Abstract
The 1856 discovery of the Neandertal type specimen (Neandertal 1) in western Germany marked the beginning of human paleontology and initiated the longest-standing debate in the discipline: the role of Neandertals in human evolutionary history. We report excavations of cave sediments that were removed from the Feldhofer caves in 1856. These deposits have yielded over 60 human skeletal fragments, along with a large series of Paleolithic artifacts and faunal material. Our analysis of this material represents the first interdisciplinary analysis of Neandertal remains incorporating genetic, direct dating, and morphological dimensions simultaneously. Three of these skeletal fragments fit directly on Neandertal 1, whereas several others have distinctively Neandertal features. At least three individuals are represented in the skeletal sample. Radiocarbon dates for Neandertal 1, from which a mtDNA sequence was determined in 1997, and a second individual indicate an age of ≈40,000 yr for both. mtDNA analysis on the same second individual yields a sequence that clusters with other published Neandertal sequences.
Thirteen kilometers east of Düsseldorf lies a nondescript valley cut through deposits of high-grade Devonian limestone by the Düssel river. Before the 1850s, the valley was defined by walls that rose as high as 50 m above the river and extended for slightly less than 1 km in an east–west direction. Originally known as “Gesteins” or “Hundsklipp,” the valley became known by about 1850 as the Neander Valley (Neandertal) in honor of Joachim Neander (1650–1680), a teacher, poet, and composer of hymns who often visited the area (1). Descriptions of this valley from the early 19th century note that numerous caves and rock shelters of varying sizes were located along both the north and south valley walls (2). One of the smaller caves located on the south wall was known as the Kleine Feldhofer Grotte, so named because of its proximity to the nearby large farm of Feldhof. This small cave (≈3 m in width by 5 m in length by 3 m in height) had a very small mouth (less than 1 m wide), which opened approximately 20 m above the valley floor (1, 3).
Before the 1850s, Neandertal limestone was important for local construction, but by mid-decade, the demands of the Prussian construction industry reached the Neandertal. In 1854, Wilhelm Beckershoff and Friedrich Wilhelm Pieper founded the Actiengesellschaft für Marmorindustrie Neanderthal to facilitate local construction projects (1). To supply limestone for these projects, Beckershoff and Pieper focused quarrying operations on the south wall of the Neander Valley. As the limestone was quarried, the south wall and much of the north wall, as well as the caves located therein, were literally removed. As the caves were encountered, the clay deposits in them had to be removed to reduce contamination of the limestone during actual quarrying.
In August 1856, removal of deposits from the Kleine Feldhofer Grotte resulted in recovery of a calotte and 15 postcranial bones. Although the detailed events of the discovery of this skeleton are not known, the circumstances that led to the recovery of the original Neandertal skeleton were chronicled by local teacher and natural historian Johann Carl Fuhlrott (4). Fuhlrott reported that the bones were found about 2 feet below the surface, and that the skeleton was originally oriented with the skull facing the cave opening. Because the clay matrix adhered tightly to the individual bones, some of them, including the calotte, were recognized only after they had been thrown out of the cave and fell the 20 m to the valley floor. Fortunately, according to Fuhlrott, Beckershoff happened to be at the locality as the bones were initially noticed and told the workers to be on the lookout for other bones. Fuhlrott noted that the bones were initially thought to be those of a cave bear, which had been found in other local caves, and that this misidentification was responsible for the bones being collected (4). At Pieper's invitation, Fuhlrott visited the area at the end of August 1856 and identified the remains of Neandertal 1 as human. Only then, perhaps 2 weeks after the specimen was found, was he able to record the circumstances of the discovery. Fuhlrott also stated that because the bones were not considered to be of any great importance at the time of recovery, the workers were not particularly careful and collected only the larger easily identifiable bones.
The Neandertal find, first reported scientifically by Fuhlrott and Schaaffhausen in 1857 (5, 6), was the subject of a detailed analysis by Schaaffhausen (7) and became a focal point in the debate about human evolution during the late 19th and 20th centuries (8). However, all of these discussions concern only aspects of Neandertal 1's skeletal anatomy and interpretation thereof, because no archaeological or faunal materials were reported from the site. Because there were no associated finds, and the cave was destroyed without a scientific geological analysis, the site has been considered undatable. Also, because no careful surveying was done, the exact location of the Kleine Feldhofer Grotte was no longer known by 1900 (1).
