Abstract
Herpes simplex virus type 1 (HSV-1) encodes 11 envelope glycoproteins, of which glycoprotein G-1 (gG-1) induces a type-specific antibody response. Variability of the gG-1 gene among wild-type strains may be a factor of importance for a reliable serodiagnosis and typing of HSV-1 isolates. Here, we used a gG-1 type-specific monoclonal antibody (MAb) to screen for mutations in the immunodominant region of this protein in 108 clinical HSV-1 isolates. Of these, 42 isolates showed no reactivity to the anti-gG-1 MAb. One hundred five strains were further examined by DNA sequencing of the middle part of the gG-1 gene, encompassing 106 amino acids including the immunodominant region and epitope of the anti-gG-1 MAb. By phylogenetic comparisons based on the sequence data, we observed two (main) genetic variants of the gG-1 gene among the clinical isolates corresponding to reactivity or nonreactivity to the anti-gG-1 MAb. Furthermore, four strains appeared to be recombinants of the two gG-1 variants. In addition, one strain displayed a gG-1-negative phenotype due to a frameshift mutation, in the form of insertion of a cytosine nucleotide. When immunoglobulin G reactivity to HSV-1 in sera from patients infected with either of the two variants was investigated, no significant differences were found between the two groups, either in a type-common enzyme-linked immunosorbent assay (ELISA) or in a type-specific gG-1 antigen-based ELISA. Despite the here-documented existence of two variants of the gG-1 gene affecting the immunodominant region of the protein, other circumstances, such as early phase of infection, might be sought for explaining the seronegativity to gG-1 commonly found in a proportion of the HSV-1-infected patients.
Herpes simplex virus (HSV) is an alpha-herpesvirus with two subtypes, HSV type 1 (HSV-1) and HSV-2, which both establish latent infections with different or similar clinical expressions during reactivation (6). The ability to discriminate between HSV-1 and HSV-2 infections by serological means has been of importance for several clinical aspects, including diagnosis and treatment of neonatal herpes as well as of complications from the central nervous system (5, 7, 9, 10, 23). Glycoprotein G-1 (gG-1), a viral envelope glycoprotein (1, 21) that was suggested to contribute to viral entry through apical surfaces of polarized cells (33), has been utilized as a prototype antigen for HSV-1 type-specific diagnosis due to lack of cross-reactivity with its counterpart in HSV-2, i.e., gG-2 (15, 29). Several commercial gG-based enzyme immunoassays have been evaluated in clinical settings (2). Furthermore, gG-1 may be a suitable target for typing of HSV isolates by monoclonal antibodies (MAb) that identify type-specific epitopes. Conservation of the gene coding for gG-1 among clinical HSV-1 isolates might therefore be a prerequisite for a reliable assessment of a type-specific antibody response as well as of correct typing of isolates in individuals infected with HSV-1.
In a previous study, several point mutations were found when the gG-1 genes of 11 HSV-1 clinical strains and two reference strains were sequenced (26), and some of these mutations were present within a recently defined immunodominant region including the epitope of a type-specific anti-gG-1 MAb (34). Genetic variability of this gene among clinical isolates may therefore be of importance for the immunoglobulin G (IgG) response within the individual host when a predefined gG-1 antigen is used for detection. Previously, a few studies have described a limited genetic variability of genes coding for envelope glycoprotein among HSV-1 strains as shown by DNA sequencing of the gB-gene (30) or the gD gene (27) after PCR amplification of cerebrospinal fluid samples. These studies observed some point mutations which were mostly silent, and the insertion or deletion of one or a few codons compared to a previously published HSV-1 sequence (21).
In contrast to this, we here report the identification of two major genetic variants of the gG-1 gene based on DNA sequencing and investigation of MAb reactivity to a type-specific gG-1 epitope in a large number of clinical isolates. Altogether, 42 strains were found to be unreactive to the anti-gG-1 MAb among the 108 HSV-1 clinical isolates, a finding that was explained by the existence of two genetic variants discovered when the strains were subjected to DNA sequencing of the middle part of the gG-1 gene. However, no differences in IgG reactivity were found when sera from patients harboring either of these variants were compared by gG-1 enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Patients and viral strains.
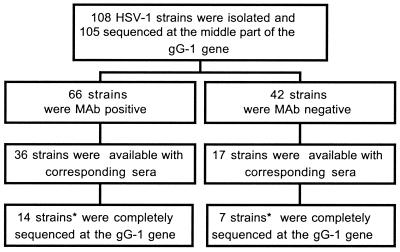
The study material is schematically presented in Fig. 1. One hundred six patients with reactivated herpetic lesions from different anatomical sites (oral, facial, genital, perianal, and others; see Table 1) were randomly chosen for investigation, and 108 virus strains derived from these 106 patients were consecutively received at the Department of Clinical Virology, Göteborg. Green monkey kidney (GMK) cells were used for isolation of the virus strains, which all were typed as HSV-1 by the use of a type-specific anti-gC MAb (17, 24). Of the 106 patients, documentation of gender was available for 76 subjects, of whom 54 were women and 22 were men. From 53 patients, a serum sample was drawn and included for serological assays. From two of these patients, two isolates were investigated: one strain from the thoracic region and one from penis in one patient, and one isolate from the lip and one from the genital region in another patient.
