Abstract
A rapidly fatal case of pulmonary tularemia in a 43-year-old man who was transferred to a tertiary care facility is presented. The microbiology laboratory and autopsy services were not notified of the clinical suspicion of tularemia by the service caring for the patient. Despite having a laboratory bioterrorism procedure in place and adhering to established laboratory protocol, 12 microbiology laboratory employees were exposed to Francisella tularensis and the identification of the organism was delayed due to lack of notification of the laboratory of the clinical suspicion of tularemia. A total of 11 microbiology employees and two persons involved in performing the patient's autopsy received prophylactic doxycycline due to concerns of transmission. None of them developed signs or symptoms of tularemia. One microbiology laboratory employee was pregnant and declined prophylactic antibiotics. As a result of this event, the microbiology laboratory has incorporated flow charts directly into the bench procedures for several highly infectious agents that may be agents of bioterrorism. This should permit more rapid recognition of an isolate for referral to a Level B laboratory for definitive identification and should improve laboratory safety.
Francisella tularensis, a fastidious gram-negative coccobacillus, is an uncommonly encountered organism in most clinical microbiology laboratories. Nevertheless, the need for diagnostic laboratories to be familiar with this organism has taken on increased importance due to its possible use as a bioterrorism agent (4, 7, 8, 10, 13). F. tularensis has been classified as a Category A critical biological agent because it can be disseminated easily, causes high mortality with the potential for major public health impact, might cause public panic and social disruption, and requires special action for public health preparedness (3). It was reportedly developed as a weapon by both the United States (10) and the Soviet Union (1).
Despite the presence in the clinical microbiology laboratory of a written procedure for working with agents of bioterrorism, including F. tularensis, the identification of F. tularensis isolated from a fatal case of pulmonary tularemia was delayed, resulting in the manipulation of the organism at the bench by laboratory workers, many of whom subsequently began taking prophylactic antibiotics. Although tularemia is rare, with approximately 200 cases annually in the United States, in Pike's study of 3,921 cases of laboratory-associated infections, it ranked second in the United States as a cause of laboratory-associated infections, behind only brucellosis, and third worldwide, behind brucellosis and typhoid (15).
We report a fatal case of culture-proven tularemia and the associated laboratory investigation prompted upon learning of the cause of the patient's demise. We provide guidelines that will assist other laboratories in suspecting and more safely dealing with infections caused by this organism.
Case report.
A 43-year-old man with no significant past medical history presented to Martha's Vineyard Hospital with a chief complaint of a sore chest for several days. He had not been feeling well for approximately 1 week, with pleuritic chest discomfort and a productive cough releasing rusty brown, thick sputum. Three days prior to presenting, he developed progressive shortness of breath and back pain. He subsequently sought care from a chiropractor, who noted an abnormality on an X-ray study and referred the patient to the hospital's emergency department for further evaluation.
The patient lived alone in a wooded area of Martha's Vineyard, Mass. He worked as a self-employed house painter and had recently worked cleaning roadside debris. He had no pets.
At Martha's Vineyard Hospital, his vital signs were a temperature of 97°F, a heart rate of 140 beats/min, a blood pressure of 70/50 mm of Hg, and a respiratory rate of 30 breaths/min. His oxygen saturation (evaluated by pulse oximetry) was 78% while breathing room air. A chest X ray was noted to be consistent with left lung whiteout. The patient received intravenous ceftriaxone. Serial arterial blood gases demonstrated the progression of metabolic acidosis.
Laboratory studies at Martha's Vineyard Hospital were otherwise notable for a white blood cell count of 6,900 cells/mm3, with 62% neutrophils and 33% band forms; a blood urea nitrogen level of 90 mg/dl; and a serum creatinine level of 7.1 mg/dl. The patient was intubated, given intravenous saline and pressors, and transferred via medevac to Boston Medical Center.
Upon arrival at Boston Medical Center, the patient's initial arterial blood gas was remarkable for a pH of 6.98, a pCO2 of 67, and a pO2 of 78 on assisted control ventilation while receiving 100% oxygen. Cultures of blood and sputum (for routine and Legionella cultures) were obtained. The patient continued on intravenous ceftriaxone and began on azithromycin, trimethoprim-sulfamethoxazole, and streptomycin.
