The microsporidia belong to the phylum Microspora within the taxonomic group Protozoa; there are more than 140 genera and 1,200 species that are parasitic in all major animal groups. Already, seven genera (Enterocytozoon, Encephalitozoon, Nosema, Pleistophora, Vittaforma, Trachipleistophora, and Brachiola) and some that are unclassified have been confirmed to cause human infection. Septata intestinalis, an organism that was identified in 1994 as belonging to a new genus, i.e., Septata (Encephalitozoon-like), has been reclassified as Encephalitozoon intestinalis, although there is not universal agreement on this generic change. The genus Microsporidium is essentially a catch-all genus for organisms that have not yet been classified (or may never be classified due to a lack of specimen). Depending on acceptance of these generic designations, there may be seven or nine microsporidial genera.
The microsporidia are obligate intracellular, spore-forming protists with no active metabolic stages outside of the host cell. The life cycle involves a proliferative merogonic stage followed by sporogony, which results in spores containing a tubular extrusion apparatus (polar tubule) for injecting infective spore contents into the host cell (Fig. 1).
FIG. 1.
Life cycle of microsporidia. Reprinted from reference 9.
Because microsporidia have a membrane-bound nucleus, an intracytoplasmic membrane system, and chromosome separation on mitotic spindles, they are considered to be true eukaryotes. However, they are somewhat unusual in that they have 70S ribosomes, have no mitrochondria or peroxisomes, and have simple Golgi membranes. Also, the microsporidial genome is smaller and less complex than those of other eukaryotes. Several characteristics, including the presence of chitin in the spore wall, suggest a potential link to the fungi.
Microsporidiosis, a disease resulting from infection with these important emerging opportunistic organisms, has been noted in human immunodeficiency virus (HIV)-infected patients, as well as in other immunocompromised patients, including transplant recipients. The first human case was reported in 1959; since then, cases of microsporidiosis in both those who are immunocompromised and those who have no underlying immune problems and are considered to be immunocompetent have been reported (Table 1) (2, 7, 21).
TABLE 1.
Microsporidia causing human infection
| Organism(s) | Clinical presentation
|
Commentsa | |
|---|---|---|---|
| Immunocompromised host | Immunocompetent host | ||
| Common | |||
| Enterocytozoon bieneusi | Chronic diarrhea, wasting, cholangitis, acalculous cholecystitis, chronic sinusitis, chronic cough, and pneumonia; isolated from 5 to 30% of HIV-positive patients with very low CD4 lymphocyte counts (one of the most important HIV-associated intestinal pathogens) and organ (liver, heart-lung, and bone marrow) transplant recipients | Self-limiting diarrhea and traveler's diarrhea; asymptomatic carriers | Short term culture only; three strains identified but not named; clinical disease based on continuous excess loss of epithelial cells, crypt hyperplasia, partial villus atrophy, decreased brush border sucrase, lactase, and maltase activities, or malabsorption; nonhuman hosts include pigs and primates |
| Encephalitozoon hellem | Disseminated infection; keratoconjunctivitis, sinusitis; bronchitis, pneumonia, nephritis, ureteritis, cystitis, prostatitis, and urethritis; nasal epithelium confirmed as infected in one AIDS patient; isolated in cases of asymptomatic respiratory system infection | Not identified or described | Grown in culture; may be acquired by inhalation; cerebral lesions similar to those seen in cases of toxoplasmosis; nonhuman hosts include psittacine birds |
| Encephalitozoon intestinalis (Septata intestinalis) | Chronic diarrhea; cholangiopathy; sinusitis, bronchitis, pneumonitis, nephritis; bone infection; nodular cutaneous lesions; often associated with AIDS patients; tends to disseminate; kidney is prominent site | Self-limiting diarrhea; asymptomatic carriers | Grown in culture; can produce multiorgan failure resembling hyperinfection syndrome (Strongyloides stercoralis); nonhuman hosts include dogs, donkeys, pigs, cows, and goats |
| Encephalitozoon cuniculi | Disseminated infection; keratoconjunctivitis; sinusitis, bronchitis, pneumonia; nephritis; hepatitis; peritonitis; symptomatic and asymptomatic intestinal infection; encephalitis; asymptomatic respiratory system infections | Not identified or described; two HIV-seronegative children with seizure disorder probably were immunocompromised | Grown in culture; nonhuman hosts include rabbits (strain I) (identified in humans in Switzerland), mice, blue foxes (strain II) (not identified in humans); domestic dogs (strain III) (identified in humans in the United States and Spain) |
| Uncommon | |||
| Brachiola algerae | Not identified or described | Keratitis | Formerly known as Nosema algerae |
| Brachiola connori | Disseminated infection | Not identified or described | Formerly known as Nosema connori; Nosema spp. often infect insects; disseminated in infant with SCID |
