Abstract
Nicotine exposure diminishes the protective breathing and arousal responses to stress (hypoxia). By exacerbating sleep-disordered breathing, this disturbance could underpin the well established association between smoking and the increased risk of sudden infant death syndrome. We show here that the protective responses to stress during sleep are partially regulated by particular nicotinic acetylcholine receptors (nAChRs). We compared responses of sleeping wild-type and mutant mice lacking the β2 subunit of the nAChR to episodic hypoxia. Arousal from sleep was diminished, and breathing drives accentuated in mutant mice indicating that these protective responses are partially regulated by β2-containing nAChRs. Brief exposure to nicotine significantly reduced breathing drives in sleeping wild-type mice, but had no effect in mutants. We propose that nicotine impairs breathing (and possibly arousal) responses to stress by disrupting functions normally regulated by β2-containing, high-affinity nAChRs.
Smoking during pregnancy significantly increases perinatal and infant morbidity and mortality. It is now the most important independent risk factor contributing to the sudden infant death syndrome (SIDS) (1, 2). The most compelling hypothesis for the link between smoking and SIDS is that nicotine in tobacco diminishes, among other things, crucial breathing and arousal responses to stress during sleep (3). Brief pauses in breathing (apneas) are common during sleep, but the accompanying stress (hypoxia) normally provokes a powerful, protective, cardiorespiratory excitation and arousal response (4). A diminished stress response exacerbates apnea and hypoxia, aggravating perinatal injury and ultimately precipitating SIDS (5–9). The effects of nicotine are mediated by its activation of highly selective nicotinic cholinergic receptors (nAChRs), which are present in the carotid bodies (the principal hypoxic sensors) and critical brainstem nuclei, such as the nucleus of the solitary tract and locus coeruleus (5, 10–12). nAChRs at these sites contribute to the cholinergic modulation of breathing and arousal (13, 14). Interference with the normal function of these nAChRs is the presumed basis of the detrimental side effects of nicotine (15). Here we investigated the role played by a particular nAChR subtype in modulating critical protective responses to stress during sleep.
The nAChRs form a family of pentameric oligomers made up of combinations of different protein subunits. Five α subunits(α2–α6) and three β subunits (β2–β4) can be associated into neuronal nAChRs with subunits of two or more different types. Specific subunit patterns confer unique biophysical and pharmacological properties on a given receptor oligomer, and determine its activation and desensitization kinetics, ion selectivity, allosteric effects, and binding properties (16). The large number of possible combinations of nAChR subunits implies that important diversity exists in the way various nAChR subunits or oligomers influence particular behaviors (e.g., breathing and arousal). We studied mutant mice in which the gene encoding the β2 nAChR subunit has been deleted (“knocked-out”) to gain insights into the regulatory roles of this particular nAChR subunit. Studies of mutant mice already indicate that the β2-containing nAChRs are crucial in regulating aspects of waking behavior (17). Here we demonstrate that crucial protective responses mounted to stress during sleep are also partially regulated by nAChRs containing this subunit.
Methods
Animals.
We used age-matched wild-type and mutant mice lacking the β2 nAChR subunit gene (17). Iffa-Credo supplied male C57BL/J6 wild-type control and male ACNβ2 mutant siblings from parents backcrossed for 12 generations to C57BL/J6 inbred mice. Mice were housed in a quiet, temperature-controlled room (22–23°C) under a 12-h light-day cycle, and were provided with water and dry food pellets ad libitum; they habituated to the laboratory for 2–3 weeks, before study at postnatal day (P) 35–P48. Ethical approval was granted by the French Ministère de l'Agriculture et de la Forêt; all procedures conformed with the guidelines of the Institut National de la Santé et de la Recherche Médicale.
Reverse Transcriptase–PCR.
Three wild-type C57-BL6 were killed by cervical dislocation, and the six carotid bodies rapidly removed. Total RNA was isolated from whole carotid bodies by using TRIZOL reagent (GIBCO/BRL, Life Technologies); 1 μg of total RNA was reverse transcribed with the Superscript preamplification system (GIBCO/BRL, Life Technologies), and 1/20 of the reaction product was amplified by PCR. The methodology and oligonucleotides selected for nAChR subunit detection were as used (12). Tyrosine hydroxylase detection was used as a positive control (5). DNA products of amplification were analyzed by gel electrophoresis stained with ethidium bromide.
