Abstract
NCCLS document M38-P describes standard parameters for testing the fungistatic activities (MICs) of established agents against filamentous fungi (molds). This study evaluated the in vitro susceptibilities of 15 Aspergillus flavus isolates, 62 A. fumigatus isolates, and 10 isolates each of A. niger, A. nidulans, and A. terreus to voriconazole, posaconazole, itraconazole, and amphotericin B by the E-test and NCCLS M38-P microdilution methods. The agreement (within 3 dilutions) between methods for voriconazole was independent of the E-test incubation time (93.3 to 100% for four of five species at both incubation times). In contrast, with amphotericin B, itraconazole, and posaconazole, E-test results were more dependent on the incubation time for certain species. For A. fumigatus, posaconazole E-test MICs had better concordance with reference values after 48 h (95.2%) than after 24 h (90%), while the highest agreement for itraconazole MICs was after 24 h (90.3 versus 74.2%) of incubation. Better agreement between the methods was also obtained with 24-h E-test amphotericin B MICs for A. flavus (73.3 versus 26.7%) and A. fumigatus (96.7 versus 64.5%). E-test MICs of the four agents had the lowest percentages of agreement with reference values for A. nidulans (60 to 80%). For isolates for which high MICs were obtained for the four agents by the reference method, high MICs were also obtained by E-test at both 24 and 48 h. The utility of in vitro results of either the E-test or the NCCLS broth microdilution (M38-P) method for Aspergillus spp. needs to be established in clinical trials.
Since the 1980s, a higher incidence of infections caused by filamentous fungi (molds) has been documented, especially in immunocompromised hosts (2, 12-15, 24, 27). Aspergillus fumigatus is responsible for the majority (85 to 90%) of different clinical manifestations of severe mold infections (4); however, other species of Aspergillus have become important emerging pathogens (12-14, 27). Paralleling the increasing incidence of fungal infection has been the development of new triazoles and echinocandins. Among these, voriconazole and posaconazole are new triazoles that have potent activity against both yeast spp. and molds. Because the number of serious infections caused by Aspergillus spp. has increased and resistance to established agents has been documented (4, 12, 15, 25, 27), determination of the in vitro susceptibilities of Aspergillus isolates to both established and investigational agents is warranted.
The National Committee for Clinical Laboratory Standards (NCCLS) Subcommittee on Antifungal Susceptibility Tests has proposed a standard procedure for antifungal susceptibility testing of molds (NCCLS document M38-P [17]). Although development of reference procedures for yeasts (18) and molds (17) was essential for standardization of results, these methods are cumbersome and time-consuming; other approaches have been evaluated for fungal testing in recent years. Among these approaches, E-test has been suggested as an alternative procedure for antifungal susceptibility testing of yeasts (8, 16, 20, 22, 23, 28), and more recently, this method has been evaluated for certain molds (6, 21, 26). Although the in vitro activities of both voriconazole and posaconazole have been evaluated by NCCLS broth dilution methods (5, 10), no comparison of the E-test procedure with the broth microdilution M38-P method (17) for testing Aspergillus spp. has been conducted. This study evaluated the activities of voriconazole, posaconazole, itraconazole, and amphotericin B by E-test against 107 Aspergillus isolates recovered from clinical specimens during the past 5 years. E-test MICs were compared to those obtained by the NCCLS M38-P broth microdilution method for filamentous fungi (17).
(Part of this work was presented at the 39th and 40th Interscience Conferences on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada,17 to 20 September 2000, and Chicago, Ill., 16 to 19 December 2001, respectively.)
MATERIALS AND METHODS
Isolates.
