Abstract
An NS1 serotype-specific indirect enzyme-linked immunosorbent assay (ELISA) was developed to differentiate primary and secondary dengue virus infections and serotypes of primary dengue virus infection. For this report, we carried out retrospective seroepidemiologic studies on serum samples collected from residents of Liuchiu Hsiang, Pingtung County, an isolated island in southern Taiwan during 1997-1998. The results demonstrated that good correlation existed between dengue virus NS1 serotype-specific immunoglobulin G (IgG) ELISA and dengue virus plaque reduction neutralization test (PRNT). Our data suggested that NS1 serotype-specific IgG ELISA could replace PRNT for seroepidemiologic studies to differentiate Japanese encephalitis and dengue virus infections and for dengue virus serotyping.
Dengue virus (DEN) is a mosquito-borne flavivirus and the most prevalent arbovirus in tropical and subtropical regions of the world. There are four distinct serotypes, DEN-1, DEN-2, DEN-3, and DEN-4. Infection induces life-long protective immunity to the homologous serotype, but there is no cross-reactive immunity to the heterologous serotypes. The global prevalence of dengue has grown dramatically in recent decades. The disease is now endemic in more than 100 countries in Africa, the Americas, the Eastern Mediterranean, Southeast Asia, and the Western Pacific (5).
There have been a number of historical dengue epidemics (either regional or island-wide) over the last century (1915, 1931, 1942-1943, 1981, 1987-1988, 1991, 1994, 1995, 1998, and 2000) in Taiwan (19, 20). Among these, the 1915, 1931, and 1942-1943 outbreaks were large island-wide epidemics (Table 1). Dengue hemorrhagic fever- and dengue shock syndrome-like syndromes were described during the 1931 and 1942-1943 outbreaks. No dengue outbreaks were reported after 1945 until a dengue fever epidemic occurred in Liuchiu Hsiang, Pingtung County, an islet about 15 km southwest of Taiwan, in the summer of 1981. It was estimated that approximately 80% of the inhabitants were infected and the serotype was identified as DEN-2 (9, 19). During the winter of 1987-1988, a small outbreak circulated in southern Taiwan, affecting Kaohsiung City, Kaohsiung County, and Pingtung County (7, 12). The serotype was identified as DEN-1. Since then, small regional epidemics have been reported almost every year in southern Taiwan, with the exception of a 1995 DEN-1 outbreak that occurred in Chungho, Taipei County, in northern Taiwan. To better understand the present status of dengue virus infection in Taiwan, we have recently initiated several seroprevalence surveys in various areas of Taiwan.
TABLE 1.
Major dengue epidemics in Taiwan between 1901 and 1988
| Year(s) | Location of index case | Epidemic area | Attack rate (size of outbreak or %) | Serotypea |
|---|---|---|---|---|
| 1901-1902 | Southern Taiwan | Large | DEN? | |
| 1915-1916 | Kaohsiung | Island-wide | 25-50 | DEN? |
| 1922 | Penghu | Penghu | 20-30 | DEN? |
| 1927 | Kaohsiung | Southern Taiwan | Small | DEN? |
| 1931 | Kaohsiung | Island-wide | Large | DEN? |
| 1942-1943 | Kaohsiung | Island-wide | Large | DEN? |
| 1981 | Liuchiu Hsiang (Pingtung County) | Liuchiu Hsiang (Pingtung County) | ∼80 | DEN-2 |
| 1987-1988 | Kaohsiung | Southern Taiwan | Small | DEN-1 |
DEN?, DEN serotype is unknown.
For seroepidemiologic study, plaque reduction neutralization test (PRNT) remains the “gold standard” for the confirmation and serotyping of past dengue virus infections in the regions where two or more flaviviruses are cocirculating (1, 15). This is due to high cross-reactivity of envelope (E)- and membrane (M)-specific immunoglobulin G (IgG) antibodies produced from Japanese encephalitis (JE) virus and yellow fever virus vaccination and sequential flavivirus infections (4). The PRNT, however, is time-consuming and difficult to perform and is not as amenable to testing large numbers of sera as the enzyme-linked immunosorbent assay (ELISA). In addition, Halstead et al. (6) first reported the “original antigenic sin” phenomenon, which led to the biased neutralizing antibody titers favoring the initial infecting DEN serotype. Makino et al. (14) later reported that the cross-reactivity among flaviviruses in different subgroups was quite often seen in the sequential flavivirus infection, even when using PRNT. Thus, DEN PRNT is only reliable for primary infection but not for secondary and multiple infections. It would be a great breakthrough if a DEN serotype-specific ELISA could be developed for seroepidemiologic study to differentiate primary and secondary and/or multiple infections and for DEN serotyping.