| Brachiola vesicularum | Myositis; also found in corneal stroma | Not identified or described | Previously called a Nosema-like species; nonhuman hosts not identified |
| Microsporidium africanum | Not identified or described | Corneal ulcer, keratitis (corneal stroma) | Catch-all genus for organisms that could not be identified; HIV-seronegative individual at autopsy; nonhuman hosts not identified |
| Microsporidium ceylonensis | Not identified or described | Corneal ulcer, keratitis (corneal stroma) | Catch-all genus for organisms that could not be identified; HIV-seronegative individual at autopsy; nonhuman hosts not identified |
| Nosema ocularum | Disseminated in athymic child | Corneal ulcer, keratitis (corneal stroma) | HIV-seronegative individual (child); nonhuman hosts not identified |
| Pleistophora sp. | Myositis (skeletal muscle) | Not identified or described | HIV-seronegative and HIV-seropositive individual, Pleistophora spp. tend to infect fish, insects, amphibians, and reptiles |
| Trachipleistophora anthropophthera | Disseminated infection in AIDS patient (brain, heart, kidney) | Not identified or described | Appears to be dimorphic: two different spore forms have been seen; ring-enhancing lesions suggestive of CNS toxoplasmosis; nonhuman hosts not identified |
| Trachipleistophora hominis | Myositis (skeletal muscle), keratoconjunctivitis; sinusitis | Not identified or described | AIDS patient; nonhuman hosts not identified |
| Vittaforma corneae | Disseminated infection | Keratitis (corneal stroma); iritis | Formerly known as Nosema corneum |
CNS, central nervous system. SCID, severe combined immunodeficiency disease.
SPECIMEN COLLECTION
It is important to remember that microsporidial spores are quite resistant to environmental conditions and can remain infectious for several years, particularly if they are protected from desiccation. Stool or duodenal drainage specimens can be submitted fresh or preserved in 5 or 10% formalin, or sodium acetate-acetic acid-formalin. Biopsy specimens are also acceptable. In cases of disseminated infection, it is recommended that urine (fresh or preserved) be submitted for analysis; other body fluids (sputum, bronchoalveolar lavage [BAL] fluid, nasal secretion, or cerebrospinal fluid [CSF]), conjunctival smears, corneal scrapings, or tissue can also be submitted (Table 2). Formalin fixation is recommended for routine histology, while gluteraldehyde is preferred for electron microscopy. Fresh specimens should be collected for cell culture and for molecular studies.
TABLE 2.
Body sites where microsporidia infecting humans have been detecteda
| Body site or specimen type | Report of detection of:
|
||||
|---|---|---|---|---|---|
| Enterocytozoon bieneusi | Encephalitozoon (Septata) intestinalls | Encephalitozoon hellem | Encephalitozoon cuniculi | Other speciesb | |
| Body fluids | |||||
| Stool | ++ | ++ | + | + | − |
| Duodenal aspirate | ++ | + | − | − | − |
| Bile | + | + | − | − | − |
| Urine | − | + | ++ | ++ | V. corneae |
| Sputum, BAL fluid | + | + | ++ | ++ | − |
| Nasal secretion or washings | + | + | + | + | T. hominis |
| CSF | − | − | − | + | − |
| Mucosal smears | |||||
| Conjunctival | − | + | + | + | − |
| Corneal | − | − | + | + | V. corneae |
| N. ocularum | |||||
| Tissue | |||||
| Intestinal tract | |||||
| Duodenum | ++ | ++ | − | + | B. connori |
| Jejunum | ++ | ++ | − | + | B. connori |
| Terminal ileum | ++ | ++ | − | + | B. connori |
| Colon | + | + | − | − | B. connori |
| Gallbladder | + | + | − | − | − |
| Biliary tract | + | + | − | − | − |
| Liver | + | − | − | − | T. anthropophthera |
| B. connori | |||||
| Pancreas | + | − | − | − | T. anthropophthera |
| Pancreatic duct | + | − | − | − | T. anthropophthera |
| Peritoneum | − | − | − | + | − |
| Respiratory system | |||||
| Bronchial epithelium | + | + | + | + | B. connori |
| Sinus | + | + | + | + | B. connori |
| Nasal epithelium | + | + | + | + | B. connori |
| Eye | |||||
| Cornea | − | + | + | + | V. corneae |
| N. ocularum | |||||
| M. ceylonensis | |||||
| M. africanum | |||||
| T. hominis | |||||
| Conjunctiva | − | + | + | + | V. corneae |
| N. ocularum | |||||
| M. ceylonensis | |||||
| M. africanum | |||||
| T. hominis | |||||
| Kidney | − | + | + | + | T. anthropophthera |
| Urinary system | B. connori | ||||
| Ureter | − | − | + | + | B. connori |
| Bladder | − | − | + | + | B. connori |
| Prostate | − | − | + | + | B. connori |
| Urethra | − | − | + | + | B. connori |
| Brain | − | +c | − | + | T. anthropophthera |
| Muscle | − | − | − | + | Pleistophora sp. |
| T. hominis | |||||
| B. vesicularumd | |||||
| Circulatory system | |||||
| Heart | − | − | − | + | T. anthropophthera |
| B. connori | |||||
| Bone marrow | − | − | − | − | T. anthropophthera |
| Immune system | |||||
| Lymph nodes | − | − | − | − | T. anthropophthera |
| Spleen | − | − | − | − | T. anthropophthera |
| Bone | − | + | − | − | T. anthropophthera |
| Endocrine system | |||||
| Thyroid | − | − | − | − | T. anthropophthera |
| Parathyroid | − | − | − | − | T. anthropophthera |
| Adrenal glands | − | − | − | + | B. connori |
Data are from references 7 and 21 to 23. Symbols: −, not reported; +, case report(s) of microsporidia detection; ++, reliable specimens for detection of microsporidia.