The set of 107 isolates evaluated included 15 Aspergillus flavus isolates, 62 A. fumigatus isolates, and 10 isolates each of A. nidulans, A. niger, and A. terreus; high MICs of itraconazole (isolates NCPF 7100, NCPF 7099, MMRL A83, and MMRL A130), posaconazole (isolates NCPF 7100 and MMRL A130), and voriconazole (isolate MMRL A130) have been documented (4, 10, 19). Each isolate originated from a different patient and was received at the Medical Mycology Research Laboratory (MMRL), Medical College of Virginia Campus, Virginia Commonwealth University, for MIC testing during the past 5 years. Isolates were maintained at −70°C until testing was performed. The reference isolate A. flavus ATCC 204304 (17) and the quality control (QC) strain Candida parapsilosis ATCC 22019 (18) were included as control isolates for both the NCCLS and E-test methods. The QC isolate Candida krusei ATCC 6258 was tested by E-test five times to obtain further reproducibility data; all controls were tested at least five times by E-test. The agents evaluated in this study have well-established microdilution MIC ranges for these QC strains (1), and preliminary E-test MIC ranges of the reference agents have been determined for these strains as well (AB Biodisk, Solna, Sweden). MIC ranges of amphotericin B and itraconazole have also been established for the A. flavus isolate ATCC 204304 (9, 17). MICs of amphotericin B and itraconazole were within expected ranges (1, 9,17, 18).
Antifungal agents.
E-test gradient strips of voriconazole, posaconazole, itraconazole, and amphotericin B were obtained from AB Biodisk. The concentration gradient for each drug ranged from 32 to 0.004 μg/ml. Amphotericin B (Bristol Myers Squibb Pharmaceutical Research Institute, Wallingford, Conn.), itraconazole (Janssen Pharmaceutica, Titusville, N.J.), voriconazole (Pfizer Inc., Central Research Division, Sandwich, United Kingdom), and posaconazole (Schering-Plough Research Institute, Kenilworth, N.J.) were provided by the manufacturers as assay powders. As described in NCCLS document M38-P (17), additive drug dilutions were prepared to yield twice the final strength required for the test. The drugs at their final concentrations (8 to 0.0078 μg/ml) for testing by the M38-P method were frozen at −70°C until needed.
Procedures.
Stock inoculum suspensions were prepared as described in NCCLS document M38-P (17) from 7-day-old cultures grown on potato dextrose agar slants at 35°C and adjusted spectrophotometrically to optical densities that ranged from 0.09 to 0.11 (82 to 80% transmittance). The final concentrations of the stock inoculum suspensions ranged from 0.5 × 106 to 4.5 × 106 CFU/ml, as demonstrated by quantitative colony counts on Sabouraud dextrose agar.
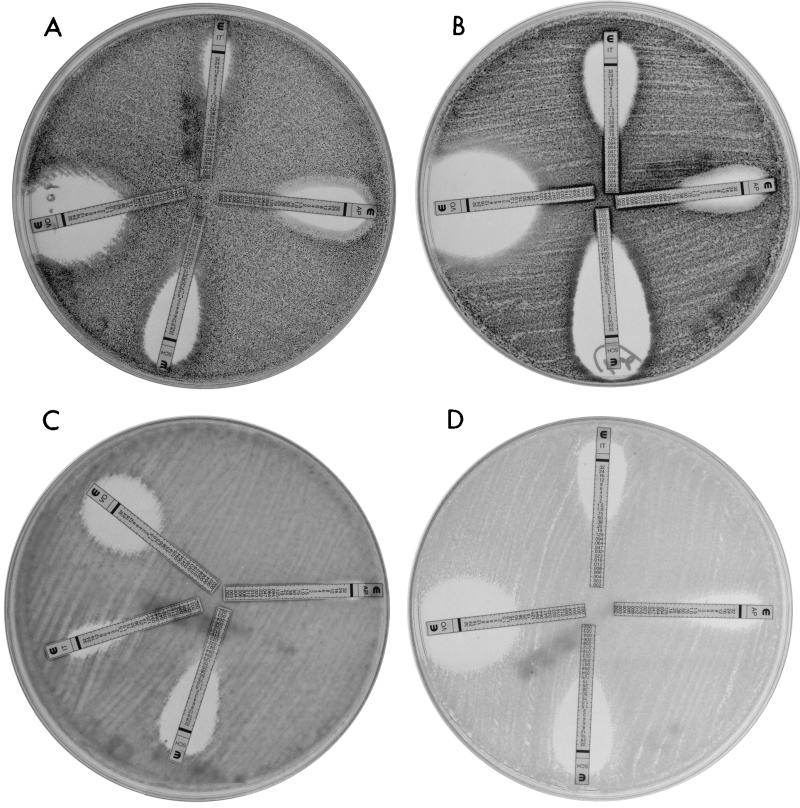
E-test was performed according to the manufacturer's instructions by using solidified RPMI 1640 medium with 2% dextrose (Remel, Lenexa, Kans.). Briefly, each 150-mm petri plate containing 60 ml of solidified medium was inoculated with 400 μl of the respective undiluted stock inoculum. Plates were incubated at 35°C, and MICs were determined following incubation times of 24 and 48 h. The E-test MIC was the lowest drug concentration at which the border of the elliptical inhibition intercepted the scale on the antifungal strip (Fig. 1).
