE 10 tubing) for the administration of i.v. fluids or the NOS inhibitor Nω-nitro-l-arginine-methyl ester hydrochloride (l-NAME, Sigma, 25 mg/kg i.v.).
For measurement of pulmonary arterial pressure, the anesthetized mice were strapped in a supine position to a fluoroscopic table. A single-lumen catheter (Nu-Med, Hopkinton, NY) 145 mm in length and 0.25 mm in o.d., with a curved tip, was passed through the right heart into the main pulmonary artery and into the left or right pulmonary artery. Before introduction, the catheter curve was straightened with a 0.010-inch angioplasty guide wire to facilitate passage from the right jugular vein into the right atrium to the tricuspid valve under fluoroscopic guidance (Picker Surveyor, Cleveland). After the straight wire was removed, the natural curve facilitated entry of the catheter into the right ventricle. A 0.010-inch soft-tip coronary artery angioplasty guide wire then was inserted, and the catheter was passed over the guide wire into the main pulmonary artery. Pressure in the main pulmonary artery was measured with a pressure transducer (Schneider/Namic, Glenn Falls, NY), and mean pressure was derived electronically and recorded on a Grass Instruments Model 7 polygraph (Quincy, MA) as described (12, 13, 19).
Cardiac output was measured by the thermodilution technique. A known volume (20 μl plus catheter dead space) of 0.9% NaCl solution at 23°C was injected into the right atrium, and changes in blood temperature were measured in the root of the aorta. A computer (Cardiotherm 500, Columbus Instruments, Columbus, OH) with a small-animal interface was used to measure cardiac output. The thermistor microprobe (Columbus Instruments, Fr-1) was inserted into the right carotid artery and advanced to the aortic arch, where changes in aortic blood temperature were measured. A catheter in the right jugular vein was advanced to the right atrium, and the indicator was injected with a constant-rate syringe (Hamilton) to ensure rapid, repeatable indicator injection. Thermodilution curves were recorded on a chart recorder (12, 13, 19, 20).
Statistics.
The data are expressed as mean ± SE and were analyzed by using a one-way ANOVA with repeated measure and the Neumann–Keuls or paired t tests. A P value of less than 0.05 was used as the criterion for statistical significance.
Results
Pulmonary Hemodynamics.
Hemodynamic measurements were made by a right-heart catheterization procedure in eNOS+/+ and eNOS−/− mice, and these data are summarized in Table 1. Under baseline conditions in anesthetized mice, mean systemic and pulmonary arterial pressures were significantly higher in eNOS−/− mice when compared with values in eNOS+/+ mice (Table 1). Cardiac output, heart rate, and right atrial and pulmonary arterial wedge pressures were not significantly different in the two groups of mice (Table 1). Pulmonary vascular resistance and systemic vascular resistance were significantly higher in eNOS−/− mice when compared with values in eNOS+/+ mice (Table 1).
Table 1.
Baseline cardiovascular and pulmonary hemodynamic values in anesthetized eNOS+/+ and eNOS−/− mice
| Hemodynamic value | Mice
|
|
|---|---|---|
| eNOS+/+ | eNOS−/−* | |
| Mean systemic arterial pressure, mmHg | 85 ± 10 | 133 ± 12† |
| Mean right atrial pressure, mmHg | 4.1 ± 0.5 | 4.2 ± 0.6 |
| Heart rate, beats per min | 401 ± 14 | 409 ± 12 |
| Cardiac output, ml/min | 10.6 ± 0.5 | 10.1 ± 0.5 |
| Mean pulmonary arterial pressure, mmHg | 12.4 ± 1.0 | 19.2 ± 1.8† |
| Mean pulmonary arterial wedge pressure, mmHg | 4.2 ± 0.7 | 4.6 ± 1.1 |
| Total peripheral resistance, mmHg⋅ml−1⋅min | 8.1 ± 0.7 | 12.7 ± 1.0† |
| Pulmonary vascular resistance, mmHg⋅ml−1⋅min | 0.77 ± 0.07 | 1.44 ± 0.1† |
| n = 11 | n = 10 | |
1 mm Hg = 133 Pa.
eNOS−/− mice were treated with vehicle.
P < 0.05 when compared with values in eNOS+/+ mice.