Plethysmography.
Ventilation was measured by whole-body plethysmography (18). Pairs of mice of the same genotype were studied on alternate days. To facilitate sleep onset, all mice were permitted a long period of pretest familiarization inside the measurement chamber (overnight before study). Mice were studied at environmental thermoneutrality (26–28°C). No restraint was used; mice explored the plethysmograph, groomed, and so forth, until sleep ensued. Recording commenced at sleep onset.
Acute effects of nicotine.
At sleep onset, baseline data (breathing air) were recorded for 10 min; the plethysmograph was then opened, and the mouse injected i.p. with either 100 μl of saline (morning studies), or the same volume of saline +0.5 mg⋅kg−1 nicotine tartrate (afternoon studies) (17). The mouse was then returned to the plethysmograph, which was resealed, and recording recommenced. The response to a single 10-min hypoxic challenge was recorded at 13 min after injection. Sleep resumed at 30–40 min after injection, and the test was repeated at 60 min (i.e., during sleep); at the end of the second hypoxic challenge, we added O2 to measure peripheral chemoreflex (i.e., carotid body-mediated) respiratory drive.
Response to two cycles of episodic hypoxia.
At sleep onset baseline breathing was recorded for 20 min; two to three brief O2 pulses were administered during this period to measure peripheral drive. Then two cycles of 10-min hypoxia–5-min normoxia were administered, followed by an O2 pulse at the end of the second hypoxic cycle. Hypoxia consisted of 13% O2 + 2% CO2, balance N2. Each mouse was tested twice (morning and afternoon) on the same day, and a mean response calculated.
Response to five cycles of episodic hypoxia.
Using similar methods, we administered five cycles of 10-min hypoxia–5-min normoxia to a different group of mice; each mouse was studied only once by using this protocol, and no O2 pulses were administered.
Data Analysis.
Sleep state was scored by using behavioral criteria; periods of arousal lasting ≥1 s were identified by the pressure artifact associated with sudden movements (18). For the analysis of ventilation, we only used parts of the record where breaths were clearly evident. From breath-by-breath arrays of tidal volume (VT), inspiratory (TI), expiratory (TE), and total (TTOT) breath time, and minute ventilation [VE; = VT × 1,000/(TI + TE)], we calculated mean VE each minute. The hypoxic ventilatory response (HVR) was the average VE during the third to fifth and eighth to tenth minute (inclusive) of the first of the two to five challenges administered; respiratory volumes were expressed as absolute values normalized for body weight (microliters per gram), and as a percent of control baseline (= final 3min in air preceding hypoxia). The decline in VE in O2 (ΔVE) was the 10-point minimum moving average VE during the initial 20 s of O2; (baseline = 20 s preceding the O2 switch) (18). Data were analyzed by using a repeated-measures ANOVA (STATVIEW 5.0; SAS Institute, Cary, NC), and are presented as group means ± SD in the text and tables, and (for improved clarity) means ± SEM in figures. A P ≤ 0.05 was considered significant.
Results
Nicotine Diminishes Breathing Efforts in Wild-Type but Not β2 Mutant Mice.