FIG. 1.
Clear E-test growth inhibition ellipses for voriconazole (strip VO), posaconazole (strip SCH), itraconazole (strip IT), and amphotericin B (strip AP) at 48 h. (A) A. niger; (B) A. fumigatus; (C) A. terreus; (D) the QC strain C. krusei ATCC 6258.
For the NCCLS broth microdilution method (document M38-P), each microdilution well containing 100 μl of the (twofold) diluted drug concentration was inoculated with 100 μl of the (twofold) diluted conidial inoculum suspension (final volume in each well, 200 μl). Microdilution trays were incubated at 35°C and examined at 48 h for MIC determination. MICs were determined by visual inspection as described in NCCLS document M38-P (17) for complete, or 100%, growth inhibition (10). C. parapsilosis ATCC 22019 and A. flavus ATCC 204304 were tested each time a set of isolates was evaluated by both methods.
Data analyses.
Because the E-test strips contain a continuous gradient instead of an established twofold drug dilution schema, E-test MICs were elevated to the next twofold dilution concentration matching the drug dilution schema of the NCCLS M38-P method (8 to 0.0078 μg/ml). Elevation of E-test MICs facilitated the comparisons and presentation of results. Both on-scale and off-scale MICs were included in the analysis. As analyzed in previous studies (6-10), discrepancies between MIC end points of no more than 3 dilutions (e.g., 0.5, 1.0, and 2 μg/ml) were used to calculate the percent agreement. E-test and NCCLS MIC ranges and corresponding geometric mean values were obtained for each species-drug combination. E-test and NCCLS MIC90s (MICs at which 90% of isolates were inhibited) were also determined.
RESULTS AND DISCUSSION
Our study represents the first evaluation of the suitability of E-test for determining the susceptibilities of Aspergillus isolates to two new triazoles, voriconazole and posaconazole. Voriconazole and posaconazole data for the QC strain C. krusei by E-test were within the accepted ranges (Fig. 1D). Posaconazole E-test MICs for the QC isolate C. parapsilosis were 1 dilution lower (0.03 to 0.06 μg/ml) than the range established for this isolate (0.06 to 0.25 μg/ml), while voriconazole results were within the established range (0.03 to 0.12 μg/ml) (1). Results for the reference isolate A. flavus also were within acceptable ranges on 5 different days (e.g., 0.25 to 0.5 μg/ml and 0.01 to 0.06 μg/ml for voriconazole and posaconazole, respectively). In addition, isolates for which high MICs were obtained had the same value or values within 1 dilution upon repetitive testing by both procedures. These preliminary results indicate the potential reproducibility of E-test for testing isolates of Aspergillus spp.
In our study, the solidified RPMI 1640 medium supported growth of four of five Aspergillus species at both 24 and 48 h. The exceptions were isolates of A. terreus; at 24 h, five of these isolates did not grow well in RPMI 1640 broth, and seven did not grow well on solidified medium, but MICs could be determined at 48 h for all 107 isolates. Lack of growth at 24 h for A. terreus has been documented with RPMI 1640 broth (10). Trailing growth (presence of growth above the MIC) was not a major problem for any of the antifungal agents tested. All E-test inhibition ellipses were clear, but triazole ellipses, especially those of voriconazole, were wider than amphotericin B ellipses for most isolates (Fig. 1A to C). Clear E-test ellipses were expected, since reference MIC end points for all triazoles and amphotericin B corresponded to 100% growth inhibition (10, 17). In addition, it has been reported that solidified RPMI 1640 with 2% dextrose minimizes the trailing effect in testing of voriconazole and posaconazole by E-test against Candida spp. (22, 23).