If detection of another species has been reported, the species name is given. The species listed may include one or more of the following: Vittaforma corneae, Trachipleistophora hominis, Nosema ocularum, Brachiola connori, Trachipleistophora anthropophthera, Microsporidium ceylonensis, Microsporidium africanum, and Brachiola vesicularum.
Detected in pituitary gland.
Formerly known as Nosema-like microsporidian.
LABORATORY METHODS
There are a number of methods available for the recovery of microsporidial spores and confirmation of microsporidiosis (Table 3). Most of these methods were developed in order to diagnose infections in the immunocompromised population. Microsporidial infections in the immunocompetent patient may result in self-cure after mild symptoms appear, a situation similar to that seen with cryptosporidiosis and isosporiasis. As awareness of microsporidiosis increases and more-sensitive techniques become available, we may find that these infections are not uncommon in the immunocompetent host. The recommendations for the use of current, less-sensitive methodology suggest that multiple diagnostic methods may be required to diagnose a microsporidial infection, particularly when fecal specimens are examined. Since microsporidial infections often involve multiple body sites, detection of organisms from any clinical specimen should be followed by examination of other body tissues and fluids. In patients for whom disseminated microsporidiosis is suspected, urine specimens should always be examined.
TABLE 3.
Microsporidia: Recommended diagnostic techniquesa
| Technique | Use | Comments |
|---|---|---|
| Light microscopy | ||
| Stool specimens | ||
| Modified trichromeb | ++ | Reliable, available; light infections difficult to identify |
| Giemsa | − | Not recommended for routine use, hard to read |
| Chemofluorescence | ++ | Calcofluor, Fungifluor, Unitex 2B; sensitive but nonspecific |
| Immunofluorescence | (++) | Commercial availability limits use; products in development |
| Other bodily fluids | ||
| Modified trichrome | ++ | Reliable, available; light infections difficult to identify |
| Giemsa | + | Urine, conjunctival swab, BAL, CSF, duodenal aspirate |
| Chemofluorescence | ++ | Calcofluor, Fungifluor, Unitex 2B; sensitive but nonspecific |
| Immunofluorescence | (++) | Commercial availability limits use; products in development |
| Cytology testingc | ||
| Modified trichrome | ++ | Reliable, available; light infections difficult to identify |
| Giemsa | + | Urine, conjunctival swab, BAL, CSF, duodenal aspirate |
| Gram | + | Recommended, especially for specimens with little debris |
| Chemofluorescence | ++ | Calcofluor, Fungifluor, Unitex 2B; sensitive but nonspecific |
| Immunofluorescence | (++) | Commercial availability limits use; products in development |
| Routine histology | ||
| Hematoxylin & eosin | + | Sensitivity uncertain with low parasite numbers |
| PAS | + | Controversy over effectiveness; PAS-positive granule |
| Modified Gram stains (Brown Brenn, Brown-Hopps) | ++ | Sensitive, generally recommended |
| Giemsa | + | Sensitivity uncertain with low parasite numbers |
| Warthin-Starry | + | Not standardized, may not be necessary, but often used |
| Modified trichrome | ++ | Reliable, sensitive |
| Immunofluorescence | (++) | Commercial availability limits use; products in development; have been used in research setting for conformation of microsporidia to species level |
| Plastic-embedded sections | ||
| Toluidine blue | ++ | Recommended; sensitive method |
| Methylene blue-azure II-basic fuchsin | ++ | Recommended as alternative to toluidine blue |
| Electron microscopy | ||
| Body fluids | + | Specific, sensitivity unknown; used for identification to species level (some exceptions) |
| Tissue sections | ++ | Gold standard for confirmation, but sensitivity lower than for detection of spores in stool or urine; used for identification to species level (some exceptions) |
| Molecular testing | − | Availability limited to research laboratories; studies ongoing and appear promising; molecular identification to the species level possible |
| Serologic testing (serum) | − | Reagents not commercially available; preliminary results controversial; have been reported for Encephalitozoon; not available for Enterocytozoon; not relevant for immunocompromised patients |
| Culture | − | Generally used in the research setting; continued advances in culture and organism survival and growth; Encephalitozoon, Nosema, Trachipleistophora, Vittaforma can be isolated; delivery to specialty lab within 2 to 3 days recommended; use Universal Precautions when handling specimens |
Abbreviations: BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid. Symbols: −, not available or not recommended for routine use; +, reported; ++, techniques in general use (most widely used); (++), excellent results, but reagents not yet commercially available.
