Abstract
Plants frequently respond to herbivorous insect attack by synthesizing defense proteins that deter insect feeding and prevent additional herbivory. Maize (Zea mays L.) lines, resistant to feeding by a number of lepidopteran species, rapidly mobilize a unique 33-kDa cysteine protease in response to caterpillar feeding. The accumulation of the 33-kDa cysteine protease in the maize mid-whorl was correlated with a significant reduction in caterpillar growth that resulted from impaired nutrient utilization. Black Mexican Sweetcorn callus transformed with mir1, the gene encoding the 33-kDa cysteine protease, expressed the protease and growth of caterpillars reared on the transgenic callus was reduced 60–80%. Scanning electron microscopy was used to examine the effect of plant material expressing the 33-kDa cysteine protease on the structure of the caterpillar peritrophic matrix. Because the peritrophic matrix surrounds the food bolus, assists in digestive processes, and protects the caterpillar midgut from physical and chemical damage, disruption of peritrophic matrix may reduce caterpillar growth. The results indicated that the peritrophic matrix was severely damaged when caterpillars fed on resistant maize plants or transgenic Black Mexican Sweetcorn. The accumulation of the 33-kDa cysteine protease in response to caterpillar feeding, and its ability to damage the insect peritrophic matrix, represents an unusual host–plant resistance mechanism that may have applications in agricultural biotechnology.
Although the world's most important food crops are the monocotyledenous plants, wheat, rice, and corn, little is known about insect-defense response mechanisms in these species. However, maize lines with genetic resistance to several lepidopteran species appear to have a novel defense mechanism (1). Within 1 h of fall armyworm (Spodoptera frugiperda) feeding, resistant plants mobilize a unique 33-kDa cysteine protease that accumulates at the wound site in the mid-whorl region and increases in abundance up to 7 days after infestation (1). The protease accumulates more rapidly than other insect-induced plant defense proteins, which typically appear 4–8 h after insect attack (2). It is most abundant in the yellow-green region of the mid-whorl, which is the preferred caterpillar-feeding site (1). Yellow-green mid-whorl tissue inhibits caterpillar growth in bioassays and physiological indices indicated that it significantly impairs caterpillar nutrient utilization (3). The properties of the 33-kDa cysteine protease are distinct from those of the typical insect-induced plant defense proteins, such as protease inhibitors, cell wall proteins, lectins, oxidative enzymes, and enzymes catalyzing the production of secondary products (4). Understanding how resistant maize plants mobilize this protease and determining its effect on caterpillar growth may provide information that can be used to enhance insect resistance in other plants.
Exotic germplasm from Antigua was used to develop maize lines resistant to feeding by fall armyworm (S. frugiperda) and southwestern corn borer (Diatraea grandiosella) (5). These lines have shown some level of resistance to all Lepidoptera tested, including corn earworm (Heliocoverpa zea), tobacco budworm (Heliothis virescens), sugarcane borer (Diatrea saccharalis), European corn borer (Ostrinia nubilalis), and other lepidopteran pests (6). Fall armyworm caterpillars reared on resistant lines weighed approximately 50% less than those reared on susceptible lines (7). They had a lower initial growth rate, and attained their maximum weight later and pupated later than those reared on susceptible genotypes (3). A similar growth reduction occurred when caterpillars were fed on callus initiated from mature embryos of the resistant lines (8). The 33-kDa cysteine protease also was abundant in this callus, and genetic analysis demonstrated a significant negative correlation between protease concentration in callus and caterpillar weight (9).