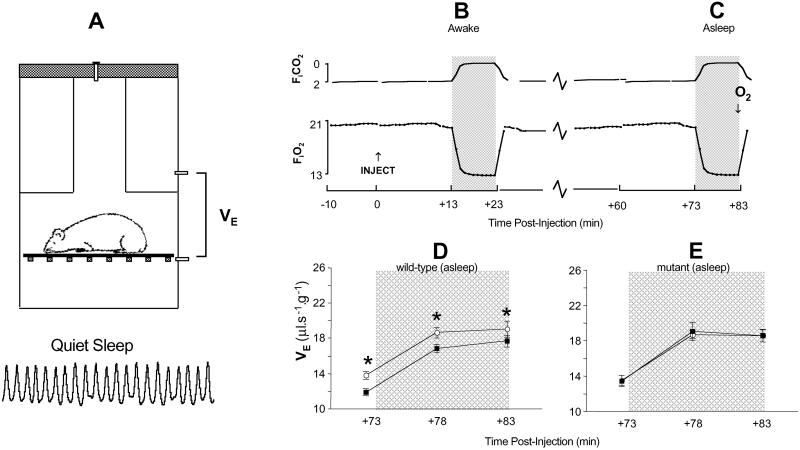
Nicotine exposure attenuates the increase in breathing (ventilatory) efforts during hypoxia (5, 6). We compared how brief exposure to nicotine (0.5 mg⋅kg−1) alters breathing patterns of mutant and wild-type mice. Mice were studied by using a noninvasive technique (Fig. 1A). Breathing (minute ventilation, VE) during a 10-min hypoxic challenge was measured soon after nicotine was administered(awake; Fig. 1B), and again one-hour later (during behavioral sleep; Fig. 1C). No differences existed between the immediate effects of nicotine on breathing in awake mutant and wild-type mice (data not shown). When mice were re-tested during sleep, however, the mean VE response curve was displaced downwards in wild-type mice, indicating that the drive to breathe had diminished significantly in these mice (Fig. 1D). Nicotine had no effect on breathing in sleeping mutant mice (Fig. 1E).
Figure 1.
Nicotine diminishes the drive to breathe in sleeping wild-type mice. Ventilation (VE) was measured by whole-body plethysmography (A) after a single i.p. injection of saline (○, morning studies) or nicotine (■, 0.5 mg⋅kg−1, afternoon studies). The HVR (period of hypoxia = shaded panels) was then recorded at +13 min, (B, mice awake) and again 1 h after injection (asleep, C). Only HVRs elicited during sleep are shown (D and E). The downward displacement of the curve of wild-type mice signaled diminished ventilatory (breathing) drive after nicotine exposure (D; *, P = 0.015), an effect not evident in mutant mice (E; P = 0.8 nicotine vs. saline).
The Arousal Response to Episodic Hypoxia Is Attenuated in β2 Mutant Mice.
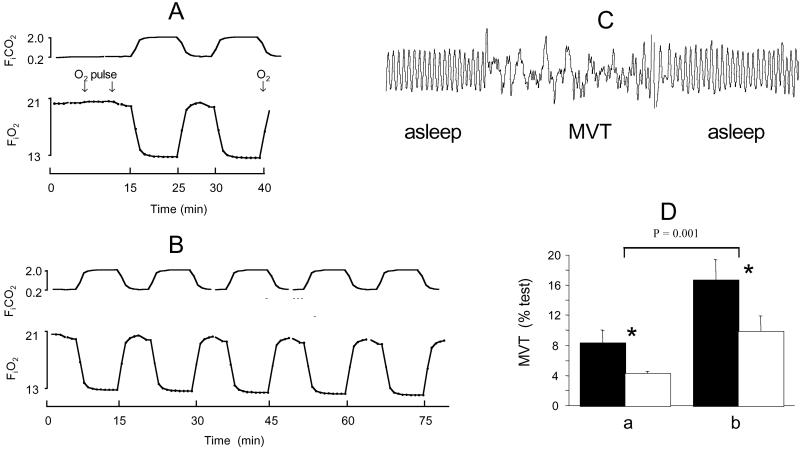
Hypoxia is normally a powerful arousal stimulus (4, 18). We compared the arousal response of mutant and wild-type mice to episodic hypoxia to clarify the role of β2-containing nAChRs in this important defense-alerting response. We used a stress paradigm designed to mimic (over a longer cycle time) the sorts of episodic hypoxic insults which occur clinically e.g., in repetitive sleep apnea. Either two (Fig. 2A) or five (Fig. 2B) cycles of hypoxia were administered during sleep. Arousal was defined by agitation (movement lasting ≥1 sec; Fig. 2C). Hypoxia elicited a dose-dependent increase in arousal time from all mice, but mutants were consistently less aroused, indicating that arousal thresholds were higher in these animals (Fig. 2D).
Figure 2.
The arousal response to episodic hypoxia is attenuated in β2 mutant mice. Either 20 min (A) or 50 min (B) of episodic hypoxia were administered; arousal from sleep was defined by movement (MVT) artifact (C). The arousal response from mutants (□) was consistently lower than from wild-type mice (■) to both stimuli (*, P = 0.015).
Respiratory Responses to Hypoxia Are Accentuated in β2 Mutant Mice.