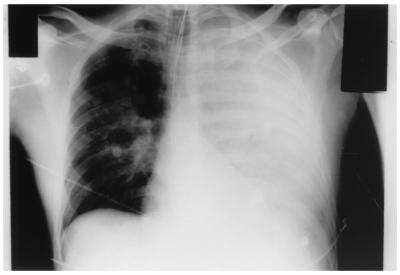
The patient was emergently dialyzed. He was found to have a coagulopathy and was persistently acidotic despite hemodialysis. He remained hypotensive despite aggressive intravenous fluid resuscitation and pressors. The chest X ray progressed and demonstrated a completely opacified left hemithorax (Fig. 1). The patient suffered a cardiac arrest the following morning and was pronounced dead at 10:45 a.m. An autopsy was performed.
FIG. 1.
Portable chest X ray of patient demonstrating complete whiteout of the left hemithorax and diffuse patchy opacities in the right lung field.
The autopsy service was not notified of the medical service's suspicion of tularemia. The autopsy service did, however, have access to the patient's medical record in which tularemia was noted as a possibility. As a result of the attending pathologist's concern for the possibility of tularemia, tissue sampling was performed rather than a full autopsy. At the autopsy, the findings included bilateral hemorrhagic necrotizing pneumonia with lobar consolidation. Microabscesses were found in the spleen and liver, and bilateral acute tubular necrosis of both kidneys was present.
Lung, liver, and spleen tissues were sent to the Centers for Disease Control and Prevention (CDC; Fort Collins, Colo.) for direct fluorescent-antibody assay (DFA) and cultures for F. tularensis. The anatomic pathology service was subsequently notified by CDC of a positive DFA for F. tularensis in lung and spleen tissue and notified the clinical microbiology laboratory of the positive DFA study. At CDC, F. tularensis was subsequently isolated from upper and lower lung tissue. These isolates were characterized as type A based on positive glycerol fermentation. Mouse inoculation studies with the isolate were lethal. PCR with F. tularensis-specific primers of both lung specimens, of liver tissue, and of spleen tissue were all positive.
Clinical microbiology laboratory investigation.
The notification of the positive DFA study prompted an investigation within the microbiology laboratory. Blood cultures had been placed on the blood culture instrument, and respiratory cultures from the patient as well as autopsy specimens of pleural fluid and spleen tissue had been processed in biological safety cabinets and cultured per standard laboratory protocol. The laboratory had not been alerted to the suspicion of F. tularensis. Multiple cultures from the patient were positive and were being worked up on open benches without any additional personal protective equipment for what had been thought to be most consistent with a Haemophilus species.
The aerobic bottles (ESP 80A; Trek Diagnostic Systems, Inc., Westlake, Ohio) in two sets of blood cultures were flagged for growth, which was detected after 10.8 h of incubation. Blood culture processing, including subculture of the broth and the preparation of smears for Gram's staining, was performed in the biological safety cabinet in accordance with the laboratory procedure for the initial workup of positive blood cultures. Personal protective equipment consisted of gloves and a fluid-impermeable gown. No organisms were seen on the Gram's stain of broth from these bottles. A subculture from these blood cultures yielded growth on chocolate agar, but not on sheep blood agar or MacConkey agar, of small colonies that looked similar to those of a Haemophilus species, and these colonies were being worked up on the open bench. The Gram’s stain demonstrated small gram-negative coccobacilli. Respiratory cultures were growing gram-negative coccobacilli on both chocolate and on buffered chocolate yeast extract agar (Legionella cultures) that were morphologically identical to those isolated from the blood. Autopsy specimens of pleural fluid yielded growth of morphologically identical gram-negative coccobacilli.
An emergency laboratory meeting was called for all microbiology employees, and the laboratory procedure for dealing with suspected agents of bioterrorism was reviewed with the staff. All cultures from the patient were placed in shrink seal and labeled as biohazardous, to be worked with only in a biological safety cabinet in one of our two negative-pressure rooms. The hospital employee health service was notified of the exposure of employees to F. tularensis, and arrangements were made to have laboratory workers evaluated. An assessment of the level of exposure of the technologists to the positive cultures was made, and those technologists with any exposure to the cultures were seen by a physician at the employee health service. Since some of the exposure occurred over the weekend, weekend employees were contacted by telephone.
Exposures of laboratory personnel occurred while employees were subculturing positive broth blood cultures to agar plates in a biological safety cabinet; performing Gram's staining of the blood culture broth; examining agar plates containing colonies of F. tularensis, including examinations with a hand lens close to the face; performing Gram's staining of the colonies; and making suspensions of F. tularensis for X- and V-factor assays.