Various stain modifications have been developed: Chromotrope (original modified trichrome stain [Weber green]) trichrome blue (modification of original counterstain from fast green to aniline blue, different concentration of photphotungstic acid [Ryan blue]); modified chromotrope stain (staining temperature increased to 50°C, staining time reduced to 10 min [Kokoskin]); modified chromotrope stain (staining temperature increased to 37°C, staining time 30 min [Didier]); quick-hot Gram-chromotrope stain [Moura]); acid-fast trichrome stain (can detect coccidia and microsporidia [Ignatius]) (5, 7, 10, 14, 16, 19, 20, 22).
Requires high-speed centrifugation.
LIGHT MICROSCOPY
Modified trichrome stains.
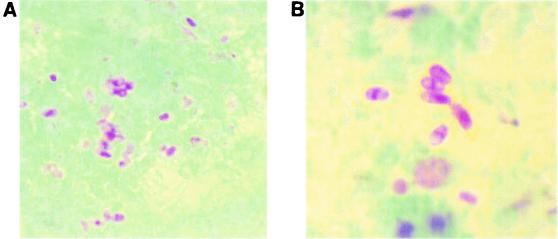
Smears are prepared using 10 to 20 μl of concentrated specimen (stool specimen or urine or other fluid) that is thinly spread onto the slides. Some laboratories use concave well slides; these are very helpful when examining the stained preparations. It is important to remember to centrifuge the specimen for 10 min at 500 × g prior to smear preparation. There is also some evidence to indicate that pretreatment of fecal specimens (1:1) with 10% KOH may provide a better-quality smear when modified trichrome stains are used. Specimens can be fresh or preserved (with 5 or 10% formalin or with sodium acetate-acetic acid-formalin or by using one of the newer single-vial systems). The modified trichrome stain methods are based on the fact that stain penetration of the microsporidial spore is very difficult; thus, the dye content in the chromotrope 2R component of the formula is greater than that used to prepare Wheatley's modification of the Gomori's trichrome stain, and the staining time is much longer (90 min) (11, 12, 19, 20). Several of these stains (e.g., Weber-Green modified trichrome stain [Fig. 2 ] and Ryan-Blue modified trichrome stain [Fig. 3 ]) are available commercially from a number of suppliers. The use of positive control material is highly recommended. Spore detection requires adequate illumination and magnification (oil immersion; total magnification, ×1,000).
FIG. 2.
Weber-Green modified trichrome staining of microsporidial spores. (A) Spores in stool specimen; (B) higher magnification of the image shown in panel A.
FIG. 3.
Ryan-Blue modified trichrome staining of microsporidial spores. (A) Spores in stool specimen; (B) spores in intestinal tract tissue.
The spore wall should stain pinkish to red, with the interior of the spore being clear or perhaps showing a horizontal or diagonal stripe, which represents the polar tube. The background will appear green or blue, depending on the method. Other bacteria, some yeast cells, and some debris will also be evident (stained shades of pink and red); the shapes and sizes of the artifacts may be helpful in differentiating the spores from other structures. Results should be reported only if the positive control smears are acceptable.
The choice of counterstain, with either fast green or aniline blue dyes in the stain formulation, depends on laboratory preference and does not change the color results of the actual microsporidial spores, which stain pink. In addition to the counterstain (green or blue), several modifications of the original chromotrope staining solution have been proposed, including changes in temperature of the staining solution and staining time. Results indicate that staining at 50°C for 10 min or staining at 37°C for 30 min may improve the detection of spores; the background appears to contain less debris, and the spores may stain more intensely (5, 7, 13, 22).
An acid-fast trichrome method stains both acid-fast cryptosporidial oocysts and microsporidial spores in the same smear (Fig. 4) (10). Also, a rapid-hot Gram-chromotrope staining method with a staining time of 5 min is available; the microsporidial spores stain dark violet against a pale-green background (16).
FIG. 4.

Single smear stained by an acid-fast trichrome stain method showing both an Isospora belli oocyst (modified acid-fast positive stain) and microsporidial spores (modified trichrome stain).
Giemsa stain.