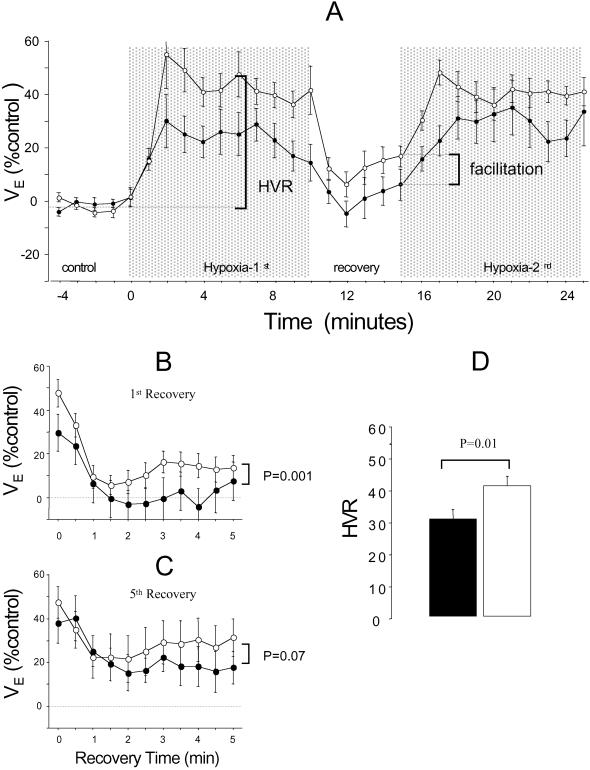
Deficits in the control and regulation of breathing during sleep can accentuate respiratory instability and failure, and are implicated in SIDS pathophysiology (5–8). We compared breathing patterns of wild-type and mutant mice for evidence of abnormal respiratory control during sleep. At rest (in air), mutants were significantly hypopneic: the weight-adjusted minute volume was less in mutant than in wild-type mice (VE; 16 ± 2 vs. 18 ± 2 μl⋅s−1⋅g−1; P = 0.007), because of the smaller breath volume (tidal volume, VT, = 5.8 ± 0.7 vs. 6.2 ± 0.6 μl⋅g−1; P = 0.035); breath duration was comparable (362 ± 51 vs. 347 ± 40 ms, P = 0.3). Episodic hypoxia caused VE to rise and fall (Fig. 3A). The first hypoxic cycle always elicited a persistent ventilatory facilitation (19) during the first (and subsequent) recovery periods in mutant, but not wild-type mice (Fig. 3A). Facilitation in wild-type mice required exposure to repetitive, intermittent hypoxia cycles (Fig. 3 B and C). Although mutants were less aroused by hypoxia (Fig. 2D), their ventilatory response (HVR) was accentuated (Fig. 3D).
Figure 3.
Respiratory responses to hypoxia are accentuated in β2 mutant mice. Mean ventilatory responses to 20-min episodic hypoxia illustrate persistent facilitation during the first posthypoxic recovery cycle (A) in mutants (○) but not wild-type mice (●). Comparison of the first (B) and fifth (C) recovery periods during 50-min episodic hypoxia illustrates that, in wild-type mice, significant facilitation was only evident after exposure to five cycles of repetitive hypoxia (C). Note the greater HVR of the mutants (D).
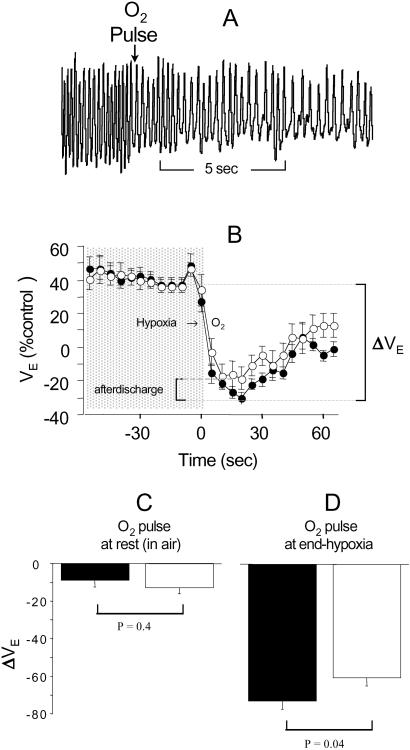
Sudden hyperoxia diminishes breathing efforts because of so-called “physiological denervation” of the carotid body (peripheral) chemosensors. This hyperoxic test indirectly measures the strength of peripheral respiratory drive; residual breathing reflects ongoing central drive (Figs. 4 A and B). We used this method to compare the peripheral contribution to respiratory drive of mutant and wild-type mice. Brief O2 pulses were administered at rest (air) and end-hypoxia (Fig. 2A). Peripheral drive was comparable between genotypes at rest (Fig. 4C). At end-hypoxia, however, peripheral drive was significantly less in the mutant, although these mice remained relatively hyperpneic (“afterdischarge”; Fig. 4D). The presence of afterdischarge in mutants is indicative of a long-lasting, posthypoxic augmentation of central respiratory drive in mutants (20).