Recently, we reported the development of an NS1-specific indirect ELISA to detect and differentiate JE virus and dengue virus infections using monoclonal antibody and NS1 antigens secreted in the culture supernatants of Vero cells infected with DEN or JE virus (16, 17). Specificity analysis showed that NS1-specific antibodies induced by JE virus and dengue virus infections do not cross-react with each other (17). Huang et al. (10) also reported a DEN NS1-specific antibody response using recombinant DEN NS1 protein. When recombinant DEN NS1 was evaluated for various flavivirus infections, relatively low cross-reactivity was found from sera of JE and yellow fever patients. More recently, we have reclassified the criteria of primary dengue virus infections, using the E- and M-specific capture IgM/IgG ratio (≧1.2) instead of hemagglutination inhibition titer (≦1,280), and analyzed more than 100 convalescent-phase sera from primary dengue patients covering all four serotypes (reference 11 and unpublished data). The results showed that more than 90% of the sera tested could be correctly serotyped using NS1 serotype-specific IgG ELISA. Comparison between NS1-specific IgG serotyping and PRNT showed good correlation (unpublished data).
The serum samples used in this study were obtained from volunteers of residents in Liuchiu Hsiang, Pingtung County, in southern Taiwan during 1997-1998. These sera were collected for seroepidemiologic study in an attempt to understand the age-specific seroprevalence of JE virus and DEN in Taiwan. A total of 1,317 serum samples were collected and analyzed for this report.
D2/8-1 is an NS1-specific monoclonal antibody generated and analyzed as previously described (2). It recognizes a linear epitope on the NS1 antigen common to JE virus and four DEN types. Monoclonal antibodies were purified from ascitic fluid by protein A-Sepharose 4B Fast Flow affinity chromatography (Pharmacia Biotech) as described previously (3). NS1-specific indirect ELISA was performed as previously described (16). This assay can be used to analyze isotype- and serotype-specific antibody responses to NS1 antigens. In this study, serum samples were first screened for DEN NS1-specific IgG antibodies using pooled NS1 antigens from culture supernatants of DEN-1-, DEN-2-, DEN-3-, or DEN-4-infected Vero cells. DEN NS1 serotype-specific IgG antibodies were then analyzed using culture supernatant from each of the DEN-1-, DEN-2-, DEN-3-, or DEN-4-infected Vero cells.
The PRNTs were performed in BHK-21 cells as previously described (15). The prototype strains of DEN-1, DEN-2, DEN3, and DEN-4 (Hawaii, New Guinea C, H87, and H241, respectively) were used in the assay. The titer of a serum was considered to be the reciprocal of the highest dilution which neutralized ≧50% of the average number of plaques present in control wells.
The optical densities (OD) read from culture supernatants of Vero cells with or without dengue virus infection were assigned as the test absorbance and negative control, respectively, for each sample in the NS1-specific indirect ELISA. Positivity was determined by comparison to individual negative controls. A positive sample was defined as having a test absorbance/negative control ratio of ≧2.0 and a negative sample was defined as having a ratio of <2.0. Serotype specificity was defined as positive if the test absorbance ratio of homologous virus (the highest OD value) to heterologous virus (the second highest OD value) was ≧120%. All serum specimens were tested at least twice for reproducibility. Kappa statistics were used to evaluate the correlation between NS1 serotype-specific IgG and PRNT results (8).
In this report, we show the results of serum samples collected from residents of Liuchiu Hsiang, Pingtung County, an isolated island in southern Taiwan, during 1997-1998. DEN antibody-positive sera were first screened by DEN NS1-specific IgG ELISA. Positive sera were then analyzed by DEN NS1 serotype-specific IgG ELISA and DEN PRNT. Table 2 shows the results of NS1-specific IgG ELISA in arbitrarily specified age groups in order to fit the recorded dengue outbreaks. The results show an age-dependent increase in seroprevalence, in that 93.6, 87.5, 73.1, and 24.6% of serum samples were positive from individuals born before 1931 and in 1932-1941, 1944-1980, and 1982-1986, respectively. These data correlated very well with the known epidemics in Taiwan since the 1931 and 1942-1943 outbreaks were indeed dengue epidemics and the 1981 and 1987-1988 outbreaks occurred in Liuchiu Hsiung, affecting a large percentage of residents.