New Excavations in the Neander Valley
Although it was generally assumed that the deposits removed from the Feldhofer caves were dumped somewhere in the area, attempts to discern their location were unsuccessful until 1997. On the basis of careful archival research, Schmitz and his archaeological colleague, Jürgen Thissen, were able to deduce the likely location of these deposits. In 1997, excavations by the Rheinisches Amt für Bodendenkmalpflege in Bonn under the direction of Schmitz and Thissen began in this area. These excavations led to the discovery that the base of the south valley wall was left intact, and cave sediments were located adjacent to this remnant. Excavation of these deposits resulted in the recovery of Paleolithic artifacts, Pleistocene faunal remains, and some 24 fragments of human bone from the clay sediments on the valley side of the remnant (9). That these must at least in part represent remains from the Kleine Feldhofer Grotte was confirmed when a small piece of human bone (NN 13) was found to fit exactly onto the lateral side of the left lateral femoral condyle of Neandertal 1 (1). In 2000, additional excavations were conducted at this location, and a much larger series of fauna, artifacts, and human skeletal fragments was recovered. Two cranial fragments from these excavations were found to fit onto the original Neandertal 1 calotte.
Preliminary analysis of the thousands of lithic artifacts recovered from these deposits has shown that two specific Paleolithic assemblages are represented: Micoquian artifacts typical of the late Middle Paleolithic and Upper Paleolithic artifacts from the Gravettian (1, 9). More systematic analysis of the artifacts is underway, but the presence of Gravettian artifacts means that not all of the human skeletal fragments necessarily represent Neandertals. Study of the faunal remains also is at the incipient stage, but the fauna indicates a Late Pleistocene age for the deposits. Also, systematic evidence of human modification in the form of cut marks and impact fractures is evident on many bones.
Human Skeletal Remains
The 1997 and 2000 excavations have resulted in the recovery of 62 new human skeletal pieces. Table 3 (which is published as supporting information on the PNAS web site, www.pnas.org) lists and provides brief information on all human skeletal fragments recovered. The new specimens are all smaller and/or more fragmentary than the bones recovered in 1856. This is not surprising, because these specimens were thrown down an approximately 20-m rock face and subjected to breakage by subsequent quarrying activity while on the valley floor. Although fragmentary, many pieces of bone have been refitted to form more complete elements (see Table 3). Although a complete discussion of these specimens is not possible here, several issues are of immediate interest. First, are these specimens the remains of Neandertals? Second, do any of these elements represent additional portions of Neandertal 1? Third, are individuals other than Neandertal 1 represented?
Six teeth and seven skull fragments have been identified, none of which duplicates preserved portions of Neandertal 1. Two cranial pieces, a left zygomatic and partial maxillary body (NN 34, Fig. 1), and a right piece of temporal bone (NN 35) fit clearly on the Neandertal 1 calotte. The zygomaticomaxillary fragment exhibits a number of distinctive features and would have been identified as a Neandertal even without the fit to Neandertal 1. These features include an oblique zygomaticoalveolar margin, a markedly enlarged maxillary sinus (even extending into the root of the zygomatic arch), a columnar lateral orbital margin, multiple zygomaticofacial foramina, marked muscle attachments for fibers of the temporalis muscle in the anterior temporal fossa, and a parasagittal orientation of the infraorbital plate. This complex of features is distinctive for Neandertals, although the individual traits are not unique to them (10–14). NN 35 is a posterior mastoid portion of the temporal preserving the posterior terminus of a large digastric sulcus exteriorly and a well-excavated sulcus for the sigmoid sinus internally. Both of these features also are characteristic but not specifically diagnostic of Neandertals.
Figure 1.
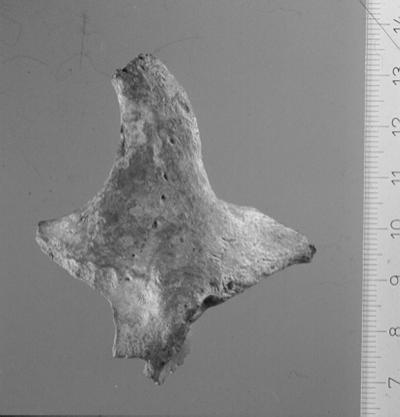
The NN 34 left zygomaticomaxillary specimen. Scale is in millimeters. Numbers designate centimeters.
The other cranial fragments do not physically connect to, but represent elements missing from, Neandertal 1. Two fragments each of sphenoid (NN 19 and 56) and mandible (NN 52 and 61) and a piece of right occipital (NN 40) also exhibit features indicative of Neandertals. The sphenoid (NN 19) exhibits the elongated body and sinus typical of Neandertals (15). The occipital fragment exhibits a distinctive V-shaped sulcus that connects the foramen magnum rim to the inferiorly positioned occipitomastoid region typical of Neandertals (see ref. 12). NN 52 is an inferior portion of a mandibular symphysis with short segments of the anterior and lingual symphyseal walls (Fig. 2). This specimen has expansive digastric fossae, partially separated by a crest for the genioglossus muscle that extends up the lingual face. In each of these features, NN 52 is particularly similar to the Krapina Neandertal mandibles (10). The anterior face is flat, with no evidence of structures associated with a mental trigone or even a mentum osseum, morphology consistent with a Neandertal mandible. NN 61 is a segment of the mandibular incisure preserving an intersection of the crest toward the center of the condylar neck, another feature that tends to be characteristic of Neandertals but is not unique to them (16).