A total of 11 employees in the clinical microbiology laboratory received prophylaxis with doxycycline, 100 mg orally twice daily, and were placed on a fever watch. One technologist was pregnant and was placed on a fever watch only. None of the employees developed a clinical illness compatible with tularemia.
Autopsy service investigation.
During the autopsy, double gloving was employed and there were no accidental cuts that occurred. Transmission of tularemia by cutaneous inoculation has been documented during autopsy (19). A face shield was not used by the prosecting resident pathologist or the diener. Both the resident and the diener received prophylactic doxycycline.
Additional clinical microbiology issues.
The Massachusetts State Public Health Laboratory was contacted about the suspected tularemia isolates from blood, sputum, and pleural fluid, and arrangements were made to ship the clinical isolate from the blood to the Massachusetts State Public Health Laboratory, Jamaica Plain, Mass. The presence of an ongoing outbreak of tularemia on Martha's Vineyard during the summer of 2000 (9) was known to the public health authorities at that time. As Martha's Vineyard, Mass., is known to be a place where tularemia is endemic and the cases occurred over the course of many weeks, bioterrorism was not suspected in this case.
Upon notification from the Massachusetts State Public Health Laboratory that the clinical isolate had been confirmed as F. tularensis, all clinical isolates from the patient were autoclaved. This was done to ensure that no stock culture of this organism remained in the laboratory.
Discussion.
Although the medical service caring for this patient was concerned enough about the possibility of tularemia to give him intramuscular streptomycin, the microbiology laboratory and the autopsy service were not informed of this clinical suspicion. As a result, there was both a delay in sending the clinical isolate for definitive identification and an increased risk to the microbiology staff. Although a specific bioterrorism procedure was in place in the microbiology laboratory, it was separate from, and had not been sufficiently integrated into, the specific bench procedures for the workup of blood, respiratory, and sterile body fluid cultures. As a result, technologists working with the isolate on these benches did not suspect F. tularensis. It has been the standard procedure in our microbiology laboratory to subculture all positive blood cultures within a biological safety cabinet. This procedure, which involves a broth culture, is one that can potentially result in the production of an infectious aerosol.
The autopsy service was not notified by the medical service of the suspicion of tularemia either. The service did, however, have access to the patient's medical record in which tularemia was listed as a considered possibility. As a result of the attending pathologist's suspicion of tularemia, tissue sampling was performed rather than a full autopsy and specimens were sent to CDC. Autopsy specimens sent to the hospital clinical microbiology laboratory, however, were not accompanied by requests culture for F. tularensis. Communication between anatomic pathology and clinical microbiology has been incorporated into the laboratory procedures in the setting of suspected infectious agents that could present a particular hazard to workers in either the microbiology laboratory or the autopsy service.
The clinical microbiology laboratory at Boston Medical Center is currently designated a Level A laboratory. This classification means that the laboratory should not attempt the identification of potential bioterrorism agents such as F. tularensis, but it does require the ability to rapidly rule out such agents and to forward those isolates which cannot be ruled out to a Level B laboratory (12, 13). Although relevant information on these organisms has been published to assist clinical microbiology laboratories in this regard (13), flow charts that simplify the process for microbiologists have only recently been prepared by the American Society for Microbiology, the CDC, and the Association of Public Health Laboratories and are now available at the American Society for Microbiology website.
The misidentification or preliminary identification of F. tularensis as a Haemophilus species has been noted in a number of published reports (2, 11, 18). F. tularensis is characteristically isolated as small, poorly staining gram-negative rods seen mostly as single cells which yield mostly pinpoint colonies on chocolate agar and often on sheep agar at 48 h, do not grow on either MacConkey or eosin-methylene blue agar, are oxidase negative, and have a weakly positive or a negative catalase test. Perhaps most notably, the satellite test is negative with F. tularensis, a test that we now include in a flow chart in our modified procedure. When an organism has these properties, further manipulation is performed within the biological safety cabinet by a technologist wearing gloves and a gown. Similarly, isolates that grow only on buffered chocolate yeast extract and chocolate agar should be manipulated in a biological safety cabinet. The bacteriology bench procedures now include flow charts for this organism as well as for Brucella species, another potentially hazardous agent that has been made into a weapon and is a possible bioterrorism agent. When a small gram-negative coccobacillus is seen on a Gram's stain of a positive blood culture in which the time to detection is greater than 24 h, all further work should be performed in a biological safety cabinet by a technologist wearing gloves and a gown until the isolate is shown not to be a Brucella species.