FIG. 1.
Schematic presentation of the virus isolates, DNA sequencing of the gG-1 gene, and availability of corresponding serum samples. *, in two additional strains in each group lacking corresponding sera, the gG-1 gene was also completely sequenced.
TABLE 1.
Sites of lesions and anti-gG-1 MAb reactivities of 108 clinical HSV-1 isolates
| Site of lesion | No. of isolates | No. of MAb− isolates |
|---|---|---|
| Lip | 25 | 12 |
| Genital | 58 | 24 |
| Perianal | 2 | 1 |
| Faciala | 5 | 0 |
| CNS/Eyeb | 3 | 1 |
| Unknown | 15 | 4 |
Indicates extra-labial lesions not affecting the eyes.
CNS, central nervous system.
The study was approved by the Medical Ethical Committee in Göteborg, Sweden, (approval no.: S 266-00).
MAbs.
The following HSV-1 type-specific MAbs were used: a commercially available anti-gG-1 MAb (Advanced Biotechnologies Incorporated), which was previously mapped by a pepscan technique to the amino acids AFPL at position 110 to 113 (34), the anti-gC-1 MAb B1C1B4, and the anti-gB-1 MAb 1B11D8 (4). For typing of HSV-2 strains, the anti-gG-2 MAb O1C5B2 (17) was used.
Type-specific serology.
An ELISA was used to determine type-common and HSV-1 and HSV-2 type-specific antibody reactivity in serum samples. In brief, the type-common antigen was based on sodium deoxycholate-solubilized membranes from cells infected with HSV-1 strain F (12). The HSV-1 type-specific antigen was based on the gG-1 protein (kindly provided by SmithKline Beecham Biologicals), and seroreactivity was analyzed by ELISA as previously described (34). The HSV-2 type-specific assay was based on Helix pomatia lectin-purified gG-2 (25, 31). For the type-common and HSV-2 ELISA assays, serum samples were diluted to 1/100, and for the HSV-1 ELISA, serum samples were diluted to 1/50 for further titration. Alkaline phosphatase-conjugated, affinity-purified F(ab)2 fragment goat anti-human IgG (Jackson ImmunoResearch Lab) was used at a 1/3,500 dilution. p-Nitrophenyl phosphate was used as a substrate at a concentration of 1 mg/ml. The A405 value was measured with a reference wavelength of 650 nm against a substrate blank. The cutoff value was calculated using the optical density (OD) value of the negative controls (previously found to be HSV-negative by HSV-1 and HSV-2 Western blot) plus 0.2 OD U for the type-common, as well as for the gG-1, ELISA. In the gG-2 ELISA the OD value of high-titrating HSV-1-positive sera plus 0.1 OD U was used as a cutoff value. When comparing the two groups, the netto-absorbance (absorbance minus background, which was bovine serum albumin) value was used, in order to give consideration to differences that might occur when developing the plates.
Indirect immunofluorescence.
To investigate whether subjects with HSV-1 IgG-negative sera experienced a primary infection, IgM antibodies were analyzed by an in-house immunofluorescence assay using HSV-2-infected GMK cells as antigen. The assay was performed as described previously (34).
ELISA with infected cells.
For screening of mutations within type-specific epitopes of gG-1 by the use of a MAb, an ELISA was performed with cells infected with the different virus strains. GMK cells were grown on 96-well plates in Eagle's minimal essential medium supplemented with 1% PEST. The cells were infected with the HSV-1 strains at an infectious dose of 106 PFU/ml. The anti-gG-1 MAb was used at a dilution of 1:500, and the MAbs B1C1B4 (reactive with gC-1) and O1C5B2 (reactive with gG-2) were used at a dilution of 1:50. As conjugate for the MAbs, alkaline phosphate- conjugated F(ab)2 goat anti-mouse IgG was used at a dilution of 1:2,000 (Jackson ImmunoResearch Lab). As the substrate, p-nitrophenylphosphate was used at a concentration of 1 mg/ml. The A405 value was measured with a reference wavelength of 650 nm against a substrate blank.
DNA sequencing of the gG-1 gene.