Figure 2.
The NN 52 inferior mandibular symphysis. Scale as in Fig. 1.
The teeth include a single permanent mandibular element (left I1), four permanent maxillary teeth (right P4, right M2, left M1 or M2, and left M3), and a single deciduous tooth (right dm2). No teeth were recovered in 1856, so no dental specimen duplicates elements from Neandertal 1. All teeth but the left M3 (NN 33) exhibit moderate-to-heavy occlusal and interproximal wear. In terms of size (Table 1), NN 55 (left I1) appears small compared with other Neandertal samples. Molars are somewhat less worn, but mesiodistal diameters are certainly reduced by interproximal wear in all teeth except NN 33. Crown size and morphology do not permit attribution of the teeth to any specific Late Pleistocene human group (17). Radiological analysis of the three permanent molars showed no unusual expansion of the pulp cavities (taurodontism), but the presence of this feature is highly variable in Neandertals, especially in maxillary teeth (18). NN 31 (right M2), exhibits a 6.8-mm-long interproximal “toothpick” groove mesially, a feature increasingly found in (but not unique to) Neandertals (19).
Table 1.
Dental metrics
| NN no. | Tooth | B(L)-L | M-D | Krapina B(L)-L Mean ± SD (n) (10) | European Würm Neandertal B(L)-L Mean ± SD (n) (20) | European Early Upper Paleolithic B(L)-L Mean ± SD (n)* |
|---|---|---|---|---|---|---|
| 16 | M2 (L) | 13.3 | 10.7 | 12.4 ± 1.2 (17) | 12.3 ± 1.2 (19) | 12.4 ± 0.9 (29) |
| 31 | M2 (R) | 13.1 | 10.5 | 12.4 ± 1.2 (17) | 12.3 ± 1.2 (19) | 12.4 ± 0.9 (29) |
| 33 | M3 (L) | 12.7 | 10.5 | 11.6 ± 1.4 (6) | 12.0 ± 1.0 (16) | 11.6 ± 1.3 (24) |
| 50 | dm2 (R) | 10.2 | 8.5 | 10.5 ± 0.7 (11) | 10.2 ± 0.7 (13)* | 10.4 ± 0.6 (11) |
| 51 | P4 (R) | 9.0 | 6.6 | 10.8 ± 1.3 (14) | 9.9 ± 0.6 (20) | 10.0 ± 0.8 (22) |
| 55 | I1 (L) | 6.2 | 4.2 | 7.7 ± 0.5 (7) | 7.2 ± 0.3 (11) | 6.3 ± 0.5 (24) |
B(L)-L, buccal (labial)–lingual dimension; M-D, mesio-distal dimension.
Data courtesy of E. Trinkaus.
The only new postcranial piece that derives definitely from Neandertal 1 is the small portion of left lateral condyle (NN 13) mentioned above. Additionally, there is an adult right partial ischium (NN 14), including the superior half of the ischial tuberosity and inferior acetabulum. Neandertal 1 lacks the right os coxae, and the similarities in size and morphology between NN 14 and the Neandertal 1 left ischium strongly suggest that both specimens represent the same individual. Several other postcranial specimens were recovered that do not duplicate elements found in 1856 (see Table 3) and that could represent portions of Neandertal 1.
At least four postcranial specimens demonstrate the presence of a minimum of one additional adult individual in this sample, because they duplicate elements known for Neandertal 1. NN 1 is a ≈108-mm-long segment of a second right humerus, reconstructed from four fragments. This specimen is slightly smaller and more gracile than the right humerus of Neandertal 1, but three features suggest NN 1 is a Neandertal. First, the midshaft index (minimum shaft diameter = 18.2 mm/maximum shaft diameter = 25.5) of 70.7 indicates the moderate platymeria characteristic of Neandertals but rarer for early modern humans. This value lies just below the mean of a Neandertal sample of right humeri (73.8 ± 4.7, n = 12) but further below the mean of an early Upper Paleolithic right humerus sample (79.6 ± 5.1, n = 19) (data courtesy of E. Trinkaus). Second, the deltoid tuberosity is markedly less pronounced than the pectoralis major tuberosity. This pattern is more characteristic of Neandertals, whereas the reverse is more often true of early Upper Paleolithic humeri (21, 22). The pronounced pectoralis major tuberosity suggests that NN 1 likely represents an adult. Maximum breadth of the tuberosity is 10.4 mm, just above the mean for a sample of adult European Neandertals of 8.7 ± 1.4 mm (n = 6) and well above the mean for a sample of adult Early Upper Paleolithic humeri (5.1 ± 0.9 mm, n = 6) (ref. 12). Third, the deltoid tuberosity extends broadly parallel to the shaft's long axis rather than more obliquely across the shaft. The former condition is characteristic of Neandertal and earlier humeri in Europe but can also be found in other samples (23). There is also a distal right humeral metaphysis (NN 24), which might represent the same individual as NN 1, but no direct refitting of the two is possible at this time.