Table 1 summarizes the in vitro susceptibilities of 107 isolates of Aspergillus spp. to voriconazole, posaconazole, itraconazole, and amphotericin B as determined by the E-test and NCCLS M38-P reference methods. Of the three triazoles, voriconazole had the least-variable E-test results between the two incubation times and the highest overall degree of agreement between the methods at both incubation times (93.8 and 96.3% for voriconazole versus 90.7 and 95.3% for posaconazole at 24 and 48 h, respectively). Although the agreement between methods was good to excellent for voriconazole and posaconazole (90.3 to 100% agreement between reference method MICs and 24- or 48-h E-test MICs), to a certain degree this was dependent on the species tested and the incubation time (Table 2). Prior valuations for the two new triazoles against Candida spp. by E-test also have demonstrated good reproducibility (≥91%) between E-test and M27-A results for most species tested (22, 23), as well as species dependency for posaconazole (23). Lower percentages (50 to 85%) of agreement were obtained for Candida guilliermondii, C. parapsilosis, and Candida lusitaniae (23).
TABLE 1.
MICs obtained by E-test and NCCLS broth microdilution (M38-P)a for 107 Aspergillus isolates
| Fungus (no. of isolates tested) | Antifungal agentb | Incubation time | MICc (μg/ml) by:
|
|||||
|---|---|---|---|---|---|---|---|---|
| E-test
|
NCCLS M38-P method
|
|||||||
| Range | G mean | MIC90 | Range | G mean | MIC90 | |||
| A. flavus (15) | V | 24 h | 0.12-0.25 | 0.17 | 0.25 | |||
| 48 h | 0.25-0.5 | 0.22 | 0.25 | 0.12-0.5 | 0.34 | 0.5 | ||
| P | 24 h | 0.03-0.12 | 0.07 | 0.12 | ||||
| 48 h | 0.06-0.25 | 0.14 | 0.25 | 0.03-0.12 | 0.06 | 0.12 | ||
| I | 24 h | 0.06-0.25 | 0.18 | 0.25 | ||||
| 48 h | 0.12-1.0 | 0.35 | 0.5 | 0.03-0.25 | 0.14 | 0.25 | ||
| A | 24 h | 0.25-8 | 3.4 | 8 | ||||
| 48 h | 0.5->8 | >8 | >8 | 0.5-4 | 1.26 | 2 | ||
| A. fumigatus (62) | V | 24 h | 0.03->8 | 0.32 | 0.5 | |||
| 48 h | 0.03->8 | 0.46 | 0.5 | 0.03-8 | 0.42 | 1.0 | ||
| P | 24 h | <0.01-4 | 0.17 | 0.25 | ||||
| 48 h | <0.01-4 | 0.2 | 0.25 | 0.01-0.5 | 0.07 | 0.12 | ||
| I | 24 h | 0.06-8 | 0.84 | 1 | ||||
| 48 h | 0.12->8 | 1.57 | 2 | 0.03->8 | 0.71 | 0.5 | ||
| A | 24 h | 0.25->8 | 1.34 | 2 | ||||
| 48 h | 0.25->8 | 5.5 | >8 | 0.25-4 | 1.1 | 2 | ||
| A. nidulans (10) | V | 24 h | 0.01-0.25 | 0.08 | 0.25 | |||
| 48 h | 0.01-0.25 | 0.08 | 0.25 | 0.03-1.0 | 0.26 | 0.5 | ||
| P | 24 h | 0.01-0.06 | 0.031 | 0.03 | ||||
| 48 h | 0.03-0.12 | 0.09 | 0.12 | 0.01-0.25 | 0.08 | 0.25 | ||
| I | 24 h | 0.06-0.25 | 0.12 | 0.25 | ||||