mir1 encodes the 33-kDa cysteine protease, and it has a derived amino acid sequence of 398 aa (ref. 10; GenBank accession no. AF019145). The 372 amino acids starting at the amino terminus have a high degree of similarity with other cysteine proteases in the papain superfamily (10). However, the sequence of the 25 remaining amino acids at the carboxyl terminus, has no match in the databases (10). The effect of this unique sequence on protease function is currently unknown. In addition, the amino acid sequence from position 188 to 236 has approximately 70% similarity to the chitin-binding domains present in a Volvox cysteine protease (11), wheat germ agglutinin (12, 13), and hevein (14, 15), and recent experiments confirmed that the 33-kDa cysteine protease has chitin-binding activity (unpublished data). mir1 has been mapped to chromosome 6 (bin 6.02) on the maize genome, where there is a significant quantitative trait locus for resistance to European corn borer leaf feeding (16). Growth of both fall armyworm and tobacco budworm on Black Mexican Sweetcorn (BMS-33) cells transformed with mir1 and expressing the 33-kDa cysteine protease was reduced 60–80% (1). These results suggest that the 33-kDa cysteine protease plays some role in retarding caterpillar growth. A likely target for the protease is the caterpillar peritrophic matrix (PM). The PM is a chitin network embedded with glycoproteins and proteoglycans that lines the midgut epithelium of most insects (17). It protects the midgut epithelium from mechanical damage, pathogens, and toxins, and plays an active role in digestion and nutrient absorption (18). It is generally accepted that PM damage can be very deleterious to the insect (17, 18). This study was conducted to determine the effect of resistant maize whorl tissue and transgenic BMS expressing 33-kDa cysteine protease on caterpillar PM structure.
Materials and Methods
Susceptible (Tx601) and resistant (Mp708) maize lines (5) were grown outside in pots for approximately 5 weeks (1). Neonate S. frugiperda caterpillars were reared on artificial diet (19) for 4 days and then transferred to the maize mid-whorl region. Plants were maintained outdoors during the experiment. Four-day-old caterpillars also were transferred to Black Mexican Sweetcorn (BMS) callus and BMS transformed with mir1 (BMS-33) that ectopically expressed the 33-kDa cysteine protease (1). Four days after transfer to plants or callus, 5–10 caterpillars per treatment were collected, immobilized on ice, weighed, and dissected under ice cold PBS (80 mM Na2HPO4/20 mM NaH2PO4/100 mM NaCl). The gut was removed and gently teased open with fine forceps. The inner gut surface was pulled back to expose the villi and microvilli, as well as the PM. The gut and PM were placed in 50–100 μl of PBS and fixed overnight at 4°C in Karnovsky's fixative.¶ Postfixation was carried out in 2% OsO4. For scanning electron microscopy, dehydrated tissue was treated with pentane, air-dried, and mounted on aluminum stubs. Mounted stubs were coated with gold/palladium at 2.5 kV and examined by using a Cambridge S360 scanning electron microscope. At least three individual caterpillars per treatment were used to prepare samples of PM for scanning electron microscopy. Each sample was thoroughly examined at several magnifications, and more than 100 photographs were taken to document typical PM morphology.
Results
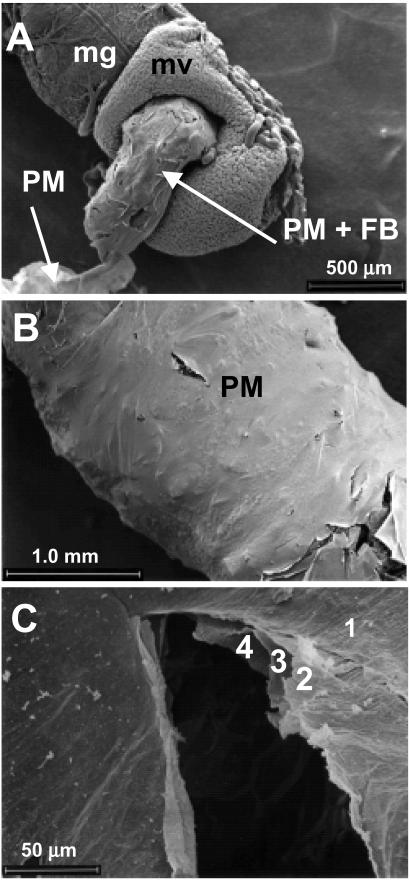
Fig. 1A shows the ectoperitrophic layer of the S. frugiperda midgut dissected from a caterpillar that fed in the whorl of a resistant maize plant for 4 days. The PM extruding from the midgut is partially filled with a food bolus. In this caterpillar, only a short section of the PM was filled with food. All caterpillars collected from resistant plants had incompletely filled PMs. The food bolus did not appear to be preferentially located in the anterior or posterior region of the gut in these insects. Caterpillars collected from susceptible plants had completely filled PMs.