The other two postcranial specimens that duplicate elements in Neandertal 1 are an adult left proximal ulna (NN 60), preserving most of the olecranon and coronoid processes, and a right ulnar shaft (NN 42), reconstructed from six fragments. NN 60 exhibits a trochlear notch that faces anteriorly like many Neandertals rather than proximoanteriorly, as in most modern human ulnae (12, 24). NN 42 lacks both articular surfaces, but the apparently truncated length of the shaft suggests the forelimb shortening characteristic of Neandertals (12, 25), and the rugosity of muscle attachments on the proximal shaft indicates the specimen is adult.
Although it is not certain whether the NN 1 humerus, NN 24 distal humerus, NN 42 right ulna, and NN 60 left ulna belong to the same individual, these specimens demonstrate that at least one additional adult Neandertal individual, in addition to Neandertal 1, is present. However, the NN 50 deciduous molar could not derive from Neandertal 1 or from any other adult represented in the sample. The heavy wear and resorbed roots indicate that this tooth was from an ≈11- to 14-year-old individual by recent human standards (26). Thus, NN 50 demonstrates the presence of minimally a third individual, a subadult, in the sample.
Accelerator Mass Spectrometry (AMS) Radiocarbon Dating
AMS radiocarbon dating was conducted on samples of three specimens: the right humerus of Neandertal 1, the second right humerus (NN 1), and a right tibia fragment (NN 4). The sample from the Neandertal 1 right humerus had to be taken with care, due to the use of preservatives to conserve the Neandertal 1 skeleton. Investigation of the conservational history of the specimen reveals no indication that the specimen had been saturated with preservatives, which is confirmed by the histological analysis of M. Schultz (University of Göttingen). Thus, the preservative is restricted only to the bone surface. To avoid surface contamination, the probe for dating was taken by coring into the humeral compacta from a point exposed where a segment of bone was removed for the original DNA analysis (27) and parallel to the shaft's long axis. No preservatives were used on the recently excavated specimens (NN 1, NN 4), so that cores into the compacta for samples could be initiated directly from the bone surface.
A few small cylinders 2.5 mm in diameter and ≈2–3 mm in length were removed from each bone with a crown drill. The organic fraction referred to as collagen was extracted from the core samples by dissolving the inorganic part of the bones (calcium hydroxyapatite and calcium carbonate) with dilute hydrochloric acid (0.5 M HCl) at room temperature over several hours. The bone in all samples was relatively well preserved, with normal collagen content. After the complete dissolving of the inorganic part, the collagen was rinsed to pH 7 with ultra-pure distilled water. After the chemical treatment, the samples were dried in an oven at 60°C, then combusted to CO2 for 2 hours at 950°C in evacuated and sealed quartz tubes together with copper oxide and silver powder. In the presence of hydrogen, the purified carbon dioxide gas was reduced to filamentous graphite over a cobalt catalyst by using Vogel's method (28, 29). The resulting graphite–cobalt mixtures were pressed into copper discs to be used as targets in the ion source.
The 14C/12C and 13C/12C ratios of the samples were determined relative to the respective National Institute of Standards and Technology oxalic acid I standard values (30). Radiocarbon ages were calculated following the procedure described by Stuiver and Polach (31). The natural mass fractionation corrections of the samples were derived from the measured 13C/12C ratios and were normalized to δ13C = −25‰ relative to the reference standard value. The radiocarbon ages are reported in years before present (1950), and the errors are at the 1σ level (Table 2). The obtained δ13C values of about −20‰ are in accordance with expectations (Table 2).
Table 2.
Uncalibrated and calibrated AMS 14C ages for human remains from the Neander Valley
| Lab. no. (sample) | AMS-14C age, years before present | δ13C, ‰ | Calibrated age, years BC |
|---|---|---|---|
| ETH-19660 (NN 1) | 39,240 ± 670 | −20.0 ± 1.2 | 40,052 ± 409 |
| ETH-19661 (NN 4) | 40,360 ± 760 | −18.8 ± 1.2 | 40,734 ± 682 |
| ETH-20981 (Nean 1) | 39,900 ± 620 | −19.6 ± 1.1 | 40,394 ± 512 |
Calibration was accomplished by using the CalPal program by O. Jöris and B. Weninger, University of Cologne, Cologne, Germany.