Effect of eNOS Gene Transfer on Pulmonary Hemodynamics.
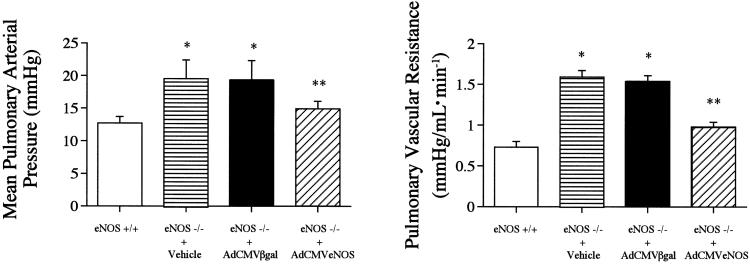
One day after transfection with AdCMVeNOS or AdCMVβgal, which served as control adenoviral vectors, baseline parameters were measured in eNOS−/− mice transfected with AdCMVβgal and AdCMVeNOS, and these data are summarized in Table 2. Mean pulmonary arterial pressure and pulmonary vascular resistance were significantly higher in eNOS−/− mice treated with vehicle or transfected with AdCMVβgal (Fig. 1). In eNOS−/− mice transfected with AdCMVeNOS, mean pulmonary arterial pressure and pulmonary vascular resistance were decreased significantly when compared with values in eNOS−/− mice transfected with AdCMVβgal (Fig. 1). In eNOS−/− mice treated with vehicle or transfected with AdCMVβgal, mean systemic arterial pressure and systemic vascular resistance were significantly higher than values in eNOS+/+ mice (Tables 1 and 2). Transfection with AdCMVeNOS did not significantly change mean systemic arterial pressure or systemic vascular resistance when compared with values in eNOS−/− mice transfected with AdCMVβgal (Tables 1 and 2). The reduction in pulmonary arterial pressure and pulmonary vascular resistance in eNOS−/− mice transfected with AdCMVeNOS was attenuated after administration of the NOS inhibitor Nω-nitro-l-arginine-methyl ester hydrochloride in a dose of 25 mg/kg i.v. (data not shown).
Table 2.
Baseline cardiovascular and pulmonary hemodynamic values in anesthetized eNOS−/− mice 1 day after intratracheal administration of AdCMVβgal or AdCMVeNOS
| Hemodynamic value | Mice
|
|
|---|---|---|
| eNOS−/− + AdCMVβgal | eNOS−/− + AdCMVeNOS | |
| Mean systemic arterial pressure, mmHg | 135 ± 11 | 130 ± 8 |
| Mean right atrial pressure, mmHg | 6.1 ± 0.8 | 5.8 ± 0.4 |
| Heart rate, beats per min | 410 ± 15 | 407 ± 16 |
| Cardiac output, ml/min | 10.1 ± 0.8 | 10.4 ± 0.6 |
| Mean pulmonary arterial pressure, mmHg | 19.4 ± 1.8 | 14.1 ± 2.0* |
| Mean left atrial pressure, mmHg | 4.8 ± 0.8 | 4.3 ± 0.8 |
| Total peripheral resistance, mmHg⋅ml−1⋅min | 12.7 ± 0.7 | 12.1 ± 0.8 |
| Pulmonary vascular resistance, mmHg⋅ml−1⋅min | 1.45 ± 0.09 | 0.89 ± 1.2* |
| n = 9 | n = 10 | |
, P < 0.05 when compared with eNOS−/− + AdCMVβgal.
Figure 1.
Comparison of mean pulmonary arterial pressure (Left) and pulmonary vascular resistance (Right) in eNOS+/+ mice and eNOS−/− mice 1 day after administration of vehicle or 1 day after transfection with AdCMVβgal or AdCMVeNOS. n = 7. *, P < 0.05 when compared with eNOS+/+; **, P < 0.05 when compared with eNOS−/− + AdCMVβgal group.
Expression of eNOS Protein, Activity, and Immunolocalization.