Figure 4.
Hypoxia increases central respiratory drive in β2 mutant mice. Sudden hyperoxia rapidly diminished breathing efforts (A and B; ○, mutants; ●, wild-type mice). The fall in ventilation (ΔVE) measured peripheral drive, which was comparable for both groups of mice at rest (C), but after a period of hypoxia, was less in mutants (D). The persistent hyperpnea of mutants in O2 (“afterdischarge,” B) probably originated centrally.
Carotid-Body nAChR Subunit Expression.
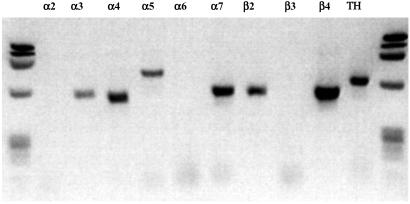
The activity of the carotid bodies, the principal hypoxic sensors, is partly regulated by nAChRs. Only two nAChR subunits (α4 and α7) are so far known to be present in this organ (21, 22). If the β2 subunit is also normally expressed, dysfunction of nAChR oligomers containing this subunit could result in abnormalities in particular aspects of peripheral responsiveness to hypoxia. We analyzed carotid bodies from C57-BL6 wild-type mice by reverse transcriptase–PCR to determine which subunits are normally expressed in this structure. Transcripts of 6 nAChR subunits were detected in carotid-body total RNA (α3, α4, α5, α7, β2, and β4; Fig. 5). Thus, multiple subtypes of nAChRs, including high-affinity β2-containing nAChRs, may be present and play a functional role in regulating carotid-body activity.
Figure 5.
Agarose gel electrophoresis of the reverse transcriptase–PCR products from total RNA of murine carotid bodies. Transcripts for the nAChR subunits α3, α4, α5, α7, β2, and β4, and tyrosine hydroxylase (TH) were detected.
Discussion
We have found that β2-containing nAChRs play a crucial role in modulating vital elements of the protective responses which are believed to guard against respiratory failure during sleep.
Nicotine is known to reduce the drive to breathe under certain circumstances, an action which is believed to exacerbate or precipitate stress-related cardiorespiratory failure during sleep (5–7). Our data demonstrate that the mechanism underlying this effect of nicotine involves activation of high-affinity, β2-containing nAChRs. Whereas in newborn animals nicotine depresses breathing by attenuating peripheral (carotid body) drive (5–7), our data (obtained during sleep) suggest that nicotine also attenuates central respiratory drive (Fig. 1D). It seems likely, given that nAChRs are present in the carotid bodies as well as brainstem centers regulating breathing, that nicotine influences breathing by multiple actions at either or both sites (5, 15). Because breathing and sleep mechanisms both interact to modulate breathing during sleep, part of nicotine's action in reducing breathing drives could also be mediated by subtle alterations to particular aspects of the sleep cycle (e.g., its depth; ref. 23).
Nicotine had little or no effect on respiratory drives in mutant mice, which is possibly because the lack of high-affinity nAChRs reduced nicotine binding in critical structures regulating breathing (17). This reduction in nicotine binding could, in turn, diminish the abnormal consequences of nAChR overactivity, including “downstream” activation of inhibitory (e.g., dopaminergic) circuits, which are regulated by nAChRs (24, 25). Activation of inhibitory circuits by nicotine is an important mechanism depressing respiratory drive, possibly contributing to SIDS (5). Absence of the β2 nAChR subunit, by disengaging critical signaling cascades normally triggered by nicotine, could be neuroprotective (17, 26).