| 48 h | 0.06-1.0 | 0.23 | 0.5 | 0.06-0.5 | 0.18 | 0.5 | ||
| A | 24 h | 0.25-8 | 1.5 | 1 | ||||
| 48 h | 0.5->8 | 6.1 | >8 | 0.25-4 | 0.87 | 2 | ||
| A. niger (10) | V | 24 h | 0.06-0.25 | 0.12 | 0.25 | |||
| 48 h | 0.12-0.25 | 0.16 | 0.25 | 0.12-1.0 | 0.35 | 0.5 | ||
| P | 24 h | 0.01-0.25 | 0.07 | 0.12 | ||||
| 48 h | 0.12-0.25 | 0.17 | 0.25 | 0.06-0.25 | 0.09 | 0.12 | ||
| I | 24 h | 0.5 | 0.5 | 0.5 | ||||
| 48 h | 1.0 | 1.0 | 1.0 | 0.25-1.0 | 0.48 | 1.0 | ||
| A | 24 h | 0.5-1.0 | 0.75 | 1.0 | ||||
| 48 h | 1.0 | 1.0 | 1.0 | 0.5-1.0 | 0.7 | 1.0 | ||
| A. terreus (10) | V | 24 h | 0.25 | 0.25 | N/A | |||
| 48 h | 0.12-1.0 | 0.4 | 1.0 | 0.12-1.0 | 0.26 | 0.5 | ||
| P | 24 h | 0.01-0.06 | 0.028 | N/A | ||||
| 48 h | 0.01-0.06 | 0.035 | 0.06 | 0.03-0.06 | 0.033 | 0.03 | ||
| I | 24 h | 0.03-0.5 | 0.18 | N/A | ||||
| 48 h | 0.01-0.5 | 0.19 | 0.5 | 0.03-0.5 | 0.13 | 0.25 | ||
| A | 24 h | 0.25-4 | 2.05 | N/A | ||||
| 48 h | 0.5-8 | 2.85 | 4 | 0.5-4 | 1.7 | 4 | ||
For the NCCLS M38-P broth microdilution method, see reference 17.
V, voriconazole; P, posaconazole; I, itraconazole; A, amphotericin B.
G mean, geometric mean MIC. N/A, not applicable; five to seven isolates did not grow well at 24 h on either the solidified or the broth medium.
TABLE 2.
Distribution of differences in MICs for 107 Aspergillus isolates and percent agreement within 3 dilutions for the E-test and M38-P method
| Fungus (no. of isolates tested) | Antifungal agenta | Incubation time | No. of isolates with E-test MICs different from the NCCLS M38-P method MICs
|
% Agree- mentb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| >+2 | +2 | +1 | 0 | −1 | −2 | >−2 | ||||
| A. flavus (15) | V | 24 h | 3 | 6 | 2 | 3 | 1 | 93.3 | ||
| 48 h | 2 | 1 | 5 | 7 | 100 | |||||
| P | 24 h | 2 | 2 | 7 | 3 | 1 | 100 | |||
| 48 h | 7 | 5 | 1 | 1 | 1 | 100 | ||||
| I | 24 h | 3 | 5 | 4 | 3 | 100 | ||||
| 48 h | 7 | 4 | 4 | 100 | ||||||
| A | 24 h | 3 | 3 | 3 | 3 | 2 | 1 | 73.3 | ||
| 48 h | 11 | 3 | 1 | 26.7 | ||||||
| A. fumigatus (62) | V | 24 h | 4 | 26 | 27 | 1 | 2 | 2 | 96.8 | |
| 48 h | 1 | 6 | 30 | 22 | 1 | 2 | 98.3 | |||
| P | 24 h | 2 | 17 | 35 | 4 | 4 | 90.3 | |||
| 48 h | 2 | 3 | 34 | 14 | 7 | 1 | 1 | 95.2 | ||
| I | 24 h | 5 | 10 | 15 | 22 | 7 | 2 | 1 | 90.3 | |
| 48 h | 16 | 11 | 18 | 16 | 1 | 74.2 | ||||
| A | 24 h | 4 | 12 | 14 | 22 | 8 | 2 | 96.7 | ||
| 48 h | 22 | 15 | 7 | 18 | 64.5 | |||||
| A. nidulans (10) | V | 24 h | 1 | 1 | 1 | 2 | 2 | 3 | 70 | |
| 48 h | 1 | 1 | 2 | 1 | 2 | 3 | 70 | |||
| P | 24 h | 2 | 6 | 2 | 80 | |||||
| 48 h | 1 | 5 | 2 | 2 | 80 | |||||
| I | 24 h | 1 | 2 | 1 | 4 | 2 | 80 | |||