TABLE 2.
Age-dependent seroprevalence of dengue virus infection in Liuchiu Hsiang, Pingtung County, analyzed by NS1-specific IgG ELISA
| Age group (year of birth)a | Seroprevalence of DEN in Liuchiu Hsiang, Pingtung County (southern Taiwan)
|
|
|---|---|---|
| No. of cases | No. of DEN NS1-specific IgG-positive individuals (%) | |
| Before 1931 | 47 | 44 (93.6) |
| 1931 | 11 | 10 (90.9) |
| 1932-1941 | 96 | 84 (87.5) |
| 1942-1943 | 20 | 18 (90.0) |
| 1944-1980 | 342 | 250 (73.1) |
| 1981 | 18 | 3 (16.7) |
| 1982-1986 | 525 | 129 (24.6) |
| 1987-1988 | 100 | 14 (14.0) |
| 1989-1998 | 158 | 0 (0.0) |
| Total | 1,317 | 552 (41.9) |
Age groups were arbitrarily assigned in order to fit recorded dengue epidemics.
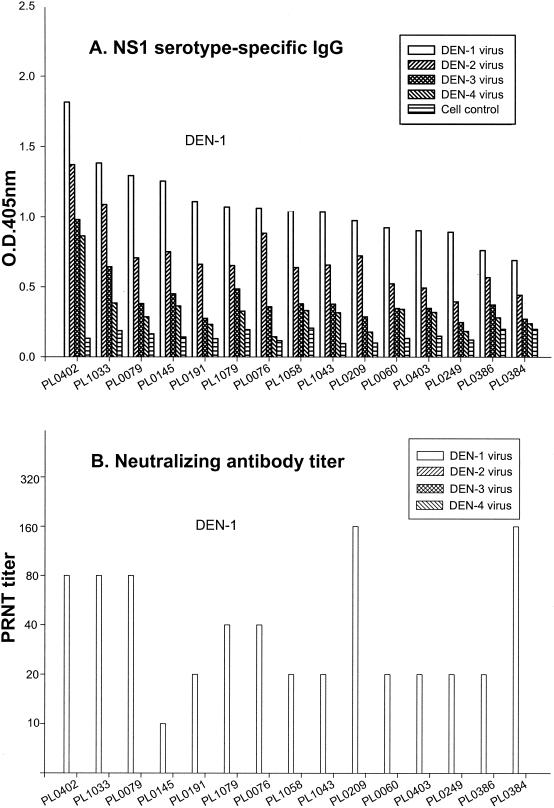
To evaluate the potential application of NS1 serotype-specific IgG ELISA in DEN serotyping, positive sera from the above screening were selected for serotype analysis. The results were then compared with the standard DEN serotyping method, PRNT. To simplify the analysis, serum samples from individuals born before 1931 and in 1932-1941, 1944-1980, and 1982-1986 were grouped and tested in accordance with the 1931, 1942-1943, 1981, and 1987-1988 outbreaks. Figure 1 shows representative data from 52 randomly selected DEN-positive serum samples from patients born between 1982 and 1986. The results clearly show significantly higher levels of DEN-1 NS1-specific IgG antibodies than other serotypes (Fig. 1A). These data correlated very well with PRNT in that only DEN-1-specific neutralizing antibodies were detected (Fig. 1B).
FIG. 1.
Serotype analysis of DEN-positive sera from residents of Liuchiu Hsiang, Pingtung County, born between 1982 and 1986. Serum samples from 52 randomly selected DEN-positive individuals were analyzed and representative data are shown. (A) NS1 serotype-specific IgG ELISA was used to analyze the NS1-specific IgG antibodies to various DEN serotypes. (B) PRNT was used to measure neutralizing antibody titers to various DEN serotypes.