Although Yersinia pestis and Bacillus anthracis, two agents that have been classified as a Category A critical biological agents, have only rarely been reported to cause laboratory infections, we have incorporated flow charts for the identification of these organisms into our procedures in order to prevent a delay in their identification. In the clinical virology laboratory, we have incorporated a flow chart for those situations in which cytopathic effect is seen and which is consistently demonstrated upon passage but cannot be identified with our standard laboratory procedures. This is of importance as there are a number of viral agents that cannot be diagnosed with routinely available procedures that are potentially very hazardous and will grow in tissue culture cells that are commonly used in clinical virology laboratories.
Other pitfalls in the identification of F. tularensis include the presence of nonfastidious strains that do not require cysteine (2), strains that grow well on sheep blood agar and Trypticase soy agar (5), strains for which kit identifications may incorrectly suggest an identification of Actinobacillus actinomycetemcomitans (5) or Neisseria meningitidis (5), and the fact that standard textbook descriptions of the identification of the organism rely on tests that are not routinely available in hospital laboratories (21).
This patient received azithromycin for the possibility that his pneumonia was due to infection with a Legionella species. Clinically, it is possible to confuse pulmonary tularemia with legionellosis. Similar difficulties may be encountered in the laboratory. F. tularensis and Legionella pneumophila may both grow on buffered charcoal yeast extract agar, which contains enough cysteine to support the growth of F. tularensis (20). In addition, a false-positive DFA for Legionella has been reported in tularemia pneumonia and both illnesses may respond to erythromycin (16).
It was not known if any of the laboratory workers had aerosol or percutaneous exposure that would result in infection, but given that the median infective dose for both aerosol and percutaneous challenge is on the order of 10 organisms for F. tularensis, this was regarded as a distinct possibility. A number of the activities were regarded as potentially risky, especially making and plating suspensions of bacteria for X- and V-factor testing, which was performed outside biological safety cabinets and could have resulted in the generation of infectious aerosols. There are limited data on the efficacy of prophylaxis against tularemia with tetracyclines. One published study with volunteers who were exposed to an airborne challenge of F. tularensis demonstrated the failure of prophylaxis with 1 g of tetracycline daily (given 24 h after airborne challenge) for 15 days and of 2 g of tetracycline given for 10 days. In this study, 2 g of tetracycline given for 15 days was shown to be effective prophylaxis (17). As tetracycline is normally given four times per day, the decision was made to use 100 mg of doxycycline orally twice per day for 14 days, as the compliance rate for doxycycline is likely to exceed that for tetracycline.
Infectious agents that have been transmitted during postmortem examinations include Blastomyces dermatitidis, F. tularensis, Toxoplasma gondii, Streptococcus pyogenes, Salmonella species, and Mycobacterium tuberculosis (6). Transmission in the autopsy suite can occur via the percutaneous, mucocutaneous, and inhalational routes. The role of performing autopsies in the possible detection of cases of bioterrorism is an important one (14). Under ideal circumstances, autopsies in cases of suspected bioterrorism should be performed in a specially designated morgue rather than in a routine hospital-based setting to minimize the risk of transmission of exotic agents, such as those causing viral hemorrhagic fevers. Clear lines of communication between the clinicians and the staff performing the autopsy are essential.
Despite having a laboratory bioterrorism procedure in place, which had been discussed at laboratory meetings, and adhering to established procedures, 12 microbiology employees were exposed to F. tularensis and the identification of the organism was delayed due to the lack of notification of the lab of suspected tularemia. Since this occurred, we have incorporated flow charts for several highly infectious agents that may be agents of bioterrorism directly into each of the bench procedures. This should permit more-rapid recognition of the need to refer a clinical isolate to a Level B laboratory for definitive identification. Additional steps that have been taken include the education of physicians on bioterrorism and the development of a comprehensive institutional plan on bioterrorism. Education of the healthcare providers on the need to alert the laboratory when these agents are suspected is important, but notification will be inconsistent. The laboratory, including the autopsy suite, must have procedures in place to handle unexpected infectious agents.
Acknowledgments
We thank John Kasznica for his review of the autopsy results.
REFERENCES
- 1.Alibek, K., and S. Handelman. 1999. Biohazard: the chilling true story of the largest covert biological weapons program in the world, told from the inside by the man who ran it, 1st ed. Random House, New York, N.Y.