Although Giemsa staining of stool can be performed and results in a light-blue staining of microsporidial spores, the spores can be very difficult to differentiate from debris in the smear. However, body fluid cytology preparations or intestinal biopsy specimens containing less debris and artifact material can be stained with Giemsa stain; identification of spores will be much easier with these types of specimens than with preparations from stool (Fig. 5).
FIG. 5.
Giemsa staining of microsporidial spores in intestinal tract cells. The images show the development of the spores.
Chemofluorescent (optical brightening) agents.
Chemofluorescent (optical brightening) agents are chitin stains that require the use of a fluorescent microscope (8). Depending on the agent used as well as the wavelength, the walls of the microsporidial spores fluorescence. Using Calcofluor White 2MR (American Cyanamid Corp., Princeton, N.J.) and a wavelength of 395 to 415 nm (observation light, 455 nm), the spores appear as bluish-white or turquoise oval halos. It is important to remember that this type of stain is nonspecific; small fungi and some artifact material present in stool may also fluoresce. However, when this approach is used with specimens other than stool, the microsporidial spores tend to be much easier to detect and identify (Fig. 6). Many laboratories tend to use one of the modified trichrome stains as well as the Calcofluor method for clinical specimens. The sensitivity of both methods is relatively good, and small numbers of spores that might be missed by the modified trichrome methods might be seen with the optical brightening agents. Again, clinical specimens other than stool tend to contain less debris and artifact material and are much easier to examine. Other optical brightening agents that can be used include Fungi-Fluor and Cellufluor (both from Polysciences Inc., Warrington, Pa.), Fungiqual A (Medical Diagnostics, Kandern, Germany), and Uvitex 2B (not commercially available; Ciba Geigy, Basel, Switzerland).
FIG. 6.
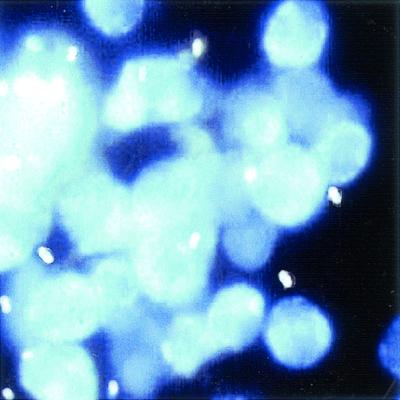
Calcofluor white staining of microsporidial spores in urine sediment.
Immunofluorescent reagents.
Although immunofluorescent reagents for the detection of microsporidial spores have been used, they are not yet commercially available and most have been limited to Encephalitozoon species spores (Fig. 7) (3, 15). Unfortunately, background staining, cross-reactions with yeast species and bacteria, and overall lower sensitivity than is observed with chromotrope or chemofluorescent staining prevent the polyclonal antibodies from being applicable to routine diagnostic use. Fluorescing microsporidial spores have a darker cell wall, and the polar tubule can be seen as cross or diagonal lines. With the use of an antiserum, it was observed that 9 of 27 patients (30%) who were positive for cryptosporidiosis also had microsporidial spores in the stool (8). Although there is some cross-reactivity with bacteria, immunofluorescent staining is more-sensitive than the routine staining methods currently available.
FIG. 7.
Encephalitozoon spp. detected with immunofluorescent reagent. (A) Urine sediment; (B) positive control spores.
More specific reagents are in various stages of development and testing; it can be hoped that these will become commercially available in the near future. With the development of monoclonal antibodies to Encephalitozoon intestinalis and Enterocytozoon bieneusi, it can also be hoped that diagnostic reagents will become available soon (1, 6). Since E. intestinalis and E. bieneusi are the two most important microsporidian pathogens in humans, the availability of commercial reagents for these organisms would enhance diagnostic capabilities of the routine clinical laboratory.
As clinicians begin to suspect these infections and microbiologists become more familiar with the diagnostic methods, the number of patients who test positive for microsporidial infection may increase dramatically. It is strongly suspected that with the use of more sensitive and specific methods many of these cases will be found in the immunocompetent population, as well as in immunocompromised patients.
CYTOLOGY
Techniques that do not require tissue embedding are becoming more widely used. Touch preparations of fresh biopsy material that are air dried, methanol fixed, and Giemsa stained have been used; however, microscopic examination must be performed using the oil immersion objective (100× objective; total magnification, ×1,000). This approach can also be used for smears prepared from conjunctival swabs and for sputum and nasal discharge fluids.
Cytocentrifugation followed by Giemsa staining has also been recommended. Spores have been detected in centrifuged sediment of duodenal aspirate, bile, biliary aspirates, urine, BAL fluid, and CSF. Oil immersion examination of stained smears of duodenal aspirates is recommended for the diagnosis of intestinal microsporidiosis. Because disseminated disease often involves multiple organs, detection of microsporidia in any tissue or fluid should be followed by examination of specimens from other body sites. The examination of urine should always be included in cases of disseminated infection. High-speed centrifugation (at least 1,500 × g for 10 min) may be required to concentrate the spores, particularly if the specimen contains debris, mucus, or other particulate material.