All calibrated dates fall at ≈40,000 14C years before present and are not statistically different from each other (Table 2). Therefore, Neandertal 1 and the individual(s) represented by NN 1 and NN 4 could well have lived at the same time. The dates furthermore show that the Neandertal 1 specimen is comparable in geological age to other European Neandertals from oxygen isotope stage 3 (32) and demonstrate that both specimens are not from a time period where possible contact with early modern humans is indicated (33).
Genetic Analysis
To gauge macromolecular preservation of these fossils, a small fragment of a right tibial shaft (NN 4), which could possibly derive from Neandertal 1, and a fragment of a right humeral shaft (NN 1), which cannot represent Neandertal 1, were analyzed for amino acid composition and extent of amino acid racemization. From each bone, a fragment of 10 mg was removed and hydrolyzed under acid conditions. The amino acids were analyzed by high-performance liquid chromatography with o-phtaldialdehyde precolumn labeling as described (34). NN 4 showed a d/l ratio for aspartic acid of 0.21 and a d/l ratio for alanine of 0.123, indicating a high level of racemization. The ratio of glycine to aspartic acid was 1.9, an unusually low value that could indicate that most of the amino acids analyzed do not come from collagen proteins. Thus, DNA was not expected to be preserved in NN 4 (35). By contrast, NN 1 had a d/l ratio for aspartic acid and alanine of 0.077 and 0.023, respectively, whereas no d-leucine was detectable. The total amino acid content of the bone was high, and the ratio of glycine to aspartic acid was 6.9, indicating that the majority of the proteins could be collagen. Thus, the latter bone fulfills the criteria found to be compatible with retrieval of endogenous DNA from paleontological remains (34), whereas the former bone does not.
A sample of 0.5 g of bone was removed from NN 1, and DNA was extracted in a laboratory exclusively dedicated to ancient DNA work by using methods previously described (27). An amplification, using primers matching segments of the hypervariable region I of the mitochondrial control region that are highly conserved among humans, Neandertals, chimpanzees, and other great apes was performed twice independently. Both amplifications yielded products that were cloned, and multiple clones were sequenced. The possible amplification of nuclear insertion of mtDNA was excluded because primers specific to the Neandertal sequence do not yield a PCR product when used in amplifications from DNA extracted from 23 contemporary humans (27). Furthermore, different primer pairs produced identical DNA sequences in regions where they overlap, and it is highly unlikely that different primer pairs would all preferentially amplify one particular nuclear insertion of mtDNA rather than the organellar mtDNA (36).
Three different types of DNA sequences were seen (Fig. 4, which is published as supporting information on the PNAS web site). Two of these were identical to contemporary human DNA sequences, whereas the third, which was seen in both amplifications, carried two substitutions relative to the mtDNA reference sequence (37). These two substitutions are seen in the mtDNA sequences of the Neandertal 1 individual (27) as well as in a Neandertal individual from Vindija, Croatia (38). Six consecutive overlapping amplifications, each using one or two primers that preferentially amplify the DNA sequences determined from previous amplifications from NN 1, were used to determine a total of 357 bp of the hypervariable region I (Fig. 5, which is published as supporting information on the PNAS web site). Each fragment was cloned, and multiple clones from two or more independent amplifications were sequenced. When different nucleotides were observed at any position in two amplifications, one or more additional amplifications covering that position were performed. The DNA sequence found to be reproducible in all clones from at least two amplifications was deemed to be the DNA sequence endogenous to the bone. Although damage is frequent in DNA extracted from ancient remains, this procedure is expected to result in a maximal error rate of about 0.12% even under the unlikely scenario that each amplification starts from single DNA strands (39).
Because it has recently been shown that miscoding DNA lesions can be a frequent phenomenon in amplifications from some ancient DNA extracts (39), all Neandertal sequences previously determined by the Munich laboratory (27, 38, 40) have been reviewed. Two sets of apparently linked substitutions stand out as possible problems. These are positions 16107 and 16108, and 16111 and 16112, respectively, all of which carry C to T substitutions relative to the mtDNA reference sequence (37) in the Neandertal 1 sequence (27). These substitutions were observed together in all clones of two independent amplifications, whereas a third amplification had three clones with none of these substitutions. The six remaining clones had two of the substitutions (positions 16111 and 16112). According to the strategy used (27, 39), these four substitutions are reproducible and were therefore deemed to represent the endogenous sequence. However, there are several reasons why these positions should be regarded with caution. They are unusual in that they have been observed neither in Neandertal mtDNA sequences determined subsequent to that of Neandertal 1 nor in mtDNA sequences from contemporary humans. Furthermore, they are clustered in an unusual pattern and result in an apparent acceleration of the Neandertal 1 DNA sequence relative to the other Neandertal DNA sequences determined to date. Thus, three possibilities exist. The C to T substitutions could be the endogenous DNA sequence of Neandertal 1 and thus represent an unusual pattern of substitutions. Alternatively, because two of the substitutions occur in all three amplifications, they may represent a heteroplasmic state in Neandertal 1. A final possibility is that they may represent deaminated cytosin residues resulting in nucleotide misincorporation during PCR. Until more samples from Neandertal 1 become available for further analyses, this issue cannot be resolved. For the purposes of this analysis, we have excluded these substitutions. However, their inclusion would not affect any conclusions drawn.