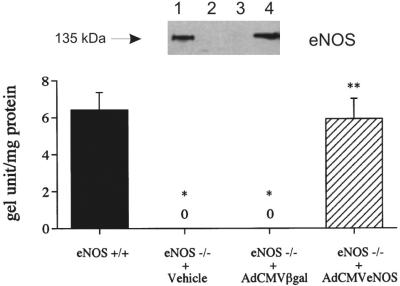
The expression of eNOS protein was compared in lung tissue from eNOS+/+ mice or eNOS−/− mice treated with vehicle or transfected with AdCMVβgal and AdCMVeNOS, and these data are shown in Fig. 2. Western blot analysis detected abundant eNOS (135 kDa) protein in eNOS+/+ mice (Fig. 2, lane 1). The expression of eNOS protein was not detected in eNOS−/− mice treated with vehicle or transfected with AdCMVβgal (Fig. 3, lanes 2 and 3). Transfection with AdCMVeNOS increased the expression of eNOS protein in eNOS−/− mice to levels that were not significantly different from values in eNOS+/+ mice (Fig. 2, lanes 1 and 4).
Figure 2.
Western blot analysis of eNOS protein in the lung of eNOS+/+ mice (lane 1) and eNOS−/− mice 1 day after intratracheal administration of vehicle (lane 2), transfection with AdCMVβgal (lane 3), or transfection with AdCMVeNOS (lane 4). eNOS protein levels are expressed as gel unit per mg protein. n = 6. *, P < 0.05 when compared with eNOS+/+; **, P < 0.05 when compared with the eNOS−/− + AdCMVβgal group.
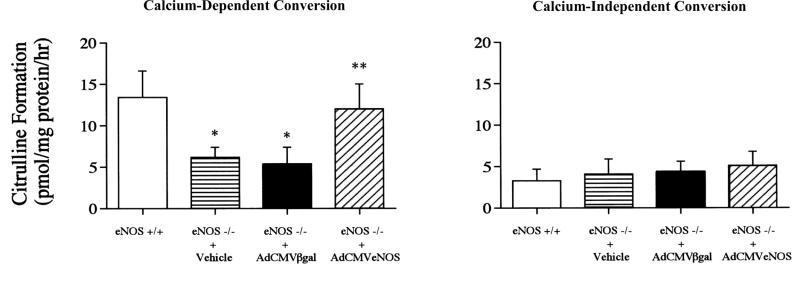
Figure 3.
Comparison of calcium-dependent (Left) and calcium-independent (Right) l-[H3]arginine conversion to l-[H3]citrulline in lung tissue from eNOS+/+ mice and eNOS−/− mice 1 day after administration of vehicle or transfection with AdCMVβgal or AdCMVeNOS. Values are pmol/mg protein/h citrulline formation. n = 6. *, P < 0.05 when compared with eNOS+/+; **, P < 0.05 when compared with the eNOS−/− + AdCMVβgal group.
The activity of the eNOS transgene was determined by measuring the conversion of l-[3H]arginine to l-[3H]citrulline in lung tissue from eNOS+/+ mice or eNOS−/− mice treated with vehicle or transfected with AdCMVβgal and AdCMVeNOS. Calcium-dependent l-[3H]arginine to l-[3H]citrulline was significantly lower in lung tissue from eNOS−/− mice treated with vehicle or transfected with AdCMVβgal when compared with values in eNOS+/+ mice (Fig. 3 Left). eNOS transfection significantly increased calcium-dependent l-[3H]arginine conversion in eNOS−/− mice to values not different from values in eNOS+/+ mice (Fig. 3 Left).
Calcium-independent conversion of l-[3H]arginine to l-[3H]citrulline, an indicator of inducible NOS activity, was measured in lung tissue from the four groups of mice, and these data are summarized in Fig. 3 Right. Calcium-independent conversion was not significantly different in eNOS+/+ mice or eNOS−/− mice treated with vehicle or transfected with AdCMVβgal or AdCMVeNOS (Fig. 3 Right).
The immunohistochemical localization of eNOS was investigated in eNOS−/− mice transfected with AdCMVeNOS. One day after transfection, eNOS staining was observed in airway epithelial cells, lung parenchyma, and the wall of resistance-sized arteries (100–300 μm; data not shown). eNOS staining was detected in smooth muscle cells, and some eNOS staining was observed in the endothelium of these arteries. There was little if any eNOS expression in resistance-sized arteries in eNOS−/− mice transfected with AdCMVβgal (data not shown).