| 48 h | 2 | 1 | 5 | 2 | 80 | |||||
| A | 24 h | 1 | 2 | 2 | 3 | 1 | 1 | 80 | ||
| 48 h | 4 | 2 | 4 | 60 | ||||||
| A. niger (10) | V | 24 h | 1 | 7 | 2 | 100 | ||||
| 48 h | 1 | 3 | 5 | 1 | 100 | |||||
| P | 24 h | 1 | 3 | 3 | 2 | 1 | 90 | |||
| 48 h | 3 | 5 | 2 | 100 | ||||||
| I | 24 h | 1 | 9 | 100 | ||||||
| 48 h | 1 | 9 | 100 | |||||||
| A | 24 h | 2 | 5 | 3 | 100 | |||||
| 48 h | 3 | 7 | 100 | |||||||
| A. terreus (10)c | V | 24 h | N/A | |||||||
| 48 h | 6 | 1 | 3 | 100 | ||||||
| P | 24 h | N/A | ||||||||
| 48 h | 4 | 4 | 2 | 100 | ||||||
| I | 24 h | N/A | ||||||||
| 48 h | 1 | 2 | 1 | 2 | 1 | 3 | 90 | |||
| A | 24 h | N/A | ||||||||
| 48 h | 3 | 3 | 2 | 2 | 70 | |||||
| All isolates (107) | V | 24 h | 5 | 30 | 35 | 12 | 9 | 6 | 93.8 | |
| 48 h | 1 | 15 | 34 | 34 | 15 | 5 | 3 | 96.3 | ||
| P | 24 h | 2 | 2 | 5 | 33 | 41 | 7 | 7 | 90.7 | |
| 48 h | 2 | 14 | 53 | 23 | 10 | 2 | 3 | 95.3 | ||
| I | 24 h | 5 | 14 | 23 | 36 | 14 | 2 | 3 | 91.8 | |
| 48 h | 17 | 23 | 33 | 22 | 7 | 3 | 2 | 82.2 | ||
| A | 24 h | 5 | 9 | 17 | 24 | 28 | 11 | 3 | 91.7 | |
| 48 h | 38 | 20 | 9 | 20 | 20 | 64.5 | ||||
V, voriconazole; P, posaconazole; I, itraconazole; A, amphotericin B.
Percentage of MICs obtained by the two methods that were within 3 dilutions.
N/A, not applicable; five to six isolates did not grow well at 24 h.
Voriconazole E-test MICs were usually 1 dilution lower (or the same) than those obtained by the M38-P method and even lower for A. nidulans (geometric mean MICs, 0.08 versus 0.26 μg/ml). The exceptions were results for A. terreus, where E-test MICs tended to be slightly higher. On the other hand, most posaconazole E-test MICs were lower than (or the same as) reference MICs at 24 h but higher after 48 h of incubation. High posaconazole MICs have been documented for 2 of the 107 isolates (NCPF 7100 and MMRL A130) in previous studies (10, 19), and a decreased response to posaconazole treatment was demonstrated in a murine model of invasive aspergillosis with isolate NCPF 7100 as the infecting organism (19). In our study, the distinction between the in vitro activity of posaconazole against strains NCPF 7100 and MMRL A130 and that against all other isolates tested was more obvious by E-test after both 24 and 48 h (4 versus ≤0.2 μg/ml) than by the reference method (0.5 versus ≤ 0.2 μg/ml). Voriconazole E-test MICs of 8 μg/ml were determined several times for isolate MMRL A130, while corresponding reference method MICs were >8 μg/ml. These results suggest that both methods can identify Aspergillus sp. isolates that are potentially resistant to these two new triazoles.