Figure 1.
Scanning electron micrographs showing fall armyworm midgut and PM. (A) Excised midgut (mg) from a caterpillar reared on a resistant plant showing the exposed microvilli (mv), PM containing the food bolus (PM + FB), and empty PM (magnification = ×50). (B) PM from a caterpillar reared on a susceptible plant (magnification = ×38). (C) Ectoperitrophic layer (1), midperitrophic layers (2, 3) and endoperitrophic layer (4) of a caterpillar reared on a resistant plant (magnification = ×500). The scale is indicated on each micrograph.
Examination at low magnifications (30–500×) did not reveal major differences in PM structure other than cracks or tears that were probably caused by drying or mechanical damage that occurred during the dissection (Fig. 1B). All caterpillars, regardless of their diet, exhibited this type of PM damage, and it was not correlated with a reduction in caterpillar weight. Careful examination of the PM at higher magnifications (500–3,500×) revealed four distinct PM layers in all samples (Fig. 1C). If the PM was intact, the layers appeared to be tightly appressed, but they appeared to be separated if it was damaged (Fig. 1C). Subsequent examinations of the PM focused on three areas: the ectoperitrophic layer closest to the midgut microvilli, the two middle (midperitrophic) layers that were exposed when the upper layer was cracked or pealed, and the endoperitrophic peritrophic layer facing the food bolus.
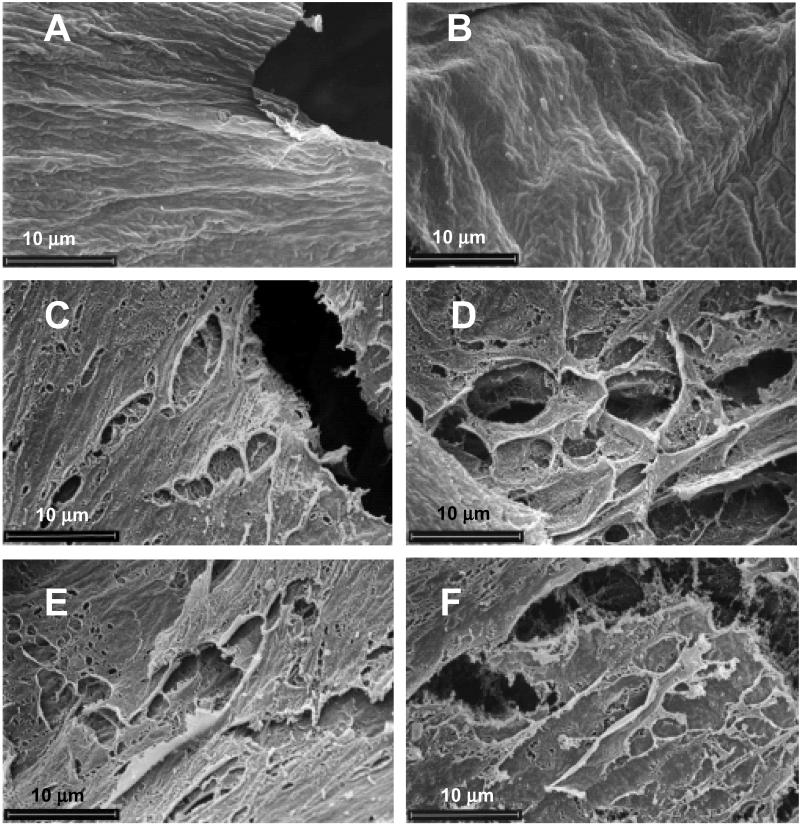
Next, PMs dissected from caterpillars that fed on resistant and susceptible plants were examined at the higher magnifications. In this experiment, caterpillars from resistant plants weighed approximately 58% less than those from susceptible plants (Fig. 2). PMs from caterpillars that fed on susceptible plants showed no apparent damage to the ectoperitrophic or endoperitrophic matrix layers (Fig. 2 A and B). The midperitrophic layers were identical in appearance to these layers and are not shown. When caterpillars fed on resistant plants, PM damage was evident only in regions where the food bolus was present. Holes, perforations, structural voids, or abrasions of variable size (up to several μm), were apparent in these regions. Damage to the ectoperitrophic layer varied from light to severe depending on the region of the PM examined. An example of severe damage is shown in Fig. 2C. The midperitrophic (Fig. 2D) and the endoperitrophic layers (Fig. 2 E and F), which were in closer contact with the food bolus, were more severely damaged (Fig. 2 D–F).