Effect on Lung cGMP Levels.
cGMP levels were measured in lung tissue from eNOS+/+ mice and eNOS−/− mice treated with vehicle or transfected with AdCMVβgal or AdCMVeNOS, and these data are summarized in Table 3. Lung cGMP concentrations were significantly lower in eNOS−/− mice treated with vehicle or transfected with AdCMVβgal when compared with values in eNOS+/+ mice (Table 3). Lung cGMP concentrations were significantly higher in eNOS−/− mice transfected with AdCMVeNOS when compared with values in eNOS−/− mice transfected with AdCMVβgal (Table 3).
Table 3.
cGMP levels in lung tissue from eNOS+/+ mice, eNOS−/− mice treated with vehicle, and eNOS−/− mice transfected with AdCMVβgal or AdCMVeNOS
| Mice and treatment | Lung cGMP, pmol/mg protein |
|---|---|
| eNOS+/+ | 0.136 ± 0.04 |
| eNOS−/− + vehicle | 0.059 ± 0.024* |
| eNOS−/− + AdCMVβgal | 0.062 ± 0.030* |
| eNOS−/− + AdCMVeNOS | 0.105 ± 0.093** |
n = 6 for each group.
, P < 0.05 vs. eNOS+/+;
, P < 0.05 vs. eNOS−/− + AdCMVβgal.
Discussion
Results of the present study show that adenovirally mediated transfer of the eNOS gene to the lung of eNOS−/− mice decreased the elevated level of pulmonary arterial pressure associated with genetic deletion of the eNOS gene. These studies show that pulmonary arterial pressure and vascular resistance are restored toward values measured in eNOS+/+ mice at the same time systemic vascular resistance is not altered. The decrease in pulmonary arterial pressure in eNOS−/− mice after eNOS gene transfer was associated with an increase in eNOS protein levels, which were not detectable in lung from eNOS−/− mice. In addition, calcium-dependent NOS activity and cGMP levels were increased toward values measured in eNOS+/+ mice. These results suggest that the eNOS transgene has activity in the lung of eNOS−/− mice and that the gene product (NO) partially corrects the pulmonary hypertension associated with a genetic deficiency of eNOS. Although eNOS protein and activity levels were restored to values not significantly different from values in eNOS+/+ mice, only partial restoration of pulmonary arterial pressure and lung cGMP levels was observed in eNOS-transfected eNOS−/− mice. The reason for the incomplete restoration is unknown but may be related to the localization of the eNOS transgene in the lung of eNOS−/− mice. After intratracheal instillation of AdCMVeNOS in eNOS−/− mice, the eNOS is expressed in airway epithelial cells, lung parenchyma, and the wall of resistance-sized intrapulmonary arteries. There was eNOS expression in the endothelium of these arteries, but it was less prominent than observed in the media or in the endothelium of eNOS+/+ mice (12). These results suggest that transfection with eNOS increases the formation of a lipophilic gene product, NO, which can regulate vasomotor tone, although eNOS expression is minimal in the endothelium of pulmonary arteries in transfected eNOS−/− mice.
It is possible that adenovirally mediated gene transfer to the lung induces inflammation, which may up-regulate inducible NOS (21, 22). In the present study the reporter gene coding for nuclear targeted β-galactosidase, which employs the same adenovirus and has no effect on vasomotor tone, was used as a control vector for the eNOS gene (12). The results of experiments with AdCMVβgal suggest that inflammation and up-regulation of inducible NOS did not account for the partial correction of pulmonary vascular resistance in the eNOS−/− mice, because pulmonary arterial pressure was not changed in control experiments with this vector. In addition, cGMP levels and calcium-dependent and independent NOS activity were not changed with AdCMVβgal. Moreover, there were no overt signs of inflammation in mice treated with AdCMVβgal. Histological examination of lung sections from animals treated with AdCMVβgal or AdCMVeNOS revealed a small number of polymorphonuclear cells, suggesting a local inflammatory response. However, this local response did not alter pulmonary vascular tone.