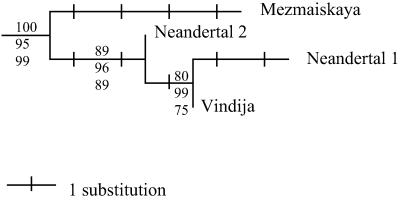
The new mtDNA sequence determined for NN 1 carries 23 differences from the contemporary human reference sequence (37) and 17 differences from the closest contemporary human DNA sequence present in GenBank. By contrast, it carries one to four nucleotide differences from the three Neandertal mtDNA sequences published to date, which stem from Neandertal 1 (27), Vindija in Croatia (38), and Mezmaiskaya cave in Russia (41). Neighbor-joining and maximum parsimony trees were constructed by using mega 2.0 (42). A quartet maximum-likelihood tree was constructed by using puzzle-tree (43). Tree reconstructions show that the NN 1 mtDNA sequence falls together with the three previously determined Neandertal DNA sequences to the exclusion of contemporary humans (Fig. 3). The mean pair-wise difference between these four Neandertal sequences is 1.7%. The mean pair-wise difference between four sequences of mtDNA drawn by chance from the databank was estimated from 10,000 replications as 1.8% (± 0.6, n = 9,309) for extant humans, 12.1% (+/−2.5, n = 28) for gorillas, and 9.6% (+/−2.7, n = 434) for chimpanzees. This observation may show that Neandertal mtDNA diversity in the Late Pleistocene was comparable to that of contemporary humans and lower than that of the great apes. Obviously considerably more than four Neandertal sequences will be necessary to test this hypothesis and establish the parameters of Neandertal mtDNA diversity.
Figure 3.
Neighbor-joining (NJ) tree rooted with 10 recent human sequences. The same topology was found by using different tree estimation methods. The numbers on each branch represent (Top to Bottom) the NJ bootstrap value, the puzzle-tree support value, and the maximum parsimony bootstrap value. The “Neandertal 2” sequence derives from specimen NN 1.
The new mtDNA sequence determined carries three differences from the DNA sequence determined from Neandertal 1 (27). Thus, it is clear that this bone stems from an individual different from and maternally unrelated to the Neandertal 1 individual. It is further noteworthy that it carries three differences from the type specimen sequence, whereas it carries only one difference from the Croatian Neandertal sequence. This observation may suggest that strong geographical clustering of mtDNA sequences is not present in Neandertals. Again, however, a much larger series of Neandertal mtDNA needs to be studied to arrive at any conclusions about the phylogeography of the Neandertal mtDNA gene pool.
Conclusion
The presence of Middle Paleolithic tools and Late Pleistocene-age fauna as well as a direct AMS date of ≈40,000 14C years establishes, to our knowledge for the first time, an appropriate context for Neandertal 1. Questions concerning the antiquity of the original Neandertal specimen led many to reject any role for it in human evolution and generally impeded late 19th century progress in the study of human evolution (8, 44). Had the fauna and artifacts been recovered in 1856, the early history of human paleontology certainly would have been altered. The new skeletal specimens belonging to the Neandertal 1 individual exhibit anatomical features consistent with other Neandertals and significantly enhance our anatomical knowledge of the Neandertal type specimen. All other specimens with diagnostic morphology also appear to derive from Neandertals. Assessment of the total sample indicates that at least one other adult and one subadult are represented. The presence of other individuals in the sample provides the opportunity to further investigate anatomical variation in the sample from which Neandertal 1 is derived.
The mtDNA sequence from a second Neandertal individual (NN 1), also directly dated to ≈40,000 14C years ago, makes the Neandertal site the first Pleistocene locality to yield sequences from more than one human. As more Neandertal genetic data are identified, it may therefore become possible to study not only the morphological but also the molecular genetic variation of the Neandertal population at a time before there is any credible evidence of early modern humans in Europe (33).