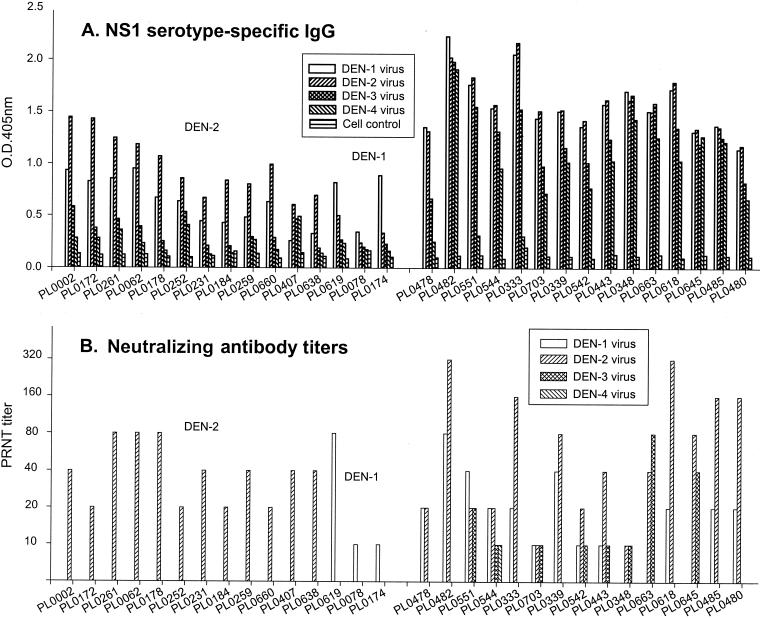
Figure 2 shows representative data from 50 randomly selected DEN-positive serum samples from residents of Liuchiu Hsiang born between 1944 and 1980. The results showed three different patterns with respect to the NS1-specific antibodies. Among these 50 DEN-positive sera tested, 4 showed significantly higher DEN-1 NS1-specific IgG antibodies, 25 showed significantly higher DEN-2 NS1-specific IgG antibodies, and 21 showed strong and complex NS1-specific IgG antibodies to at least two DEN serotypes (Fig. 2A). These data correlated very well with PRNT in that each of these DEN-1 and DEN-2 sera had their corresponding serotype-specific neutralizing antibodies detected (Fig. 2B). In addition, the PRNT results from those sera with strong and complex NS1-specific IgG antibodies showed neutralizing antibodies to at least two DEN serotypes (Fig. 2B).
FIG. 2.
Serotype analysis of DEN-positive sera from residents of Liuchiu Hsiang, Pingtung County, born between 1944 and 1980. Serum samples from 50 randomly selected DEN-positive individuals were analyzed and representative data demonstrating primary and secondary or multiple infections are shown. (A) NS1 serotype-specific IgG ELISA was used to analyze the NS1-specific IgG antibodies to various DEN serotypes. (B) PRNT was used to measure neutralizing antibody titers to various DEN serotypes.
Similar analyses were performed for DEN-positive sera from residents of Liuchiu Hsiang born between 1932 and 1941. Among 30 sera tested, 4 showed significantly higher DEN-1 NS1-specific IgG antibodies, 3 showed significantly higher DEN-2 NS1-specific IgG antibodies, and 23 showed strong and complex NS1-specific IgG antibodies to at least two DEN serotypes (data not shown). Among 12 DEN-positive sera tested from residents of Liuchiu Hsiang born before 1931, 2 showed significantly higher DEN-2 NS1-specific IgG antibodies and 10 showed strong and complex NS1-specific IgG antibodies to at least two DEN serotypes (data not shown).
Kappa statistics were applied to evaluate the correlation between NS1 serotype-specific IgG ELISA and PRNT. Among 117 serum samples available for analysis, 73 sera showed primary dengue virus infection and 32 sera showed secondary dengue virus infection, agreeing with both assays. Twelve sera were found to have discordant results. Among these, six sera showed primary infection in the NS1 assay but were classified as secondary by PRNT, and the other six showed the opposite pattern. The results suggested that good correlation existed for DEN NS1 serotype-specific IgG ELISA and DEN PRNT (κ = 0.766).