- 2.Bernard, K., S. Tessier, J. Winstanley, D. Chang, and A. Borczyk. 1994. Early recognition of atypical Francisella tularensis strains lacking a cysteine requirement. J. Clin. Microbiol. 32:551-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Biological and chemical terrorism: strategic plan for preparedness and response. Recommendations of the CDC Strategic Planning Workgroup. Morb. Mortal. Wkly. Rep. 49(RR-4):1-14. [PubMed] [Google Scholar]
- 4.Christopher, G. W., T. J. Cieslak, J. A. Pavlin, and E. M. Eitzen, Jr. 1997. Biological warfare. A historical perspective. JAMA 278:412-417. [PubMed] [Google Scholar]
- 5.Clarridge, J. E., III, T. J. Raich, A. Sjosted, G. Sandstrom, R. O. Darouiche, R. M. Shawar, P. R. Georghiou, C. Osting, and L. Vo. 1996. Characterization of two unusual clinically significant Francisella strains. J. Clin. Microbiol. 34:1995-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, C. H., and D. A. Kennedy. 1999. The postmortem room, p. 264-266. In Laboratory-acquired infections: history, incidence, causes and prevention, 4th ed. Butterworth-Heinemann, Oxford, England.
- 7.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 8.Evans, M. E., and A. M. Friedlander. 1997. Tularemia, p. 503-512. In F. R. Sidell, E. T. Takafuji, and D. R. Franz (ed.), Medical aspects of chemical and biological warfare. Textbook of military medicine. Part I: warfare, weaponry, and the casualty, vol. 3. Office of the Surgeon General, U.S. Army, Washington, D.C. [Google Scholar]
- 9.Feldman, K. A., R. E. Enscore, S. L. Lathrop, B. T. Matyas, M. McGuill, M. E. Schriefer, D. Stiles-Enos, D. T. Dennis, L. R. Petersen, and E. B. Hayes. 2001. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N. Engl. J. Med. 345:1601-1606. [DOI] [PubMed] [Google Scholar]
- 10.Franz, D. R., P. B. Jahrling, A. M. Friedlander, D. J. McClain, D. L. Hoover, W. R. Bryne, J. A. Pavlin, G. W. Christopher, and E. M. Eitzen, Jr. 1997. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 278:399-411. [DOI] [PubMed] [Google Scholar]
- 11.Fredricks, D. N., and J. S. Remington. 1996. Tularemia presenting as community-acquired pneumonia. Implications in the era of managed care. Arch. Intern. Med. 156:2137-2140. [PubMed] [Google Scholar]
- 12.Gilchrist, M. J. 2000. A national laboratory network for bioterrorism: evolution from a prototype network of laboratories performing routine surveillance. Mil. Med. 165(Suppl. 2):28-31. [PubMed] [Google Scholar]
- 13.Gilchrist, M. J. R., W. P. McKinney, J. M. Miller, and A. S. Weissfeld. 2000. Cumitech 33, Laboratory safety, management, and diagnosis of biological agents associated with bioterrorism. Coordinating ed., J. W. Snyder. ASM Press, Washington, D.C.
- 14.Nolte, K. B., S. S. Yoon, and C. Pertowski. 2000. Medical examiners, coroners, and bioterrorism. Emerg. Infect. Dis. 6:559-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pike, R. M. 1976. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab. Sci. 13:105-114. [PubMed] [Google Scholar]
- 16.Roy, T. M., D. Fleming, and W. H. Anderson. 1989. Tularemic pneumonia mimicking Legionnaires' disease with false-positive direct fluorescent antibody stains for Legionella. South. Med. J. 82:1429-1431. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer, W. D., H. G. Dangerfield, A. L. Hogge, and D. Crozier. 1966. Antibiotic prophylaxis and therapy of airborne tularemia. Bacteriol. Rev. 30:542-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro, D. S., and E. J. Mark. 2000. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 14-2000. A 60-year-old farm worker with bilateral pneumonia. N. Engl. J. Med. 342:1430-1438. [DOI] [PubMed] [Google Scholar]
- 19.Weilbacher, J. O., and E. S. Moss. 1938. Tularemia following injury while performing post-mortem examination of a human case. J. Lab. Clin. Med. 24:34-38. [Google Scholar]
- 20.Westerman, E. L., and J. McDonald. 1983. Tularemia pneumonia mimicking legionnaires' disease: isolation of organism on CYE agar and successful treatment with erythromycin. South. Med. J. 76:1169-1170. [PubMed] [Google Scholar]
- 21.Wong, J., and D. S. Shapiro. 1999. Francisella, p. 647-651. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.