Viral DNA was prepared from stock viruses by using the QIAmp blood kit (Qiagen) method, and the DNA was subjected to PCR amplification. Three overlapping oligonucleotide pairs were used as primers for complete gG-1 gene sequencing as described previously (26). Since our previous studies (26, 34) showed that the immunodominant region, as well as most of the mutations, was located within the region amplified by the second primer pair, this part was selected for sequencing of all isolates with the exception of three strains. In addition, a group of consecutively received isolates was chosen for sequencing of the complete gG-1 gene, preferentially those for which a corresponding serum sample was collected. Amplified products were separated on a 1% agarose gel prior to extraction of the amplicon bands with the QIAquick gel extraction kit (Qiagen). PCR cycle sequencing was carried out by using the fluorescence-labeled stop nucleotides with dRhodamine terminator cycle sequencing ready reaction kit (Applied Biosystems). The sequencing reaction was carried out in both sense and antisense directions for confirmation and also to act as an internal control. After precipitation with ethanol, the labeled samples were analyzed on an automated sequencer (ABI Prism 310 genetic analyzer; Applied Biosystems). In addition, DNA sequence data from the gG-1 gene in 10 HSV-1 strains from a previous study (26) were included for comparison of deduced amino acid sequences.
Phylogenetic analysis.
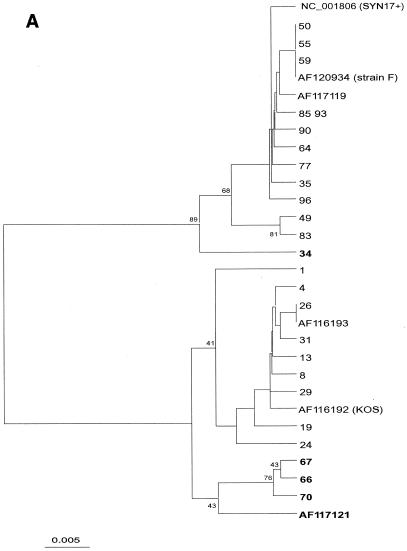
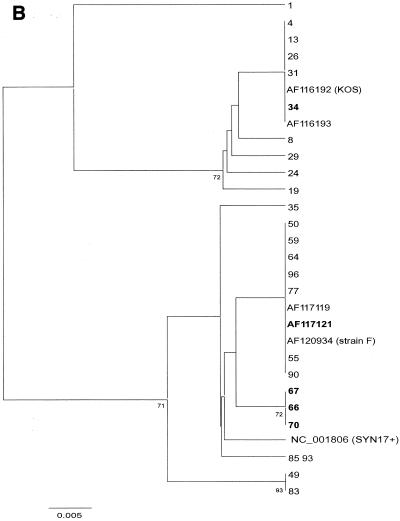
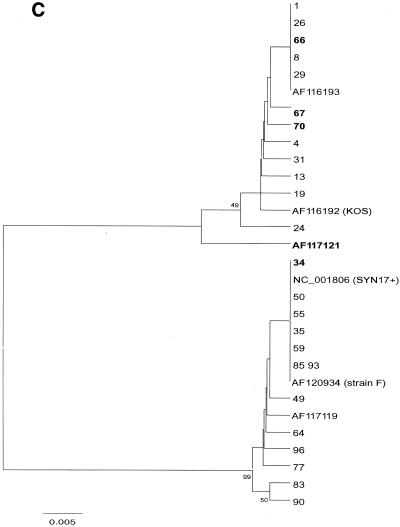
After alignment of 105 sequences from the present study and 10 database sequences (including KOS321, F, and Syn17+), phylogenetic comparison was done, after bootstrapping to 500 replicates, by distance matrix/UPGMA analysis using the MEGA2 software package available at http://www.megasoftware.net/. Trees were first constructed by comparison of an entire sequence (nucleotides [nt] 136950 to 137238 in Syn17+ according to GenBank) (Fig. 2A). For evaluation of recombination, separate trees comparing two parts, nt 1 to 100 and nt 101 to 288, was also done (Fig. 2B and C).
FIG. 2.
(A to C) Phylogenetic trees created by distance matrix/UPGMA analysis using MEGA version 2.0. Bootstrap values above 70 are displayed. The phylogenetic trees included 30 unique sequences after comparison of all 115 gG-1 sequences. The AF120934 (strain F) sequence was found in 35 further samples, NC_001806 (Syn17+) was found in 18 samples, AF116193 was found in 25 samples, 66 was found in 3 samples, and 49, 55, 64, and 72 were found in 1 additional sample each. The trees in panel A are based on the whole 288-nt sequence. Panels B and C were dated by comparing the segments 1 to 100 and 101 to 288 separately to evaluate suspected recombination in five strains (34, 66, 67, 70, and AF117121), denoted in bold.
Statistical analysis.
Statistical analyses were performed by Student's t test.
Nucleotide sequence accession numbers.