Figure 2.
Scanning electron micrographs showing peritrophic matrix (PM) of fall armyworm caterpillars that fed on susceptible and resistant corn plants. (A and B) Ectoperitrophic and endoperitrophic layers, respectively, from caterpillars reared on a susceptible plant. (C) Ectoperitrophic layer from a caterpillar reared on a resistant plant. (D) Midperitrophic layer from a caterpillar reared on resistant plant. (E and F) Endoperitrophic layer from a caterpillar reared on a resistant plant. In all cases, the magnification = ×3,000. In this experiment, caterpillars reared on susceptible and resistant plants weighed 89.0 ± 19.6 and 37.3 ± 15.0 mg, respectively. The scale is indicated in each micrograph.
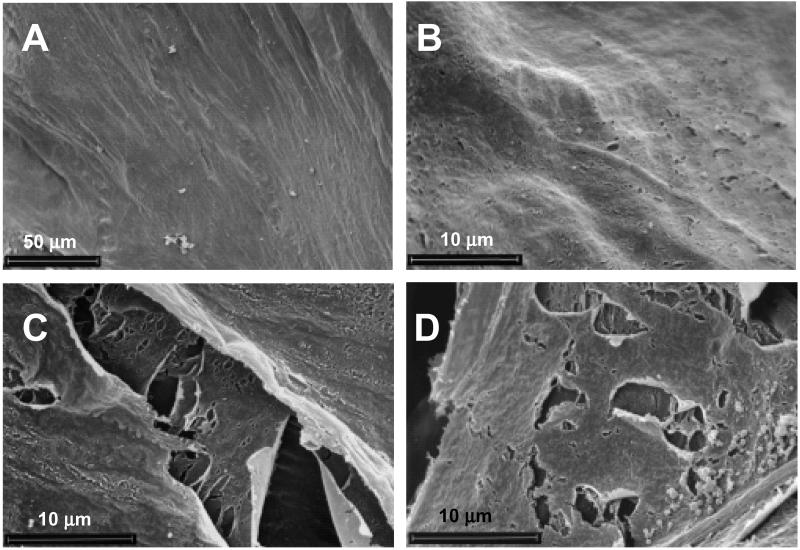
To distinguish between the effects of the resistant plants and the 33-kDa cysteine protease, caterpillars were reared on BMS-33, transgenic callus that over-expressed the protease. There was no endogenous cysteine protease activity in the BMS controls, which were either nontransformed BMS, or BMS transformed with the control vector (no mir1 insertion) (1). In this experiment, caterpillar growth was reduced approximately 74% when they were fed BMS-33 (Fig. 3). There was no damage to the ectoperitrophic (Fig. 3 A and B) or midperitrophic and endoperitrophic layers (micrographs not shown) when caterpillars were fed nontransformed BMS. Nor was there damage when insects were reared on BMS transformed with the control vector (micrographs not shown). When caterpillars were fed transgenic BMS-33, the ectoperitrophic matrix was not damaged (micrograph not shown). However, more extensive damage was apparent in the midperitrophic and endoperitrophic layers, which exhibited cracks and perforations that were similar to those found in the PMs of caterpillars that fed on resistant plants (Fig. 3 C and D). These results suggest that 33-kDa cysteine protease, or perhaps one of its catalytic products disrupts the PM when it is ingested by the caterpillar.
Figure 3.
Scanning electron micrographs showing peritrophic matrix (PM) of fall armyworm caterpillars reared on BMS-33 callus overexpressing the 33 kDa cysteine protease. (A) Ectoperitrophic from caterpillars reared on nontransformed BMS (magnification = ×500). (B) Ectoperitrophic from caterpillars reared on nontransformed BMS (magnification = ×3,000). (C) Midperitrophic layers from a larva reared on BMS-33 callus (magnification = ×3,500). (D) Endoperitrophic layer from a caterpillar reared on BMS-33 callus (magnification = ×3,500). In this experiment, caterpillars reared on BMS and BMS-33 callus weighed 264.3 ± 2.5 and 68.6 ± 7.7 mg, respectively. The scale is indicated on each micrograph.