Overall agreement between the two methods was lower for itraconazole MICs (82.2 to 91.8%) than for voriconazole and posaconazole MICs (90.7 to 96.3%) (Table 2). In contrast to that for posaconazole, agreement for itraconazole was higher between microdilution MICs and 24-h E-test results (91.8%) than between microdilution MICs and 48-h E-test results (82.2%). Itraconazole E-test MICs for A. fumigatus showed more-substantial shifting between the two incubation times (Table 1), and greater discrepancies in MICs (>2 dilutions) were found between the two methods with 48-h (16 isolates) than with 24-h (6 isolates) E-test results (Table 2). Pfaller et al. (21) and Szekely et al. (26) found that the percentages of agreement between the methods were similar for the two E-test incubation times. In agreement with our data (21), discrepancy between the methods was due in each instance to higher E-test MICs for A. fumigatus and A. flavus. The most important role of antifungal susceptibility testing is to detect potential resistance. The set of A. fumigatus isolates evaluated included four isolates (NCPF 7100, NCPF 7099, MMRL A130, and MMRL A83) for which itraconazole MICs are ≥8 μg/ml (4, 10). In the present study, corresponding E-test itraconazole MICs were 4 to ≥8 μg/ml at both incubation times. Isolates NCPF 7100 and NCPF 7099 were recovered from patients who failed appropriate itraconazole therapy.
As with itraconazole, the overall concordance between the methods for amphotericin B MICs was lower with 48-h (64.5%) than with 24-h (91.7%) E-test values, and overall agreement was greater (82.2 to 96.3%) for the triazoles than for amphotericin B (Table 2). When results for A. terreus were included in the comparison for amphotericin B, the overall agreement decreased from 91.7 to 89.7%. The reason for discrepancies was that E-test MICs were higher, especially at 48 h, than microdilution reference values. Although Szekely et al. (26) reported 100% agreement between E-test and microdilution amphotericin B results for A. terreus, their MICs were lower by both methods (MIC50, 1 μg/ml) than our results (Table 1). Low agreement (60%) between E-test and a microdilution method has also been reported for A. flavus with amphotericin B at 48 h (26). It appears that E-test amphotericin B MICs for Aspergillus spp. should be determined as soon as sufficient growth appears (before 48 h). However, the conundrum is, which incubation time would provide the most clinically relevant MIC? Death was attributed to disseminated aspergillosis in 27 of 62 patients, despite adequate treatment with amphotericin B (2, 11, 12, 15, 24), and in one of these reports (12), 6 of the 7 patients died of infections caused by A. flavus. Unfortunately, MICs were not reported for any of those infecting isolates (2, 12, 15, 24). Recently published literature regarding in vitro and clinical results also indicates that infections caused by A. terreus are refractory to treatment with amphotericin B and that in vitro resistance has been documented for this species (13, 14, 25). Future studies with our A. terreus and A. flavus isolates for which higher amphotericin B E-test MICs (Tables 1 and 2) were determined may reflect previous in vitro and clinical findings (12-14, 25). It is noteworthy that the E-test appeared to be superior to the NCCLS method for yeasts (18) in its ability to detect amphotericin B-resistant Candida spp. and Cryptococcus neoformans strains (3, 16, 28).
In conclusion, as has been demonstrated in this and other studies, the E-test has potential value for testing the susceptibilities of four of the five Aspergillus spp. evaluated to voriconazole and posaconazole. The lowest reproducibility between the methods was obtained for the activities of the four compounds against A. nidulans isolates (60 to 80%). One should keep in mind that, as for any agar antimicrobial susceptibility testing, the medium formulation and the depth of the agar can influence the MIC result. Therefore, the manufacturer's recommendations must be followed for E-test MICs. However, at present E-test strips are available for investigational use only. Collaborative studies should confirm the reproducibility of E-test results obtained in this single (our) laboratory. Evaluations of drug efficacy in experimental infections or clinical trials should provide a better assessment of the utility of both methods for use in the clinical laboratory as predictors of antifungal resistance in Aspergillus infections.
Acknowledgments
Many thanks to AB Biodisk for providing amphotericin B and itraconazole E-test strips and to Pfizer and Schering for providing voriconazole and posaconazole E-test strips.