Changes in NOS activity are inconsistent in several forms of pulmonary hypertension, and NOS inhibitors or genetic deletion of the eNOS gene induces pulmonary hypertension (6, 8, 23–26). It is well established that NO has pulmonary vasodilator activity, and inhaled NO is effective in attenuating lung injury in the rabbit and in the treatment of pulmonary hypertension in the newborn (10, 21, 22, 27). Studies in the rat and mouse show that adenovirally mediated eNOS gene transfer increases lung cGMP levels and blunts the response to ventilatory hypoxia (12, 17). eNOS gene transfer reduces pulmonary vascular resistance in bleomycin-induced pulmonary hypertension in the mouse and in chronic hypoxia-induced pulmonary hypertension in the rat, suggesting that eNOS gene transfer to the lung may be useful in the treatment of pulmonary hypertensive disorders (12, 21).
The present results show that pulmonary arterial pressure and pulmonary vascular resistance are elevated in intact-chest eNOS−/− mice and are consistent with results obtained in eNOS−/− mice using a different method to evaluate pulmonary vascular resistance (3, 26). The present results showing that eNOS gene transfer to the lung reduces pulmonary vascular resistance toward values measured in wild-type control mice suggest that transfection with AdCMVeNOS in part corrects the genetic disorder induced by eNOS deletion. These results suggest that eNOS gene transfer may represent a useful strategy for the treatment of pulmonary hypertensive disorders in which eNOS activity is decreased.
It is possible that a compensatory mechanism unrelated to enhanced NO formation may account for the decrease in pulmonary arterial pressure in eNOS−/− mice after eNOS gene transfer. However, the observation that the effect of eNOS gene transfer on pulmonary arterial pressure in eNOS−/− mice was attenuated by an NOS inhibitor suggests that the expression of eNOS and the formation of NO accounts for the restoration of pulmonary arterial pressure, although the role of a compensatory mechanism cannot be ruled out.
In summary, the present results show that adenovirally mediated transfer of the eNOS gene to the lung decreases the elevated pulmonary vascular resistance in eNOS−/− mice toward values measured in eNOS+/+ mice. The fall in pulmonary vascular resistance was associated with increased eNOS expression, eNOS protein and activity, and elevated lung cGMP levels, which were increased, toward values measured in eNOS+/+ mice. Thus, the principal, original contribution of this study is that eNOS gene transfer to the lung of eNOS−/− mice in part corrects a genetic deficiency resulting from eNOS deletion and may represent a form of therapy for the treatment of pulmonary hypertensive disorders in which eNOS activity in the lung is diminished.
Acknowledgments
We thank Ms. Janice Ignarro for editorial assistance. This study was supported by National Institutes of Health Grant HL62000 and a grant from the American Heart Association Southeast affiliate.
Abbreviations
- NOS
NO synthase
- eNOS
endothelial NOS
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Higenbottam T W, Laude E A. Chest. 1998;114:72S–79S. doi: 10.1378/chest.114.1_supplement.72s. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 3.Steudel W, Scherrer-Crosbie M, Bloch K D, Weimann J, Huang P L, Jones R C, Picard M H, Zapol W M. J Clin Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozaki M, Kawashima S, Yamashita T, Ohashi Y, Rikitake Y, Inoue N, Hirata K I, Hayashi Y, Itoh H, Yokoyama M. Hypertension. 2001;37:322–327. doi: 10.1161/01.hyp.37.2.322. [DOI] [PubMed] [Google Scholar]