Discussion
Caterpillars fed on resistant plants or BMS-33 weighed less and exhibited greater PM damage than those grown on susceptible controls. In both cases, the greatest damage was to the endoperitrophic layer that was in direct contact with the food bolus. More severe PM damage may occur in the field, where caterpillars feed in the mid-whorl from hatch to pupation. Because the PM is important in the compartmentalization of digestive events, it is highly probable that changes in PM integrity would impair digestion and nutrient absorption. A number of studies have demonstrated that increased PM permeability harms insects. For example, increased PM permeability impaired normal nutrient and enzyme cycling between the endoperitrophic and ectoperitrophic spaces (17, 20). Disruption of the chitin network with lectins or chemicals increased both PM permeability and insect mortality (21). The metalloprotease, enhancin, a product of the baculovirus, Trichoplusa ni granulosis virus, specifically degraded Insect Intestinal Mucin (IIM), a structural protein present in the T. ni PM (20, ¶). Degradation of IIM increased PM permeability and caterpillar susceptibility to subsequent baculovirus infection (22).
The 33-kDa cysteine protease has been purified from resistant maize callus and is a papain-like thiol protease (9). Its derived amino acid sequence indicates that the protease has functional similarity to baculovirus cysteine proteases that are involved in viral horizontal transmission (23). The chitin-binding activity of the 33-kDa cysteine protease may enhance its ability to cause PM damage. The protease may disrupt the PM structure by displacing another chitin-binding protein. Or the chitin-binding domain could tether the protease to the chitin network of the PM, where it could attack nearby proteins and damage PM structure. The protease has an acidic pH optimum (9), which suggests that it might not be active in the alkaline caterpillar gut. However, in fall armyworm, digestive enzymes are released from anterior midgut cells by a microapocrine process and remain associated with membranes until released into the PM jelly (20). The release of membrane contents at the PM may create microenvironment with a pH favorable for the 33-kDa cysteine protease to be active.
The PM damage and resulting growth reduction that occurs when fall armyworm caterpillars feed on resistant corn or BMS-33 expressing the 33-kDa cysteine protease, appears to be different from those of other insect-defense proteins that plants accumulate in response to insect feeding. Protease inhibitors retard caterpillar growth, not by inhibiting the digestive process, but by a feedback mechanism leading to the overproduction of digestive proteases resulting in the depletion of essential amino acids (24). Lectins may retard caterpillar growth by binding to chitin and preventing proper PM formation, or by binding to glycoconjungates exposed on midgut epithelia cells or glycosylated digestive enzymes (25). The effect of 33-kDa cysteine protease is distinct from that of the toxin produced by Bacillus thuringiensis (Bt-toxin), which is used to protect transgenic crops from Lepidoptera. Bt-toxins are lethal because they bind membrane receptors in the insect gut leading to pore formation and death (26). Our results suggest the 33-kDa cysteine protease damages the caterpillar's first line of defense, the PM. Damage to the PM probably impairs the normal highly organized digestive mechanism of caterpillar. The deterioration of digestive system organization would account for the reduction in caterpillar weight when they feed on resistant plants or transgenic callus expressing the 33-kDa cysteine protease.
Acknowledgments
This research was supported by the U.S. Department of Agriculture (USDA)-Agricultural Research Service, Mississippi Agricultural and Forestry Experiment Station (MAFES), USDA National Research Initiative Competitive Grants Program Award 9835302-6819 and National Science Foundation Award IBN-0131328.
Abbreviations
- BMS
Black Mexican Sweetcorn
- BMS-33
BMS expressing the 33-kDa cysteine protease
- PM
peritrophic matrix
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
To whom reprint requests should be addressed. E-mail: dsluthe@ra.msstate.edu.
Karnovsky, M. (1965) J. Cell Biol. 27, 137A–138A (abstr.).