The nucleotide sequences of the viruses were submitted to GenBank and given the accession numbers AF513114 to AF513218.
RESULTS
Reactivity of an anti-gG-1 MAb to clinical HSV-1 strains.
When the 108 HSV-1 isolates were investigated for reactivity using an anti-gG-1-specific MAb by ELISA on infected GMK cells, 42 clinical isolates showed no reactivity, and these strains were derived from different anatomical sites (Table 1). In contrast, all isolates were reactive with the anti-gC-1 MAb, and none were reactive with the anti-gG-2 MAb. As a likely explanation, all of the 42 HSV-1 strains contained nonsynonymous mutations (see below) within the immunodominant region harboring the epitope region of the anti-gG-1 MAb, including the amino acid F111→V mutation situated within the previously mapped AFPL epitope of the MAb (34). Where the sex of the patient could be identified, 54 strains were isolated from females and 22 were isolated from male hosts, but the reactivity to the anti-gG-1 MAb was unrelated to gender. Of the two patients who each had contributed two strains, one harbored two MAb-negative isolates while the other presented two MAb-positive isolates.
DNA sequence variations within the gG-1 gene.
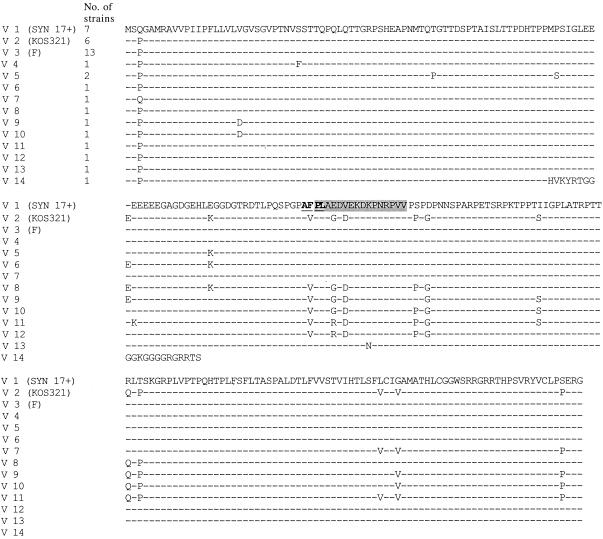
To more accurately define the extent of genetic variability among the MAb-positive and MAb-negative isolates, nucleotide sequences were determined for the middle part (106 amino acids) of the gG-1 gene including the immunodominant region and the type-specific epitope region for the anti-gG-1 MAb in 105 of the 108 strains (Fig. 1). The results from DNA sequencing showed that all 42 clinical isolates which were unreactive with the anti-gG-1 MAb showed mutations similar to those that we observed in a previous study (26) for strain KOS 321, which in concordance also was unreactive to the anti-gG-1 MAb. The results, including those from phylogenetic comparison, showed that two main genetic variants of the gG-1 gene exist among clinical and laboratory HSV-1 strains in the Western world (Fig. 2A and 3).
FIG. 3.
Aligned deduced amino acid sequences of the complete gG-1 gene for 14 variants discovered in 38 clinical and reference strains of which 13 DNA sequences were included from a previously published report (26). The number of isolates corresponding to each genetic variant is given to the left. The proposed immunodominant region of gG-1 is shaded, and the epitope of the anti-gG-1 MAb reactivity is depicted in boldface type and underlined.
The complete gG-1 gene was sequenced in 25 consecutively received isolates, 21 strains of which corresponding sera were included in the serological assays. Sequence alignment of these and previously reported sequences (26) (Fig. 3) suggested the existence of two genetic groups of HSV-1 strains, one represented by KOS321 and the other by Syn17+. This interpretation was supported by high bootstrap values in the phylogenetic tree based on 115 sequences from the middle part of the gG-1 gene (Fig. 2A). When five putative recombinants were excluded, the bootstrap value was in fact 100% (not shown). The nucleotide difference between the groups was 6.9%, compared to 1.0 and 0.7% within the KOS321 and Syn17+ groups, respectively.
From the alignment it was apparent that sequence 34 (Fig. 2 A to C) was similar to Syn17+ in the first part of the sequence (nt 1 to 100) but similar to KOS321 in the second part (nt 101 to 288). The converse was observed for sequences 66, 67, 70, and AF1177121. Phylogenetic analysis of the two segments (Fig. 2B to C) further supported that these sequences represent recombination, with sequence 34 showing a Syn17+-KOS pattern and 66, 67, 70, and AF117121 showing a KOS-Syn17+ pattern.
Detection of gG-1-negative virus due to a single frameshift mutation.