Liuchiu Hsiang has been used as a good model in the study of dengue epidemics and control in Taiwan (13, 18, 19). This small island is located about 15 km southwest of Taiwan with a total population of around 16,000. It experienced several major dengue epidemics during the past century (Table 1). Among these, the 1931, 1942-1943, 1981, and 1987-1988 outbreaks were well documented. In fact, the 1981 Liuchiu Hsiang outbreak was the first dengue epidemic in Taiwan since World War II. A total of 21 virus strains were isolated for the first time in Taiwan and the serotype was identified as DEN-2. It was estimated that approximately 80% of the inhabitants were infected during this epidemic that lasted for 5 months. The 1987-1988 DEN-1 epidemic also affected Liuchiu Hsiang, although this outbreak mainly circulated in Kaohsiung City, with more than 10,000 reported cases. Although it was generally believed that those earlier epidemics (1931 and 1942-1943) were caused by DEN, direct evidence was lacking and the serotypes involved were not known. In this study, we addressed these questions by using NS1 serotype-specific IgG ELISA and comparing it with PRNT. The results correlated very well with the known epidemics in that an age-dependent increase in seroprevalence was observed. Particularly, the DEN-positive rate was found to be 73.1% for residents born between 1944 and 1980. This is close to a previous report estimating that approximately 80% of the inhabitants were infected (19). In addition, the DEN-positive rate was found to be 24.6% for residents born between 1982 and 1986. This suggested that the infection rate of the 1987-1988 epidemic in Liuchiu Hsiang was higher than was previously recognized. The age-dependent increase in NS1-specific IgG-positive sera from older individuals provided strong support that the 1942-1943 and 1931 outbreaks were indeed dengue infections. It is interesting to find that none of the 158 serum samples from residents born after 1989 was DEN positive. This is very encouraging and suggests that integrated control measures against Aedes aegypti were very successful during the last 10 years in Liuchiu Hsiang.
DEN-positive sera were analyzed by NS1 serotype-specific IgG ELISA and PRNT. The DEN serotypes identified for each age group match correctly with the known serotypes of corresponding epidemics. For instance, all of the 52 randomly selected DEN-positive sera from residents born between 1982 and 1986 showed significantly higher DEN-1 NS1-specific IgG antibody responses than the other serotypes. This suggested that all these residents had been infected with DEN-1 during the 1987-1988 outbreak. For those individuals born between 1944 and 1980, the situation was more complicated, since they could have been infected during the 1987-1988 DEN-1 and/or 1981 DEN-2 outbreaks. Figure 2 demonstrates the different patterns of primary and secondary dengue virus infections. The results showed that NS1 serotyping correlated very well with PRNT in that each of these DEN-1 and DEN-2 sera had their corresponding serotype-specific neutralizing antibodies detected. In addition, many individuals had strong and complex NS1-specific IgG antibodies to at least two DEN serotypes. It is interesting to find that some of these individuals had neutralizing antibodies to DEN-3 in addition to DEN-1 or DEN-2. It is tempting to speculate that these individuals had been infected with DEN-3 before, since many residents are fishermen and go fishing in the northern area of the Philippines. For those individuals born during 1932-1941 and before 1931, the situation was even more complicated, since they could be infected and/or stimulated several times during the 1987-1988 DEN-1, 1981 DEN-2, 1942-1943, and 1931 outbreaks. The results did show that most of the sera tested showed strong and complex NS1 serotype-specific IgG responses.
In conclusion, we have shown the potential application of NS1-specific IgG ELISA in the study of the seroprevalence of dengue virus infection. The serotype of each dengue epidemic can be correctly identified from primarily infected sera using NS1 serotype-specific IgG ELISA. Comparison between NS1 serotype-specific IgG ELISA and PRNT demonstrated high correlation. Due to the high sensitivity, high specificity, and simplicity of NS1 serotype-specific IgG ELISA, we believe it can replace PRNT for seroepidemiologic study and DEN serotyping.
Acknowledgments
We thank Yun-Yih Chang for her expert technical assistance and Yaw-Hsiung Huang, Ching-Jung Huang, and Hsueh-Chih Tsai for serum collection.
This work was in part supported by grants NSC 88-2318-B-043B-001-M51 and NSC 89-2318-B-043B-001-M51 from the National Science Council, Taiwan, Republic of China.