Appropriate stains used for light microscopy can be applied to these clinical specimens. For body fluids that do not contain debris, other fungi or bacteria, the routine Gram stain can be used. The spores are often gram variable and may stain partially gram positive (Fig. 8). Giemsa stain and chemofluorescent stains can also be used for these types of specimens.
FIG. 8.
Cytospin preparation of bronchoalveolar lavage fluid from a patient with AIDS and intestinal E. bieneusi infection, showing intracellular gram-positive microsporidial spores (Gram stain). Reprinted from reference 19 with permission.
Urine.
Microsporidial spores are often shed sporadically; multiple single or 24-h urine specimens should be submitted for examination. A single negative specimen would not rule out the possibility of infection with the microsporidia.
Duodenal aspirate.
Microscopic examination of centrifuged aspirate sediment obtained by endoscopy can be very helpful in diagnosing intestinal infection. Duodenal aspirate specimens eliminate some of the problems inherent in stool examination, including less debris, ease of high-speed centrifugation, and easier recognition of spores.
Other body fluids.
Specimens of sinonasal washings, sputum, BAL fluid, ascites fluid, CSF, or other body fluids are prepared using high-speed centrifugation and standard smear preparation techniques. Gram, modified trichrome, or Giemsa staining, use of chemofluorescent agents, or immunofluorescent testing (if available) is recommended.
Conjunctival smears and corneal scrapings.
Confirmation of microsporidial conjunctivitis or keratitis can be made from the examination of conjunctival or corneal swab, scraping, or biopsy specimens. The staining method is optional and may include the use of Gram, Giemsa, or modified trichrome stains. In cases of confirmed ocular microsporidiosis, urine and respiratory specimens should also be submitted for examination. For immunocompetent patients who may have localized corneal infection with no disseminated disease, diagnosis can be confirmed using routine histologic methods or electron microscopy.
ROUTINE HISTOLOGY AND ELECTRON MICROSCOPY
Routine histology.
There are a number of techniques available for recovery and identification of microsporidia in tissues. Although the organisms have been identified in routine histologic tissue preparations, they do not tend to stain with predictable results. Occasionally, the spores appear as refractile gold bodies in formalin-fixed, paraffin-embedded routine hematoxylin-eosin-stained sections. Using routine hematoxylin and eosin staining on tissue sections, only experienced pathologists have consistently been able to identify microsporidia (Fig. 9).
FIG. 9.
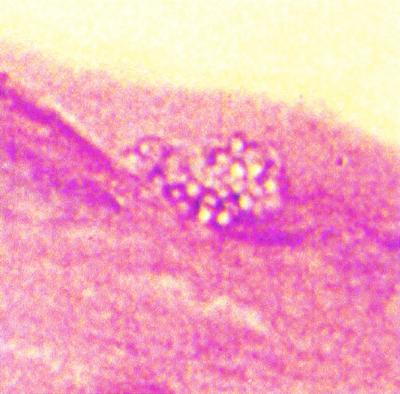
Hematoxylin-eosin staining of eye tissue (note clear spores).
However, with a variety of special staining methods, spores can be easily demonstrated in formalin-fixed and paraffin-embedded tissues. Birefringence is seen in paraffin-embedded tissue sections and results from the presence of chitin in the endospore layer. This property can be observed in sections stained with Gram and modified Warthin-Starry stains and is used to differentiate the spores from lysosomes, intracytoplasmic neuroendocrine granules, karyorrhexic debris, and mucin droplets.
Tissue Gram stains (Brown-Brenn or Brown-Hopps) have been used on routine paraffin-embedded tissue sections and appear to provide reliable results for microsporidial spores of all species; these stains are often the preferred stains for tissue specimens. Mature spores stain gram-positive (violet to purple), with some intensity variations; red spores tend to be immature. The spores may be darkly stained, but with careful examination, the spore wall and horizontal stripe representing the polar tubule can be seen in some spores.
Also, spores can occasionally be seen very well using periodic acid-Schiff (PAS) stain, silver stains (e.g., Warthin-Starry), acid-fast stains, a chromotrope-based stain, or chemofluorescent agents. The spore has a small, PAS-positive posterior body, the spore coat will stain with silver, and the spores are acid-fast variable (Fig. 10). The Warthin-Starry method stains both developing and mature stages in intestinal tissues (Fig. 11). Warrin-Starry staining also makes the spores appear larger than their actual size. One disadvantage is that the internal structure of the spore, the polar tubule, is much more difficult to see with preparations stained by the Warrin-Starry method (silver staining) than it is with Gram-stained preparations.