During the sequencing of the 42 HSV-1 MAb-negative strains, one isolate was detected that harbored a single frameshift mutation within the gG-1 gene with a subsequent lack of expression of the type-specific gG-1 epitope. A single insertion of a cytosine nucleotide within a stretch of six cytosines at the position consisting of nt 211 to 216 (amino acids 71 to 72) was observed, which resulted in a different reading frame and a premature stop codon at position 91 (Fig. 3). Interestingly, the serum sample drawn from the patient harboring the gG-1-negative isolate showed the presence of IgG antibodies reactive with the gG-1 antigen at a titer of 1/800. Furthermore, this strain was isolated from the genital region.
ELISA assay.
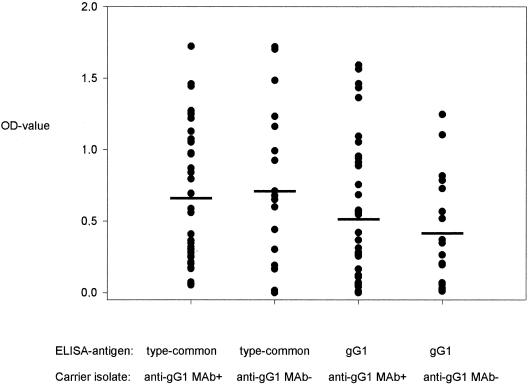
Fifty-three serum samples obtained from patients from which 55 HSV-1 strains were isolated were investigated in this study, and the OD values are shown in Fig. 4. Of these serum samples, 36 sera corresponded to anti-gG-1 MAb-reactive strains and 17 sera corresponded to MAb-unreactive isolates, respectively (Fig. 1). In total, 10 sera were negative in the type-specific gG-1 ELISA, of which 7 sera were derived from patients harboring MAb-reactive isolates and 3 sera were derived from patients carrying MAb-unreactive strains. This seronegativity to gG-1 was considered to be due to samples being collected during early seroconversion in seven cases. Of these, two sera were HSV IgM-positive by immunofluorescence. The immunofluorescence test is based on HSV-2 and has a lower sensitivity to HSV-1, which could explain why the test was negative with the other sera. Two sera were reactive in a low titer in the type-common HSV test, which sometimes shows early reactivity, and three additional patients had primary genital HSV infection as judged clinically. Of the three remaining patients with gG-1-seronegative sera, one carried a MAb-reactive isolate and two carried MAb-unreactive isolates. The remaining 43 sera were all positive in the type-common and HSV-1 type-specific gG-1 ELISA. Only two sera were positive in the HSV-2 type-specific ELISA.
FIG. 4.
Comparison of OD values in ELISA for sera drawn from patients harboring MAb-negative or MAb-positive isolates using a type-common HSV-antigen and an HSV-1 type-specific gG-1 antigen. Data are expressed as the mean of two observations. Each mean value for the two groups of sera (those drawn from patients with anti-gG-1 MAb+ or anti-gG-1MAb− strains) is indicated by a horizontal bar and did not differ significantly in either of the two serological assays when analyzed with Student's t test.
In the HSV type-common seroassay, mean OD values ± standard deviations were 0.68 ± 0.47 in sera from patients harboring anti-gG-1 MAb-reactive isolates versus 0.73 ± 0.56 in sera from hosts of anti-gG-1 MAb-unreactive isolates (Fig. 4). In the HSV type-specific gG-1 seroassay (gG-1), mean OD values ± standard deviations were 0.55 ± 0.50 in sera from patients harboring anti-gG-1 MAb-reactive isolates versus 0.43 ± 0.38 in sera from hosts of anti-gG-1 MAb-unreactive isolates. None of these differences in OD values between the two groups were statistically significant.
DISCUSSION
To ensure a firm base for the use of gG-1 as a HSV-1 type-specific antigen, we here determined the genetic variability of the gG-1 gene in a large number of clinical isolates. A recently characterized immunodominant region of the protein overlapping the epitope of an HSV-1 type-specific anti-gG-1 MAb (34) was therefore of special interest. Previously, we found several mutations within this region when the gG-1 gene was sequenced in a limited number of HSV-1 isolates (26), and reactivity to an anti-gG-1 MAb was early reported by Ackermann et al. to differ among type 1 strains (1). Here, we utilized a type-specific anti-gG-1-MAb, reactive with amino acids 110AFPL113, for screening of gG-1 variants among 108 clinical isolates. Altogether, 42 (38%) of the clinical isolates showed no reactivity to the anti-gG-1 MAb, and by DNA sequencing and phylogenetic comparison we observed two main genetic variants of the gG-1 gene that correlated to the absence or presence of MAb reactivity.