- 5.Fagan K A, Fouty B W, Tyler R C, Morris K G, Jr, Hepler L K, Sato K, LeCras T D, Asman S H, Weinberger H D, Huang P L, et al. J Clin Invest. 1999;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagan K A, Morrissey B, Fouty B W, Sato K, Harral J W, Morris K G, Hoodt-Miller M, Vidmar S, McMurtry I F, Rodman D M. Respir Res. 2001;2:306–313. doi: 10.1186/rr74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampl V C, Archer S L, Nelson D P, Weir E K. J Appl Physiol. 1993;75:1748–1757. doi: 10.1152/jappl.1993.75.4.1748. [DOI] [PubMed] [Google Scholar]
- 8.Archer S L, Tolins J P, Raij L, Weir E K. Biochem Biophys Res Commun. 1989;164:1198–1205. doi: 10.1016/0006-291x(89)91796-8. [DOI] [PubMed] [Google Scholar]
- 9.Cohen A H, Hanson K, Morris K, Fouty B, McMurtry I F, Clarke W, Rodman D M. J Clin Invest. 1996;97:172–179. doi: 10.1172/JCI118386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rimensberger P C, Spahr-Schopfer I, Berner M, Jaeggi E, Kalangos A, Friedli B, Beghetti M. Circulation. 2001;103:544–548. doi: 10.1161/01.cir.103.4.544. [DOI] [PubMed] [Google Scholar]
- 11.Zabner J, Zeiher B G, Friedman E, Welsh M J. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champion H C, Bivalacqua T J, D'Souza F M, Ortiz L A, Jeter J R, Toyoda K, Heistad D D, Hyman A L, Kadowitz P J. Circ Res. 1999;84:1422–1432. doi: 10.1161/01.res.84.12.1422. [DOI] [PubMed] [Google Scholar]
- 13.Champion H C, Bivalacqua T J, Toyoda K, Heistad D D, Hyman A L, Kadowitz P J. Circulation. 2000;101:923–930. doi: 10.1161/01.cir.101.8.923. [DOI] [PubMed] [Google Scholar]
- 14.Jeppsson A, Pellegraini C, O'Brien T, Miller V M, Tazelaar H D, McGregor C G. Ann Thorac Surg. 1998;66:318–324. doi: 10.1016/s0003-4975(98)00552-9. [DOI] [PubMed] [Google Scholar]
- 15.Schachtner S K, Rome J J, Hoyt R F, Jr, Newman K D, Virmani R, Dichek D A. Circ Res. 1995;76:701–709. doi: 10.1161/01.res.76.5.701. [DOI] [PubMed] [Google Scholar]
- 16.Muller D W, Gordon D, San H, Yang Z, Pompili V J, Nabel G J, Nabel E G. Circ Res. 1994;75:1039–1049. doi: 10.1161/01.res.75.6.1039. [DOI] [PubMed] [Google Scholar]
- 17.Janssens S P, Bloch K D, Nong Z, Gerard R D, Zoldhelyi P, Collen D. J Clin Invest. 1996;98:317–324. doi: 10.1172/JCI118795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ooboshi H, Chu Y, Rios C D, Faraci F M, Davidson B L, Heistad D D. Am J Physiol. 1997;273:H265–H270. doi: 10.1152/ajpheart.1997.273.1.H265. [DOI] [PubMed] [Google Scholar]
- 19.Champion H C, Villnave D J, Tower A, Kadowitz P J, Hyman A L. Am J Physiol. 2000;278:H8–H15. doi: 10.1152/ajpheart.2000.278.1.H8. [DOI] [PubMed] [Google Scholar]
- 20.Bivalacqua T J, Dalal A, Champion H C, Kadowitz P J. Am J Physiol. 1999;277:E838–E847. doi: 10.1152/ajpendo.1999.277.5.E838. [DOI] [PubMed] [Google Scholar]
- 21.Budts W, Pokreisz P, Nong Z, Van Pelt N, Gillijns H, Gerard R, Lyons R, Collen D, Bloch K, Janssens S. Circulation. 2000;102:2880–2885. doi: 10.1161/01.cir.102.23.2880. [DOI] [PubMed] [Google Scholar]
- 22.Kang J L, Park W, Pack I S, Lee H S, Kim M S, Lim C M, Koh Y. J Appl Physiol. 2002;92:795–801. doi: 10.1152/japplphysiol.00202.2001. [DOI] [PubMed] [Google Scholar]
- 23.Roos C M, Frank D U, Xue C, Johns R A, Rich G F. J Appl Physiol. 1996;80:252–260. doi: 10.1152/jappl.1996.80.1.252. [DOI] [PubMed] [Google Scholar]
- 24.Ichinose F, Adrie C, Hurford W E, Bloch K D, Zapol W M. Anesthesiology. 1998;84:410–416. doi: 10.1097/00000542-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Fagan K A, Tyler R C, Sato K, Fouty B W, Morris K G, Huang P L, McMurtry I F, Rodman D M. Am J Physiol. 1999;277:L472–L478. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- 26.Steudel W, Ichinose F, Huang P L, Hurford W E, Jones R C, Bevan J A, Fishman M C, Zapol W M. Circ Res. 1997;81:31–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 27.Dellinger R P, Zimmerman Z L, Taylor R W, Straube R C, Hauser D L, Criner G J, Davis K, Jr, Hyers T M, Papadakos P. Crit Care Med. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]