FIG. 10.

PAS staining of eye tissue (note PAS-positive granule at the end of each spore).
FIG. 11.

Warthin-Starry silver staining of eye tissue (note dark spores).
Plastic sections.
Regardless of the fixative used, there is evidence to indicate that ultrathin plastic sections stained with methylene blue-azure II-basic fuchsin or toluidine blue may facilitate spore detection.
Electron microscopy.
Tissue examination by electron microscopy (EM) is still considered the best approach; however, this option is not available at all laboratories (Fig. 12). The sensitivity of EM may not be equal to that of other methods when examining stool or urine. The identification of microsporidia to the genus level has been based on ultrastructural characteristics. However, in spite of the fact that electron microscopy is considered the gold standard for diagnostic confirmation and species identification, morphology alone will not always allow identification of human microsporidial pathogens to the species level. Antigenic or molecular testing may be required.
FIG. 12.
Transmission electron micrograph of a jejunal biopsy demonstrating numerous septated parasitophorous vacuoles of Encephalitozoon intestinalis, which are located in the Golgi-rich supranuclear cytoplasm. Reprinted from reference 14 with permission.
MOLECULAR METHODS
Molecular studies of the microsporidia have been limited; however DNA sequence studies on a few of the identified human pathogens have been reported (23). Although some nucleic acid-based methods have been developed, commercial products are not yet available (17, 18, 24, 25). PCR detection of E. intestinalis in intestinal biopsy specimens from AIDS patients has been reported (4). Comparison of microscopic and PCR methods for the detection of E. bieneusi spores in stool specimens of HIV-infected patients has not yet confirmed greater sensitivity for molecular tests. However, dilution studies using stool specimens seeded with Encephalitozoon spores indicated that the threshold of spore detection with PCR was 102 spores but that microscopy required 104 and 106 spores per ml (22).
CELL CULTURE
Although not relevant for routine diagnostic laboratories, in the research setting the use of in vitro culture methods continues to provide confirmatory as well as diagnostic information. This approach has been critical for the development of immunologic reagents for diagnosis and species confirmation. In vitro culture has also been used to determine the efficacy of antimicrobial agents on several microsporidia, including Encephalitozoon cuniculi, Encephalitozoon hellem, and E. intestinalis. Although the microsporidia cannot be grown axenically, Encephalitozoon spp., Trachipleistophora hominis, Vittaforma corneae, and Brachiola algerae have been grown in cell culture. A number of cell lines have been used, including monkey and rabbit kidney cell lines (Vero and RK-13), a human fetal lung fibroblasts cell line (MRC-5), and the Madin-Darby canine kidney cell line (MDCK). Unfortunately, one of the most common human microsporidial pathogens, E. bieneusi, has been propagated only in short-term cultures (7, 22).
SEROLOGIC TESTING
A variety of serologic testing methods (carbon immunoassay, indirect immunofluorescent-antibody testing, enzyme-linked immunosorbent assay, counterimmunoelectrophoresis, and Western blotting) have been used to detect immunoglobulin G and immunoglobulin M antibodies to microsporidia (primarily E. cuniculi) in animals. Antibodies to E. cuniculi and E. intestinalis have been found in HIV- and non-HIV-infected humans; however, whether this represents true infection, cross-reactivity with other species, or nonspecific reactions is unclear. Unfortunately, due to the lack of success in long-term culture and development of available antigens, there are no serologic assays available for E. bieneusi. Also, whether antibodies to this organism will cross-react with those of E. cuniculi is unknown; serologic evidence of human infection is based primarily on results for E. cuniculi as the parasite antigen. Currently, the available serologic data are interesting, but reliable tests for the diagnosis of human microsporidiosis are not yet available.
REFERENCES
- 1.Accoceberry, I., M. Thellier, I. Desportes-Livage, A. Achbarou, S. Biligui, M. Danis, and A. Datry. 1999. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J. Clin. Microbiol. 37:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canning, E. U. 1993. Microsporidia, p. 299-370. In J. P. Kreier (ed.), Parasitic Protozoa, vol. 6. Academic Press, San Diego, Calif. [Google Scholar]
- 3.Croppo, G. P., G. S. Visvesvara, G. J. Leitch, S. Wallace, and D. A. Schwartz. 1998. Identification of the microsporidian Encephalitozoon hellem using immunoglobulin G monoclonal antibodies. Arch. Pathol. Lab. Med. 122:182-186. [PubMed] [Google Scholar]
- 4.Del Aguila, C., G. P. Croppo, H. Moura, A. J. Da Silva, G. J. Leitch, D. M. Moss, S. Wallace, S. B. Slemenda, N. J. Peiniazek, and G. S. Visvesvara. 1998. Ultrastructure, immunofluorescence, Western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS. J. Clin. Microbiol. 36:1201-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Didier, E. S., J. M. Orenstein, A. Aldra, D. Bertucci, L. B. Rogers, and F. A. Janney. 1995. Comparison of three staining methods for detecting microsporidia in fluids. J. Clin. Microbiol. 33:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enriquez, F. J., O. Ditrich, J. D. Palting, and K. Smith. 1997. Simple diagnosis of Encephalitozoon sp. microsporidial infections by using a panspecific antiexospore monoclonal antibody. J. Clin. Microbiol. 35:724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia, L. S. 2001. Diagnostic medical parasitology, 4th ed., p. 87-97. ASM Press, Washington, D.C.