Since these two disparate genetic variant sequences of gG-1 resulted in up to 14 amino acid alterations (see below) and some were localized to the immunodominant region, we suspected that the two variants might also differ in their induction of IgG antibody response to gG-1. If this was the case, one could expect different serological reactions between the two groups in the HSV-1 type-specific ELISA based on gG-1 antigen currently in use (26). This could be expressed as a low sensitivity in serotesting of patients harboring strains carrying one of the other gG-1 gene variants if the antigen used as a diagnostic tool is based on the sequence of the other genetic variant. A low sensitivity of gG-1-based ELISA kits in clinical settings has been described (2, 14).
To investigate this, we compared the serological reactions in sera from patients infected with isolates representing either variant of gG-1. However, no differences in IgG reactivity were found when sera were tested by the gG-1 ELISA. In addition, only 3 of the 10 sera that were found to be negative in type-specific gG-1 ELISA were drawn from patients harboring MAb-negative isolates. Instead, a possible explanation for the seronegativity to gG-1 could be that these sera were collected early during infection before seroconversion had occurred. In fact, most of the gG-1-seronegative patients showed symptoms and/or serological signs of primary HSV-1 infection. Hence, we suggest that early infection might be a factor contributing to the reported low sensitivity to gG-1 antigen-based ELISA. The finding that only two of the sera were reactive to the gG-2 antigen (lower than expected from seroepidemiological studies [10]) could be explained by the large proportion of sera derived from what was considered to be primary HSV-1 infections. Furthermore, despite the fact that most of the corresponding viral isolates (28 of 53) were derived from the genital tract, they were typed as HSV-1. A recent epidemiological report from our geographical area has shown that genital HSV-1 infections are increasing (20), thus explaining why a lower rate of HSV-2 seropositivity might be encountered in such a selected patient group.
The clinical HSV-1 strains described herein were isolated from a number of different anatomical sites, such as the lip, ear, eye, other facial regions, genital or perianal regions, and the nervous system. Both genetic variants were proportionally isolated from females and males and were present at all of these different body regions with the possible exception of extralabial and facial manifestations, where none of five isolates were MAb negative. Thus, neither of the two variants could be associated with anatomical site or gender.
The dichotomy of the gG-1 DNA sequences found among clinical isolates was also present when reference strains originating from United States and United Kingdom, commonly used for experimental work, were sequenced and included in the phylogenetic analysis. The anti-gG-1 MAb-negative genetic variant was represented by KOS321 as previously described (26), while both the Syn17+ and F strains were found to belong to the MAb-positive branch of the phylogenetic tree. As a consequence, 14 of 238 (∼6%) of the encoded amino acid residues within gG-1 might differ between reference strains selected as prototype viruses for diagnostic or functional studies. Thus, by using either of Syn 17+ or F together with KOS321 for studies of expression and function of the gG-1 gene, the genetic diversity among clinical isolates is better represented in the experimental setting (3, 34).
Documentation of genetic variability among clinical HSV isolates is limited and was hitherto performed mostly by RE analysis (28, 35). In comparison, a huge amount of data is available at the nucleotide level for several RNA viruses such as HIV and hepatitis C virus (8, 13). However, genetic variability in specific genes of HSV-1, such as the gB-1 gene (30) and the gD-1 gene (27), after PCR amplification performed on clinical samples of cerebrospinal fluid has been reported but could not link any genetic variants to the clinical presentation in the form of encephalitis. As regards HSV-2 (22), sequence data, derived from clinical isolates, on genes encoding envelope glycoproteins such as gB, gC, gD or gG have been presented (16, 18, 32). Taken together, these studies showed the occurrence of point mutations, which were mostly silent and coded for insertion or deletion of one or a few amino acids. However, no correlation was found between clinical conditions and genetic variants.
From the results of the sequencing of the gG-1 gene, we observed additional mechanisms for generating genetic variability among the isolates: several possible recombinants of the two genetic variants have been described here as well as a frameshift mutant that most likely resulted in a gG-1-negative phenotype. The latter strain was unreactive to the anti-gG-1 MAb but showed only a single insertion of a cytosine nucleotide within a stretch of six cytosines at the N-terminal part of the gG-1 gene, resulting in a different reading frame and a premature stop codon. A similar mechanism explaining a gG-2-negative phenotype in several HSV-2 isolates was also recently described from our laboratory (16).
Sequence alignment suggested that five sequences might represent recombination of two segments of gG-1. Sequence 34 appeared to be a recombinant with a Syn17+-KOS321 pattern. Conversely, the sequences 66, 67, 70, and AF117121 showed a KOS321-Syn17+ pattern, apparently with the recombination point occurring at the same position or a nearby position. Phylogenetic analysis carried out for each segment of the gG-1 gene further supported recombination (Fig. 2B and C). In all, 4% (5 of 115) of the strains from clinical samples showed signs of recombination, indicating that this event is relatively frequent and also that these recombinants are viable and circulate in the population. Although recombinants for genes coding for glycoproteins among clinical HSV-1 isolates have not, to our knowledge, been described previously, homologous recombination of the gB gene was reported after coinfection of mice with two different HSV-1 strains (19). Furthermore, a study of CMV DNA in clinical samples has shown that homologous recombination of the gB gene has occurred and exists in several variants (11).