REFERENCES
- 1.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chumpitazi, B. F., C. Pinel, B. Lebeau, P. Ambroise-Thomas, and R. Grillot. 2000. Aspergillus fumigatus antigen detection in sera from patients at risk for invasive aspergillosis. J. Clin. Microbiol. 38:438-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy, C., and M. H. Nguyen. 1999. Correlation between in vitro susceptibility determined by Etest and response to therapy with amphotericin B: results from a multicenter prospective study of candidemia. Antimicrob. Agents Chemother. 43:1289-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A. 2001. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J. Clin. Microbiol. 39:1360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A., K. Dawson, M. Pfaller, E. Anaissie, B. Breslin, D. Dixon, A. Fothergill, V. Paetznick, J. Peter, M. Rinaldi, and T. Walsh. 1995. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob. Agents Chemother. 39:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., M. Pfaller, M. E. Erwin, and R. N. Jones. 1996. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J. Clin. Microbiol. 34:848-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, C. Cooper, Jr., A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff, A., M. Bartlett, V. Chaturvedi, M. A. Ghannoum, K. Hazen, M. A. Pfaller, M. G. Rinaldi, and T. J. Walsh. 2001. Optimal susceptibility testing conditions for detection of azole resistance in Aspergillus spp.: NCCLS collaborative evaluation. Antimicrob. Agents Chemother. 45:1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical use. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 12.Iwen, P. C., M. E. Rupp, and S. H. Hinrichs. 1997. Invasive mold sinusitis: 17 cases in immunocompromised patients and review of literature. Clin. Infect. Dis. 24:1178-1184. [DOI] [PubMed] [Google Scholar]
- 13.Iwen, P. C., M. E. Rupp, L. N. Langnas, E. C. Reed, and S. H. Hinrichs. 1998. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of literature. Clin. Infect. Dis. 26:1092-1097. [DOI] [PubMed] [Google Scholar]
- 14.Lass-Florl, C., G. Kofler, G. Kropshofer, J. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 15.Lortholary, O., M.-C. Meyohas, B. Dupont, J. Cadranel, D. Salmon-Ceron, D. Peyramond, D. Simonin, et al. 1993. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. Am. J. Med. 95:177-187. [DOI] [PubMed] [Google Scholar]
- 16.Lozano-Chiu, M., V. L. Paetznick, M. A. Ghannoum, and J. H. Rex. 1998. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J. Clin. Microbiol. 36:2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Oakley, K. L., G. Morrissey, and D. W. Denning. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., S. A. Messer, A. Karlsson, and A. Bolmstrom. 1998. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 36:2586-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., S. A. Messer, A. Houston, K. Mills, and A. Bolmstrom. 2000. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference microdilution methods for determining itraconazole MICs. J. Clin. Microbiol. 38:3359-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., S. A. Messer, A. Houston, K. Mills, A. Bolmstrom, and R. N. Jones. 2000. Evaluation of Etest method for determining voriconazole susceptibilities of 312 clinical isolates of Candida species by using three different agar media. J. Clin. Microbiol. 38:3715-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., S. A. Messer, A. Houston, K. Mills, A. Bolmstrom, and R. N. Jones. 2001. Evaluation of Etest method for determining posaconazole susceptibilities of 312 clinical isolates of Candida species. J. Clin. Microbiol. 39:3952-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribaud, P., C. Chastang, J.-P. Latge, L. Baffroy-Lafitte, N. Parquet, A. Devergie, H. Esperou, F. Selimi, V. Rocha, F. Derouin, G. Socie, and E. Gluckman. 1999. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin. Infect. Dis. 28:322-330. [DOI] [PubMed] [Google Scholar]
- 25.Sutton, A. A., S. E. Sanche, S. G. Revankar, A. W. Fothergill, and M. G. Rinaldi. 1999. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J. Clin. Microbiol. 37:2343-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szekely, A., E. M. Johnson, and D. W. Warnock. 1999. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J. Clin. Microbiol. 37:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verweij, P. E., M. F. Q. van den Bergh, P. M. Rath, B. E. de Pauw, A. Voss, and J. F. G. M. Meis. 1999. Invasive aspergillosis caused by Aspergillus ustus: case report and review. J. Clin. Microbiol. 37:1606-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanger, A., K. Mills, P. W. Nelson, and J. H. Rex. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 39:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]