References
- 1.Pechan T, Ye L, Chang Y, Mitra A, Lin L, Davis F M, Williams W P, Luthe D S. Plant Cell. 2000;12:1031–1040. doi: 10.1105/tpc.12.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan C A. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y-M, Luthe D S, Davis F M, Williams W P. J Econ Entomol. 2000;93:477–483. doi: 10.1603/0022-0493-93.2.477. [DOI] [PubMed] [Google Scholar]
- 4.Constabel C, Bergey D R, Ryan C A. In: Induced Plant Defenses Against Pathogens and Herbivores. Agrawal A A, Tuzun S, Bent E, editors. St. Paul: Am. Phytopathol. Soc.; 1999. pp. 137–166. [Google Scholar]
- 5.Williams W P, Buckley P M, Davis F M, Windham G L. Crop Sci. 1990;30:757. [Google Scholar]
- 6.Davis F M, Williams W P, Mihm A, Barry B E, Overman L J, Wiseman B R, Riley T J. Miss Agric Exp Stn Tech Bull. 1988;157:1–6. [Google Scholar]
- 7.Williams W P, Buckley P M. J Econ Entomol. 1992;85:2039–2042. [Google Scholar]
- 8.Williams W P, Buckley P M, Davis F M. Agric Ecosyst Environ. 1987;18:85–90. [Google Scholar]
- 9.Jiang B, Siregar U, Willeford K O, Luthe D S, Williams W P. Plant Physiol. 1995;108:1631–1640. doi: 10.1104/pp.108.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pechan T, Jiang B, Steckler D, Ye L, Lin L, Luthe D S, Williams W P. Plant Mol Biol. 1999;40:111–119. doi: 10.1023/a:1026494813936. [DOI] [PubMed] [Google Scholar]
- 11.Amon P, Haas E, Sumper M. Plant Cell. 1998;10:781–789. doi: 10.1105/tpc.10.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raikhel N, Lee H I, Broekaert W F. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:591–615. [Google Scholar]
- 13.Shen Z J-L. J Mol Evol. 1999;48:341–347. doi: 10.1007/pl00006478. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Olmedo F, Molina A, Alamillo J M, Rodriguez-Palenzuela P. Biopolymers. 1998;47:479–491. doi: 10.1002/(SICI)1097-0282(1998)47:6<479::AID-BIP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Koo J C, Lee S Y, Chun H J, Cheong Y H, Choi J S, Kawabata S, Miyagi M, Tsunasawa S, Ha K S, Bae D W, et al. Biochim Biophys Acta. 1998;1382:80–90. doi: 10.1016/s0167-4838(97)00148-9. [DOI] [PubMed] [Google Scholar]
- 16.Jampatong C, McMullen M D, Barry D B, Darrah L L, Byrne P, Kross H. Crop Sci. 2001;42:584–593. [Google Scholar]
- 17.Terra A R. Arch Insect Biochem Physiol. 2001;47:47–61. doi: 10.1002/arch.1036. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Granados R R. Arch Insect Biochem Physiol. 2001;47:110–118. doi: 10.1002/arch.1041. [DOI] [PubMed] [Google Scholar]
- 19.Williams W P, Buckley P M, Hedin P A, Davis F M. J Econ Entomol. 1990;83:1578–1571. [Google Scholar]
- 20.Bolognesi R, Ribeiro A F, Terra W R, Ferreira C. Arch Insect Biochem Physiol. 2001;47:62–75. doi: 10.1002/arch.1037. [DOI] [PubMed] [Google Scholar]
- 21.Harper M, Hopkins T, Czapla T. Tissue Cell. 1998;30:166–176. doi: 10.1016/s0040-8166(98)80065-7. [DOI] [PubMed] [Google Scholar]
- 22.Peng J, Zhong J, Granados R R. J Insect Physiol. 1999;45:159–166. doi: 10.1016/s0022-1910(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa T, Majima K, Maeda S. J Virol. 1994;68:6619–6625. doi: 10.1128/jvi.68.10.6619-6625.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broadway R, Duffey S. J Insect Physiol. 1986;32:827–833. [Google Scholar]
- 25.Peumans W, Van Damme E J M. Plant Physiol. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]