In conclusion, the earlier-described variability of the gG-1 DNA sequence among clinical isolates and reference strains (26) was here confirmed and further defined as a dichotomy in a large study of HSV-1 clinical isolates. Ongoing studies are investigating whether this form of genetic diversity is also present in other HSV-1 genes. Based on the hypothesis that HSV-1 coevolves with the human host, a DNA sequence comparison with strains isolated from subjects in distant parts of the world would be of interest(35). Furthermore, recent studies relating gG-1 to apical entry of polarized cells (33) raise the question of whether the two gG-1 variants differ in their function when contributing to HSV-1 infectivity.
Acknowledgments
Financial support was received from the Swedish Medical Research Council (grant no. 11225), the LUA foundation at Sahlgrenska University Hospital in Göteborg, the Medical Society of Göteborg, and the Swedish Society for Medical Research.
We greatly appreciate the excellent technical assistance of Volda Gabro.
REFERENCES
- 1.Ackermann, M., R. Longnecker, B. Roizman, and L. Pereira. 1986. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology 150:207-220. [DOI] [PubMed]
- 2.Ashley, R. L., L. Wu, J. W. Pickering, M. C. Tu, and L. Schnorenberg. 1998. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J. Clin. Microbiol. 36:294-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic, N., G. Rodger, J. Arthur, and A. C. Minson. 1999. A study of primary neuronal infection by mutants of herpes simplex virus type 1 lacking dispensable and non-dispensable glycoproteins. J. Gen. Virol. 80:2403-2409. [DOI] [PubMed] [Google Scholar]
- 4.Bergström, T., E. Sjögren-Jansson, S. Jeansson, and E. Lycke. 1992. Mapping neuroinvasiveness of the herpes simplex virus encephalitis-inducing strain 2762 by the use of monoclonal antibodies. Mol. Cell. Probes 6:42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergström, T., and E. Trybala. 1996. Antigenic differences between HSV-1 and HSV-2 glycoproteins and their importance for type-specific Serology. Intervirology 39:176-184. [DOI] [PubMed] [Google Scholar]
- 6.Corey, L. 1994. The current trend in genital herpes. Progress in prevention. Sex. Transm. Dis. 21:S38-S44. [PubMed] [Google Scholar]
- 7.Corey, L. 1998. Raising the consciousness for identifying and controlling viral STDs: fears and frustrations. STD 25:58-69. [DOI] [PubMed] [Google Scholar]
- 8.Drosopoulos, W. C., L. F. Rezende, M. A. Wainberg, and V. R. Prasad. 1998. Virtues of being faithful: can we limit the genetic variation in human immunodeficiency virus? J. Mol. Med. 76:604-612. [DOI] [PubMed] [Google Scholar]
- 9.Elion, G. B. 1982. Mechanism of action and selectivity of acyclovir. Am. J. Med. 73:7-13. [DOI] [PubMed] [Google Scholar]
- 10.Forsgren, M., E. Skoog, S. Jeansson, S. Olofsson, and J. Giesecke. 1994. Prevalence of antibodies to herpes simplex virus in pregnant women in Stockholm in 1969, 1983 and 1989: implications for STD epidemiology. Int. J. STD AIDS 5:113-116. [DOI] [PubMed] [Google Scholar]
- 11.Haberland, M., U. Meyer-König, and F. T. Hufert. 1999. Variation within the glycoprotein B gene of human cytomegalovirus is due to homologous recombination. J. Gen. Virol. 48:1-23. [DOI] [PubMed] [Google Scholar]
- 12.Jeansson, S., M. Forsgren, and B. Svennerholm. 1983. Evaluation of solubilized herpes simplex virus membrane antigen by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 18:1160-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato, N., Y. Ootsuyama, H. Sekiya, S. Ohkoshi, T. M. Nakazawa, and K. Shimotohno. 1994. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J. Virol. 68:4776-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, F. K., L. Pereira, C. Griffin, E. Reid, and A. J. Nahmias. 1986. A novel glycoprotein for detection of herpes simplex virus type 1-specific antibodies. J. Virol. Methods 14:111-118. [DOI] [PubMed] [Google Scholar]
- 15.Liljeqvist, J. Å., E. Trybala, B. Svennerholm, S. Jeansson, E. Sjögren-Jansson, and T. Bergström. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G by human and mouse antibodies. J. Gen. Virol. 79:1215-1224. [DOI] [PubMed] [Google Scholar]