- 8.Garcia, L. S., R. Y. Shimizu, and D. A. Bruckner. 1994. Detection of microsporidial spores in fecal specimens from patients diagnosed with cryptosporidiosis. J. Clin. Microbiol. 32:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardiner, C. H., R. Fayer, and J. P. Dubey. 1988. An atlas of protozoan parasites in animal tissues, U.S. Department of Agriculture handbook no. 651. U.S. Department of Agriculture, Washington, D.C.
- 10.Ignatius, R., S. Henschel, O. Liesenfeld, U. Mansmann, W. Schmidt, S. Koppe, T. Schneider, W. Heise, U. Futh, E. O. Riecken, H. Hahn, and R. Ulrich. 1997. Comparative evaluation of modified trichrome and Uvitex 2B stains for detection of low numbers of microsporidial spores in stool specimens. J. Clin. Microbiol. 35:2266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isenberg, H. D. (ed.). 1992. Clinical microbiology procedures handbook, vol. 1 and 2. American Society for Microbiology, Washington, D.C.
- 12.Isenberg, H. D. (ed.). 1995. Essential procedures for clinical microbiology. ASM Press, Washington, D.C.
- 13.Kokoskin, E., T. W. Gyorkos, A. Camus, L. Cedilotte, T. Purtill, and B. Ward. 1994. Modified technique for efficient detection of microsporidia. J. Clin. Microbiol. 32:1074-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotler, D. P., and J. M. Orenstein. 1999. Clinical syndromes associated with microsporidiosis, p. 258-292. In M. Wittner (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 15.Moura, H., F. C. Sodre, F. J. Bornay-Llinares, G. J. Leitch, T. Navin, S. Wahlquist, R. Bryan, I. Meseguer, and G. S. Visvesvara. 1999. Detection by an immunofluorescence test of Encephalitozoon intestinalis spores in routinely formalin-fixed stool samples stored at room temperature. J. Clin. Microbiol. 37:2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moura, H., D. A. Swartz, F. Bornay-Linnares, F. C. Sodré, S. Wallace, and G. S. Visvesvara. 1997. A new and improved “Quick-Hot Gram-Chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch. Pathol. Lab. Med. 121:888-893. [PubMed] [Google Scholar]
- 17.Muller, A., K. Stellermann, P. Hartmann, M. Schrappe, G. Fatkenheuer, B. Salzberger, V. Diehl, and C. Franzen. 1999. A powerful DNA extraction method and PCR for detection of microsporidia in clinical stool specimens. Clinical and Diagnostic Laboratory Immunology 6:243-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raynaud, L., F. Delbac, V. Brousolle, M. Rabodonirina, V. Girault, M. Wallon, G. Cozon, C. P. Vivares, and F. Peyron. 1998. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J. Clin. Microbiol. 36:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan, N. J., G. Sutherland, K. Coughlan, M. Globan, J. Doultree, J. Marshall, R. W. Baird, J. Pedersen, and B. Dwyer. 1993. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J. Clin. Microbiol. 31:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber, R., R. T. Bryan, R. L. Owen, C. M. Wilcox, L. Gorelkin, G. S. Visvesvara, and The Enteric Opportunistic Infections Working Group. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N. Engl. J. Med. 326:161-166. [DOI] [PubMed] [Google Scholar]
- 21.Weber, R., R. T. Bryan, D. A. Swartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber, R., D. A. Swartz, and P. Deplazes. 1999. Laboratory diagnosis of microsporidiosis, p. 315-361. In M. Wittner (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 23.Weiss, L. M., and C. Vossbrinck. 1999. Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia, p. 129-171. In M. Wittner (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 24.Xiao, L., L. Li, H. Moura, I. Sulaiman, A. A. Lal, S. Gatti, M. Scaglia, E. S. Didier, and G. S. Visvesvara. 2001. Genotyping Encephalitozoon hellem isolates by analysis of the polar tube protein gene. J. Clin. Microbiol. 39:2191-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao, L., L. Li, G. S. Visvesvara, H. Moura, E. S. Didier, and, A. A. Lal. 2001. Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J. Clin. Microbiol. 39:2248-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]