This integrated biological approach, including the direct dating, provides a more complete and reliable perspective on fossil material than any of these approaches carried out in isolation can deliver. Thus the collaboration of geneticists, morphologists, archaeologists, and dating specialists should be the norm in studies of late Pleistocene human remains.
Supplementary Material
Acknowledgments
R.W.S. dedicates this article to the memory of his father, who passed away during its preparation. We are indebted to H. Koschik (Rheinisches Amt für Bodendenkmalpflege, Bonn), F. G. Zehnder, and H.-E. Joachim (Rheinisches Landesmuseum Bonn) for general support and permission to take samples; and to the Rheinisch-Westfälische Kalkwerke (1997) and the Neanderthal Museum Foundation (2000) for excavation permission support. We thank N. Conard, H.-P. Uerpmann, S. Münzel, F. Dammann, E. Schwaderer, and A. Czarnetzki (Universität Tübingen), E. Trinkaus (Washington University), and M. Schultz (Universität Göttingen) for assistance and support; and we thank H. Jensen (Universität Tübingen) for photography. Also, we thank the staff of the Neanderthal Museum (Mettmann, Germany) for support. The Deutsche Stiftung Denkmalschutz, the Ministerium für Städtebau und Wohnen, Kultur und Sport des Landes Nordrhein-Westfalen, the Verlagshaus Gruner und Jahr (Hamburg), the Hillgruber family (Hamburg), Universität Tübingen, the Leakey Foundation, and H.-W. Burgartz (Jüchen) provided financial support (R.W.S.). The work of D.S. and S.P. is supported by the Max Planck Society and the Bundesministerium für Bildung und Forschung. The participation of F.H.S. is supported by the Alexander von Humboldt Stiftung. We are deeply grateful to all of these agencies and individuals.
Abbreviation
- AMS
accelerator mass spectrometry
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY149291).
References
- 1.Schmitz R W, Thissen J. Neandertal: Die Geschichte geht weiter. Heidelberg: Spektrum; 2000. [Google Scholar]
- 2.Bongard J H. Wanderung zur Neandershöhle: Eine topographische Skizze der Gegend von Erkrath an der Düssel. Düsseldorf, Germany: Arnz; 1835. [Google Scholar]
- 3. Fuhlrott, J. C. (1868) Verh. naturhist. Ver. preuss. Rheinl. 25, Corr. Bl., 62–70.
- 4.Fuhlrott J C. Verh naturhist Ver preuss Rheinl. 1859;16:131–153. [Google Scholar]
- 5. Fuhlrott, J. C. (1857) Verh. naturhist. Ver. preuss. Rheinl. 14, Corr. Bl., 50.
- 6. Schaaffhausen, H. (1857) Verh. naturhist. Ver. preuss. Rheinl.14, Corr. Bl., 50–52.
- 7.Schaaffhausen H. Der Neanderthaler Fund. Bonn, Germany: Marcus; 1888. [Google Scholar]
- 8.Trinkaus E, Shipman P. The Neandertals. New York: Knopf; 1992. [Google Scholar]
- 9.Schmitz R W, Thissen J. In: Neanderthals and Modern Humans—Discussing the Transition. Orschiedt J, Weniger G-C, editors. Mettmann, Germany: Neanderthal Museum; 2000. pp. 267–273. [Google Scholar]
- 10.Smith F H. The Neandertal Remains from Krapina: A Descriptive and Comparative Study. Univ. of Tennessee, Knoxville: Dept. Anthropol.; 1976. [Google Scholar]
- 11.Smith F H. Trans Philos Soc London Ser B. 1992;337:243–250. doi: 10.1098/rstb.1992.0102. [DOI] [PubMed] [Google Scholar]
- 12.Trinkaus E. The Shanidar Neandertals. New York: Academic; 1983. [Google Scholar]
- 13.Trinkaus E. J Hum Evol. 1987;16:429–443. doi: 10.1006/jhev.1997.0210. [DOI] [PubMed] [Google Scholar]
- 14.Wolpoff M H. Paleoanthropology. 2nd Ed. New York: McGraw–Hill; 1999. [Google Scholar]
- 15.Silipo P, Dazzi M, Feliciani M, Guglielmi G, Guidetti G, Martini S, Massani D, Mori S, Tanfani G. In: The Circeo 1 Neandertal Skull: Studies and Documentation. Piperno M, Scichilone M, editors. Rome: Instituto Poligrafico e Zecca Dello Stato; 1991. pp. 513–538. [Google Scholar]
- 16.Quam R, Smith F H. In: Neandertals and Modern Humans in Western Asia. Akazawa T, Aoki K, Bar-Yosef O, editors. New York: Plenum; 1998. pp. 405–421. [Google Scholar]