REFERENCES
- 1.Calisher, C. H., N. Karabatsos, J. M. Dalrymple, R. E. Shope, J. S. Porterfield, E. G. Westaway, and W. E. Brandt. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antiserum. J. Gen. Virol. 70:37-43. [DOI] [PubMed] [Google Scholar]
- 2.Chen, L. K., C. L. Liao, C. G. Lin, S. C. Lai, C. I. Liu, S. H. Ma, Y. Y. Huang, and Y. L. Lin. 1996. Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology 217:220-229. [DOI] [PubMed] [Google Scholar]
- 3.Ey, P. L., S. J. Prowse, and C. R. Jenkin. 1978. Isolation of pure IgG1, IgG2a, and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry 15:429-436. [DOI] [PubMed] [Google Scholar]
- 4.Gubler, D. J. 1996. Serological diagnosis of dengue haemorrhagic fever. WHO Dengue Bull. 20:20-23. [Google Scholar]
- 5.Gubler, D. J. 1997. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem, p. 1-22. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 6.Halstead, S. B., S. Rojanasuphot, and N. Sangkawibha. 1983. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 32:154-156. [DOI] [PubMed] [Google Scholar]
- 7.Harn, M. R., Y. L. Chiang, M. J. Tian, Y. H. Chang, and Y. C. Ko. 1993. The 1991 dengue epidemic in Kaohsiung City. J. Formos. Med. Assoc. 92(Suppl. 1):S39-S43. [PubMed] [Google Scholar]
- 8.Hesketh, L., A. Charlett, P. P. Farrington, E. Miller, T. Forsey, and P. Morgan-Capner. 1997. An evaluation of nine commercial EIA kits for the detection of measles-specific IgG. J. Virol. Methods 66:51-59. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh, W. C., M. F. Chen, K. T. Lin, S. T. Hsu, C. I. Ma, and S. S. Wu. 1982. Outbreaks of Dengue fever in 1981 in Liouchyou Shiang, Pingtung County. Taiwan Yi Xue Hui Za Zhi 81:1388-1395. [PubMed] [Google Scholar]
- 10.Huang, J. L., J. H. Huang, R. H. Shyu, C. W. Teng, Y. L. Lin, M. D. Kuo, C. W. Yao, and M. F. Shaio. 2001. High-level expression of recombinant dengue viral NS-1 protein and its potential use as a diagnostic antigen. J. Med. Virol. 65:553-560. [PubMed] [Google Scholar]
- 11.Innis, B. L., A. Nisalak, S. Nimmannitya, S. Kusalerdchariya, V. Chongswasdi, S. Suntayakorn, P. Puttisri, and C. H. Hoke. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418-427. [DOI] [PubMed] [Google Scholar]
- 12.Ko, Y. C., M. J. Chen, and S. M. Yeh. 1992. The predisposing and protective factors against dengue virus transmission by mosquito vector. Am. J. Epidemiol. 136:214-220. [DOI] [PubMed] [Google Scholar]
- 13.Lin, H. M., C. S. Chen, C. C. Hsu, and C. L. Chung. 1986. Dengue vector density survey in Liuchiu, Pintung, Taiwan. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 19:218-223. [PubMed] [Google Scholar]
- 14.Makino, Y., M. Tadano, M. Saito, N. Maneekarn, N. Sittisombut, V. Sirisanthana, B. Poneprasert, and T. Fukunaga. 1994. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol. Immunol. 38:951-955. [DOI] [PubMed] [Google Scholar]
- 15.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 16.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2000. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J. Med. Virol. 62:224-232. [DOI] [PubMed] [Google Scholar]
- 17.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2001. Antibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccination. Vaccine 19:1753-1763. [DOI] [PubMed] [Google Scholar]
- 18.Wang, C. H., N. T. Chang, H. H. Wu, and C. M. Ho. 2000. Integrated control of the dengue vector Aedes aegypti in Liu-Chiu village, Ping-Tung County, Taiwan. J. Am. Mosq. Control Assoc. 16:93-99. [PubMed] [Google Scholar]
- 19.Wu, Y. C. 1986. Epidemic dengue 2 in LiouChyou Shiang, Pingtung County, in 1981. Chin. J. Microbiol. Immunol. 19:203-211. [PubMed] [Google Scholar]
- 20.Wu, Y. C. 1996. Epidemiology and control of Japanese encephalitis and dengue fever in Taiwan. WHO Dengue Bull. 20:51-54. [Google Scholar]