- 16.Liljeqvist, J. Å., B. Svennerholm, and T. Bergström. 1999. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J. Clin. Virol. 73:9796-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liljeqvist, J. Å., B. Svennerholm, and T. Bergström. 1999. Typing of clinical herpes simplex virus type 1 and 2 isolates with monoclonal antibodies. J. Clin. Microbiol. 37:2717-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liljeqvist, J. Å., B. Svennerholm, and T. Bergström. 2000. Conservation of type-specific B-cell epitopes of glycoprotein G in clinical herpes simplex virus type 2 isolates. J. Clin. Microbiol. 38:4517-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingen, M., F. Hengerer, and D. Falke. 1997. Mixed vaginal infections of Balb/c mice with low virulent herpes simplex type 1 strains result in restoration of virulence properties: vaginitis/vulvitis and neuroinvasiveness. Med. Microbiol. Immunol. 185:217-222. [DOI] [PubMed] [Google Scholar]
- 20.Löwhagen, G. B., P. Tunbäck, K. Andersson, T. Bergström, and G. Johannisson. 2000. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex. Transm. Infect. 76:179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeoch, D. J., A. Dolan, S. Dolan, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch, D. J., H. W. Moss, D. McNab, and M. C. Frame. 1987. DNA sequence and genetic content of the Hind III 1 region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J. Gen. Virol. 68:749-764. [DOI] [PubMed] [Google Scholar]
- 23.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiological and sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69:19-36. [PubMed] [Google Scholar]
- 24.Nilheden, E., S. Jeansson, and A. Vahlne. 1983. Typing of herpes simplex virus by an enzyme-linked immunosorbent assay with monoclonal antibodies. J. Clin. Microbiol. 17:677-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olofsson, S., M. Lundstrom, H. Marsden, S. Jeansson, and A. Vahlne,. 1986. Characterization of a herpes simplex virus type 2-specified glycoprotein with affinity for N-acetylgalactosamine-specific lectins and its identification as g92K or gG. J. Gen. Virol. 67:737-744. [DOI] [PubMed] [Google Scholar]
- 26.Rekabdar, E., P. Tunback, J. A. Liljeqvist, and T. Bergstrom. 1999. Variability of the glycoprotein G gene in clinical isolates of herpes simplex virus type 1. Clin. Diagn. Lab. Immunol. 6:826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozenberg, F., and P. Lebon. 1996. Analysis of herpes simplex virus type 1 glycoprotein D nucleotide sequence in human herpes simplex encephalitis. J. Neurovirol. 2:289-295. [DOI] [PubMed] [Google Scholar]
- 28.Sakaoka, H., K. Kurita, Y. Lida, S. Takada, K. Umene, Y. T. Kim, C. S. Ren, and A. J. Nahmias. 1994. Quantitative analysis of genomic polymorphism of herpes simplex virus type 1 strains from six countries: studies of molecular evolution and molecular epidemiology of the virus. J. Gen. Virol. 75:513-527. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Martinez, D., D. S. Schmid, W. Whittington, D. Brown, W. C. Reeves, S. Chatterjee, R. J. Whitley, and P. E. Pellet. 1991. Evalution of a test based on baculovirus-expressed glycoprotein G for detection of herpes simplex virus type-specific antibodies. J. Infect. Dis. 164:1196-1199. [DOI] [PubMed] [Google Scholar]
- 30.Sivadon, V., P. Lebon, and F. Rozenberg. 1998. Variations of HSV-1 glycoprotein B in human herpes simplex encephalitis. J. Neurovirol. 4:106-114. [DOI] [PubMed] [Google Scholar]
- 31.Svennerholm, B., S. Olofsson, S. Jeansson, A. Vahlne, and E. Lycke. 1984. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J. Clin. Microbiol. 19:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terhune, S., K. T. Coleman, R. Sekulovich, R. L. Burke, and P. G. Spear. 1998. Limited variability of the glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J. Infect. Dis. 178:8-15. [DOI] [PubMed] [Google Scholar]
- 33.Tran, L. C., J. M. Kissner, L. E. Westerman, and A. E. Sears. 2000. A herpes simplex virus 1 recombinant lacking the glycoprotein G coding sequences is defective in entry through apical surfaces of polarized epithelial cells in culture and in vivo. Proc. Natl. Acad. Sci. USA 97:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tunbäck, P., J. Å. Liljeqvist, G. B. Löwhagen, and T. Bergström. 2000. Glycoprotein G of herpes simplex virus type 1: identification of type-specific epitopes by human antibodies. J. Gen. Virol. 81:1033-1040. [DOI] [PubMed] [Google Scholar]
- 35.Umene, K., and H. Sakaoka. 1999. Evolution of herpes simplex virus type 1 under herpesviral evolutionary processes. Arch. Virol. 144:637-656. [DOI] [PubMed] [Google Scholar]