Endogenous β2-containing nAChRs are important in fine-tuning of respiratory control during sleep. At rest, ventilation was significantly lower in mutants, possibly reflecting abnormally low basal central nervous system (brainstem, suprapontine, and/or metabolic) drives during sleep (27, 28). Mutants, however, exhibited a more vigorous than normal ventilatory response to hypoxia, indicating that absence of β2-containing nAChRs either potentiates excitatory, diminishes inhibitory (e.g., dopaminergic) neuromodulation of hypoxic drive, or both. Hypoxic responses are highly variable within most species, including mice; a contributing factor may be variability in the expression of genes encoding the different nAChR subunits (29). Our findings indicate that variable expression of the β2 subunit partially predetermines respiratory control settings and hypoxic responsiveness during sleep.
Hypoxia typically provokes compensatory changes (“neuroplasticity”) in respiratory output, which helps maintain breathing stability and efficiency under stress (30–32). Facilitation and afterdischarge, two classical examples of hypoxic respiratory neuroplasticity (19, 20), were dramatically accentuated in mutants (Figs. 3 and 4). These phenomena reflect persistent hyperexcitability of the brainstem and/or respiratory motoneuron pools that drive breathing. The underlying cause, enhanced central synaptic transmission, is activated by a mechanism that is normally exquisitely sensitive to the pattern of hypoxia (19, 30, 31). Facilitation, for example, is a gradual response to repetitive, intermittent hypoxia, as we observed in wild-type mice, but is not usually triggered by brief sustained hypoxia, as occurred in mutants. This difference indicates that pattern sensitivity in hypoxic respiratory neuroplasticity, an important mechanism underpinning compensation during respiratory failure, is partly encoded by a β2-containing nAChR-dependent processes.
These findings indicate that a variety of responses to hypoxia are either less vigorous, or occur more slowly when the β2 subunit is expressed (i.e., in wild-type mice). This indication implies that activation of β2-containing nAChRs, either by endogenous ACh or agonists such as nicotine, up-regulates the inhibitory modulation of breathing. Excessive or prolonged activation of these nAChRs, by disturbing the carefully maintained balance between excitation and inhibition, could exacerbate sleep-disordered breathing (33). Part of the sequence of events triggered by nicotine exposure could involve (i) overstimulation of high-affinity, β2-containing nAChRs, leading to (ii) potentiation of inhibitory drives, which ultimately (iii) depresses breathing, including, perhaps, compensatory responses to repetitive hypoxia during sleep (Fig. 1D).
Deficits in arousal, the principal defense against asphyxia and cardiorespiratory failure during sleep, are often linked to specific (e.g., brainstem) abnormalities (11). Here we demonstrate that an arousal deficit can also be associated with a general abnormality in the expression of a particular cholinergic nicotinic receptor. Afferent feedback to central nervous system arousal-promoting structures, particularly from mechanoreceptors that monitor hypoxic physical distress (increased rate and depth of breathing), is the principal trigger for arousal (4). In mutants, however, we observed uncoupling of the hypoxic ventilatory (accentuated) and arousal (depressed) responses. Hypoxic arousal thresholds thus seem to be raised in the absence of functional β2-containing nAChRs, which could reflect altered synaptic transmission at peripheral and central points along the hypoxic neural arc. The latter may be more important; the exaggerated respiratory facilitation and afterdischarge shown by the mutant indirectly indicates that central processing of peripheral hypoxic drive is abnormal in these animals. It is not necessarily a contradiction that primitive brainstem reflexes regulating breathing can be excited, but complex cortical (arousal) responses can be simultaneously depressed in mutant mice. The arousal process is partly influenced by cholinergic modulation of excitatory afferent drive, but also by a variety of other (e.g., noradrenergic) mechanisms (14, 34, 35). The development of these other mechanisms may be abnormal if appropriate levels of β2-containing nAChR stimulation are absent during ontogeny (3). The long-term consequences of this could be diminished excitatory transmission or heightened inhibitory gating of hypoxic afferent drive within the central nervous system, delaying and depressing the sleep–wake transition (14, 18). If particular nAChRs affect how arousal mechanisms develop, overactivity due to nicotine exposure, like underactivity in mutants, could be similarly detrimental to the normal postnatal development of this important protective reflex (7, 36).