- 17.Frayer D W. Evolution of the Dentition in Upper Paleolithic and Mesolithic Europe. Lawrence, KS: Univ. Kansas Publ. Anthropol. 10; 1978. [Google Scholar]
- 18.Skinner M F, Sperber G H. Atlas of the Radiographs of Early Man. New York: Liss; 1992. [Google Scholar]
- 19.Frayer D W, Russell M D. Am J Phys Anthropol. 1987;74:393–405. doi: 10.1002/ajpa.1330740311. [DOI] [PubMed] [Google Scholar]
- 20.Bermúdez de Castro J-M. J Hum Evol. 1993;24:339–371. [Google Scholar]
- 21.Churchill S E, Smith F H. Am J Phys Anthropol. 2000;112:251–273. doi: 10.1002/(SICI)1096-8644(2000)112:2<251::AID-AJPA10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Hambücken A. C R Acad Sci Ser II. 1993;317:109–114. [Google Scholar]
- 23.Carretero J M, Arsuaga J L, Lorenzo C. J Hum Evol. 1997;33:357–408. doi: 10.1006/jhev.1997.0128. [DOI] [PubMed] [Google Scholar]
- 24.Churchill S E, Pearson O M, Grine F E, Trinkaus E, Holliday T W. J Hum Evol. 1996;31:213–237. doi: 10.1006/jhev.1998.0227. [DOI] [PubMed] [Google Scholar]
- 25.Holliday T. Am J Phys Anthropol. 1997;104:245–258. doi: 10.1002/(SICI)1096-8644(199710)104:2<245::AID-AJPA10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.White T D, Folkens P A. Human Osteology. 2nd Ed. San Diego: Academic; 2000. [Google Scholar]
- 27.Krings M, Stone A, Schmitz R W, Krainitzki H, Stoneking M, Pääbo S. Cell. 1997;90:19–30. doi: 10.1016/s0092-8674(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 28.Vogel J S, Southon J R, Nelson D E, Brown T A. Nucl Inst Meth Phys Res. 1984;B5:289–293. [Google Scholar]
- 29.Vogel J S, Southon J R, Nelson D E. Nucl Inst Meth Phys Res. 1987;B29:50–56. [Google Scholar]
- 30.Bonani G, Beer J, Hofmann H J, Synal H A, Suter M, Wölfli W, Pfleiderer Ch, Kromer B, Junghans C, Münnich K O. Nucl Inst Meth Phys Res. 1997;B29:87–90. [Google Scholar]
- 31.Stuiver M, Polach H A. Radiocarbon. 1977;19:355–363. [Google Scholar]
- 32.Mellars P. The Neanderthal Legacy. Princeton, NJ: Princeton Univ. Press; 1995. [Google Scholar]
- 33.Churchill S E, Smith F H. Yrbk Phys Anthropol. 2000;43:61–115. doi: 10.1002/1096-8644(2000)43:31+<61::aid-ajpa4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Poinar H N, Höss M, Bada J L, Pääbo S. Science. 1996;272:864–866. doi: 10.1126/science.272.5263.864. [DOI] [PubMed] [Google Scholar]
- 35.Krings M, Serre D, Paunović M, Pääbo S. In: The Vindija Neandertals: Catalog of the Skeletal Remains. Rabeder G, Paunović M, Grossschmidt K, editors. Vienna: Austrian Acad. Sci.; 2002. , in press. [Google Scholar]
- 36.Handt O, Krings M, Ward R H, Pääbo S. Am J Hum Genet. 1996;59:368–376. [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson S, Bankier A T, Barrell B G, de Bruijn M H, Coulson A R, Drouin J, Eperon I C, Nierlich D P, Roe B A, Sanger F, et al. Nature (London) 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 38.Krings M, Capelli C, Tschentscher F, Geisert H, Meyer S, von Haeseler A, Grossschmidt K, Possnert G, Paunović M, Pääbo S. Nat Genet. 2000;26:144–146. doi: 10.1038/79855. [DOI] [PubMed] [Google Scholar]
- 39.Hofreiter M, Jaenicke V, Serre D, von Haeseler A, Pääbo S. Nucleic Acids Res. 2001;29:4793–4799. doi: 10.1093/nar/29.23.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krings M, Geisert H, Schmitz R W, Kraininzki H, Pääbo S. Proc Natl Acad Sci USA. 1999;96:5581–5585. doi: 10.1073/pnas.96.10.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ovchinnikov I V, Götherström A, Romanova G P, Kharitonov V M, Lidén K, Goodwin W. Nature (London) 2000;404:490–493. doi: 10.1038/35006625. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis. University Park, PA: Penn. State Univ.; 1993. [Google Scholar]
- 43.Strimmer K, Von Haessler A. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 44.Spencer F. In: The Origin of Modern Humans. Smith F H, Spencer F, editors. New York: Liss; 1984. pp. 1–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.