Multiple nAChR subunit transcripts, including those of β2 subunits, were present globally in the carotid body. On the basis of this nAChR transcript profile, at least three possible types of nAChR oligomers can be present: heteromeric β2- and β4-containing, and homomeric α7 nAChRs (16). Because different cell types or groups of cells need not necessarily express all subsets of the nAChR mRNA we detected (12, 37), discrete pools of functional nAChRs oligomers could exist with a particular ultrastructural distribution within the carotid body. Only two (α4 and α7) subunits are definitely known to be present as proteins in crucial structures such as the glomus (chemosensory) cells and carotid sinus (afferent) nerve terminals (21, 22). We do not know whether the β2 subunit is also present as a protein, but if β2-containing nAChRs are present in these structures, one of their functions may be to help regulate carotid-body dopamine release (5). Absence of this nAChR subtype in mutant mice could partially disengage an important carotid-body inhibitory drive, partly explaining why hypoxic responsiveness was augmented in these animals. Whether this nAChR subtype modulates carotid-body activity after nicotine exposure is not clear from our data.
In summary, the nAChRs are crucial in fine-tuning breathing during sleep, and are essential for the normal development of arousal mechanisms. Disrupting the regulatory role of particular nAChR subtypes disturbs the delicate balance between ventilatory and arousal responses to hypoxic stress. Disturbing the balance between ventilatory and arousal responses could exacerbate respiratory failure during sleep, and may be part of sequence of events underlying the increased the risk of SIDS in unborn or newborn babies chronically exposed to nicotine. Clarifying the role played by particular (e.g., β2-containing) nAChRs in the pathophysiology associated with nicotine exposure may ultimately aid development of therapeutic approaches to prevent, treat, or reverse its side effects.
Acknowledgments
We thank Jean-Christophe Roux and Jean-Marc Pequignot for dissecting out the carotid bodies. This work was supported by Institut National de la Santé et de la Recherche Médicale, the National Health and Medical Research Council of Australia, Société de Tabacologie (France), the European Communities, the Collège de France, l'Association pour la Recherche sur le Cancer, Swedish Match, and the Swedish Medical Research Council 5234.
Abbreviations
- HVR
hypoxic ventilatory response
- nAChR
nicotinic acetylcholine receptor
- SIDS
sudden infant death syndrome
- VE
minute ventilation
References
- 1.Alm B, Milerad J, Wennergren G, Skjaerven R, Øyen N, Norvenius G, Daltveit A-K, Helweg-Larsen K, Markestad T, Irgens L M. Arch Dis Child. 1998;78:329–334. doi: 10.1136/adc.78.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDorman M F, Cnattingius S, Hoffman H J, Kramer M S, Haglund B. Am J Epidemiol. 1997;146:249–257. doi: 10.1093/oxfordjournals.aje.a009260. [DOI] [PubMed] [Google Scholar]
- 3.Slotkin T A. J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 4.Berry R B, Gleeson K. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 5.Holgert H, Hökfelt T, Hertzberg T, Lagercrantz H. Proc Natl Acad Sci USA. 1995;92:7575–7579. doi: 10.1073/pnas.92.16.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milerad J, Larsson H, Lin J, Sundell H. Pediatr Res. 1995;37:652–660. doi: 10.1203/00006450-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Lewis K W, Bosque E M. J Pediatr. 1995;127:691–699. doi: 10.1016/s0022-3476(95)70155-9. [DOI] [PubMed] [Google Scholar]
- 8.Hafström O, Milerad J, Asokan N, Poole S D, Sundell H W. Pediatr Res. 2000;47:646–652. doi: 10.1203/00006450-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Slotkin T A. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt C N, Peers C. Neuroscience. 1993;54:275–281. doi: 10.1016/0306-4522(93)90399-z. [DOI] [PubMed] [Google Scholar]
- 11.Nachmanoff D B, Panigrahy A, Filiano J J, Mandell F, Sleeper L A, Valdes-Dapena M, Krous H F, White W F, Kinney H C. J Neuropathol Exp Neurol. 1998;57:1018–1025. doi: 10.1097/00005072-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Léna C, de Kerchove d'Exaerde A, Cordero-Erausquin M, Le Novère N, Arroyo-Jimenez M, Changeux J P. Proc Natl Acad Sci USA. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald R S. Respir Physiol. 2000;120:89–104. doi: 10.1016/s0034-5687(00)00091-8. [DOI] [PubMed] [Google Scholar]
- 14.Steriade M, McCormick D A, Sejnowski T J. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 15.Shao X M, Feldman J L. J Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordero-Erausquin M, Marubio L M, Klink R, Changeux J P. Trends Pharmacol Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- 17.Picciotto M R, Zoli M, Rimondini R, Léna C, Marubio L M, Pich E M, Fuxe K, Changeux J P. Nature (London) 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 18.Cohen G, Gressens P, Gallego J, Gautier C. J Physiol. 2002;540:691–699. doi: 10.1113/jphysiol.2001.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell G S, Baker T L, Nanda S A, Fuller D D, Zabka A G, Hodgeman B A, Bavis R W, Mack K J, Olson E B. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 20.Georgopoulus D, Giannouli E, Tsara V, Argiropoulou P, Patakas D, Anthonisen N R. Am Rev Respir Dis. 1992;146:1250–1255. doi: 10.1164/ajrccm/146.5_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 21.Ishizawa Y, Fitzgerald R S, Shirahata M, Schofield B. Adv Exp Med Biol. 1996;410:253–256. doi: 10.1007/978-1-4615-5891-0_37. [DOI] [PubMed] [Google Scholar]
- 22.Shirahata M, Ishizawa Y, Rudisill M, Sham J S, Schofield B, Fitzgerald R S. Brain Res. 1998;814:213–217. doi: 10.1016/s0006-8993(98)01015-4. [DOI] [PubMed] [Google Scholar]
- 23.Salin-Pascual R J, Moro-Lopez M L, Gonzalez-Sanchez H, Blanco-Centurion C. Psychopharmacology. 1999;145:133–138. doi: 10.1007/s002130051041. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakar N R. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 25.Goiny M, Lagercrantz H, Srinivasan M, Ungerstedt U, Yamamoto Y. J Appl Physiol. 1991;70:2395–2400. doi: 10.1152/jappl.1991.70.6.2395. [DOI] [PubMed] [Google Scholar]
- 26.Huey K A, Low M J, Kelly M A, Juarez R, Szewczak J M, Powell F L. J Appl Physiol. 2000;89:1142–1150. doi: 10.1152/jappl.2000.89.3.1142. [DOI] [PubMed] [Google Scholar]
- 27.Nattie E. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan C E. In: Physiology in Sleep. Orem J, Barnes D, editors. New York: Academic; 1980. pp. 213–271. [Google Scholar]
- 29.Tankersley C G. J Appl Physiol. 2001;90:1615–1622. doi: 10.1152/jappl.2001.90.4.1615. [DOI] [PubMed] [Google Scholar]
- 30.Gozal E, Gozal D. J Appl Physiol. 2001;90:1995–1999. doi: 10.1152/jappl.2001.90.5.1995. [DOI] [PubMed] [Google Scholar]
- 31.Winder D G, Schramm N L. Physiol Behav. 2001;73:763–780. doi: 10.1016/s0031-9384(01)00514-5. [DOI] [PubMed] [Google Scholar]
- 32.Khoo M C K. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 33.Gaultier C, Guilleminault C. Sleep Med. 2001;2:281–295. doi: 10.1016/s1389-9457(01)00098-3. [DOI] [PubMed] [Google Scholar]
- 34.Léna C, Changeux J P. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svensson T H. In: Neurobiology of the Control of Breathing. Von Euler C, Lagercrantz H, editors. New York: Raven; 1986. pp. 297–301. [Google Scholar]
- 36.Franco P, Grosswasser J, Hassid S, Lanquart J P, Scaillet S, Kahn A. J Pediatr. 1999;135:34–38. doi: 10.1016/s0022-3476(99)70324-0. [DOI] [PubMed] [Google Scholar]
- 37.Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux J P. J Neurosci. 2001;21:1425–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]