Abstract
PCR products containing sequence polymorphisms were prepared from six mycobacterial genes, denatured, mixed with reference PCR products, and reannealed; the mixtures were then examined with a denaturing high-performance liquid chromatography system (WAVE) equipped with a temperature-controlled alkalated polystyrene divinyl benzene column. Mismatching of bases in heteroduplexes of the PCR products causes elution patterns of the DNA from the column to be altered. The six mycobacterial genes studied were oxyR, in which a specific polymorphism (G1031A) is found only in certain species of the Mycobacterium tuberculosis complex, and five genes in which mutations associated with antituberculosis drug resistance have been found. The resistance genes (with affected drug and PCR product sizes given parenthetically) were rpoB (rifampin; 258 bp), katG (isoniazid; 205 bp), pncA (pyrazinamide; 579 bp); rpsL (streptomycin; 196 bp), and embB (ethambutol; 185 bp). Elution patterns of heteroduplexes of all 20 polymorphisms studied shifted detectably at column temperatures ranging from 65.3 to 68°C and elution times of 3.5 to 6 min. These results show that temperature-mediated heteroduplex analysis is a potentially useful genotypic screen for mutations associated with antituberculosis drug resistance and for the G1031A polymorphism in oxyR. The method may allow users to detect novel as well as heterogeneous mutations without using expensive kits or detection labels.
Expeditious identification of pathogenic microorganisms and their antimicrobial susceptibility patterns is essential for the control of human infections. This information, however, is difficult to obtain for fastidious organisms or those with relatively long generation times such as slowly growing Mycobacterium species, including the Mycobacterium tuberculosis complex. Genotypic assays that do not depend upon luxurious growth of the organism offer logical solutions to these problems, and commercial assays including the Amplicor Mycobacterium tuberculosis Test (Roche Diagnostic Systems, Inc., Branchburg, N.J.) and the Enhanced Mycobacterium tuberculosis Direct Test (Gen-Probe, Inc., San Diego, Calif.) have been extensively evaluated and are now well-established genetic assays for identifying M. tuberculosis in many laboratories (8, 21, 22, 30). Although genotypic assays for antimicrobial susceptibility testing of M. tuberculosis are not currently available commercially within the United States, researchers have identified genes associated with antituberculosis drug resistance. Polymorphisms in pncA (23), rpoB (13), rpsL (10), katG (31), and embB (28) correlate well with phenotypic resistance to, respectively, pyrazinamide (PZA) (14, 24), rifampin (RIF) (27), streptomycin (STR) (2), isoniazid (INH) (31), and ethambutol (EMB) (26). The identification of mutations in these genes may offer a means to rapidly screen M. tuberculosis isolates for antimicrobial resistance (19, 20). The most reliable method of identifying mutations associated with drug resistance is DNA sequence analysis of PCR products that contain regions where these mutations may be found. Alternatives to sequence analysis for antituberculosis drug resistance include single-strand conformation polymorphism electrophoresis (2, 4, 27), structure-specific cleavage (5, 25), RNA protection (16), hybridization assays (5, 6), and heteroduplex analyses (29). Electrophoretic variations of heteroduplex analysis such as constant-gradient gel electrophoresis, denaturing-gradient gel electrophoresis, and temperature-gradient gel electrophoresis exploit differences in melting points of duplex DNA strands associated with differences in their nucleotide base composition that in turn affect their mobilities in gels (12). Whereas these methods are used to examine homoduplex molecules, temperature-mediated heteroduplex analysis (TMHA) involves the melting of DNA strands, combining them with melted reference strands, and evaluating the effects of temperature on resulting heteroduplexes, particularly changes in either electrophoretic mobility or binding patterns to column matrices. Denaturing high-performance liquid chromatography (DHPLC), which was first described as a TMHA method in 1998, is used to compare mixtures of PCR amplicons for polymorphisms by the differential retention of homo- and heteroduplex DNA on a reverse-phase chromatography support under partial heat denaturation conditions (17). Recognizable distinction of heteroduplex DNA molecules is dependent upon the elution strength of the organic solvent used in the mobile phase and the number of ion pairs formed between negatively charged phosphate groups on the nucleic acid backbone and positively charged triethylammonium ions adsorbed to the stationary phase of the column matrix. Transgenomic, Inc. (Omaha, Nebr.) has adapted a special-purpose DNA binding column to reverse-phase HPLC for the analyses and purification of nucleic acids. We developed methods for performing TMHA using the Transgenomic DHPLC system (WAVE) to evaluate the ability to identify nucleotide polymorphisms in five genes associated with antituberculosis drug resistance as well as in oxyR to differentiate some members of the M. tuberculosis complex.
MATERIALS AND METHODS
Bacterial strains.
We examined 19 drug-resistant isolates of M. tuberculosis from the Centers for Disease Control and Prevention stock collection, as well as strains of Mycobacterium bovis (ATCC 19210), M. bovis BCG (ATCC 35734), Mycobacterium caprae (ATCC 105776), and M. tuberculosis H37Rv, which was used as the wild-type reference strain. Homogeneity of drug resistance was ensured by culturing the resistant strains on Middlebrook 7H10 agar containing the following drugs at the indicated concentrations: RIF, 1 μg/ml; INH, 1 μg/ml; STR, 10 μg/ml; EMB, 5 μg/ml; or PZA, 25 μg/ml. Drug-resistant mutants (Table 1) included nine rpoB mutants (3), two katG mutants (1), two embB mutants (1), two rpsL mutants (1, 2), and four pncA mutants (14). We confirmed mutations in drug resistance genes and the oxyR polymorphisms by fluorescent DNA sequence analyses of both strands of PCR products using an ABI model 373 sequencing apparatus and the Big Dye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems, Inc., Foster City, Calif.). Templates for PCR and sequence analyses were prepared by the glass bead agitation method (18).
TABLE 1.
Mycobacterial PCR products evaluated by TMHA
| Gene | Protein (drug affected) | Nucleotide (amino acid) of polymorphism | PCR product
|
||||
|---|---|---|---|---|---|---|---|
| Nucleotides | No. of nucleotides in primers
|
Size (bp) | Reference (accession no.a) | ||||
| Forward | Reverse | ||||||
| rpoB | β-Subunit RNA polymerase (RIF) | A2377T (Q513L); TTC2379-2381 insertion (F514); A2385T (D516V); AAC2390-2392 deletion (N518); CA2415-16TG (H526C); C2415T (H526Y); C2415G (H526D); C2431 T(S531L); T2437C(L533P) | 2209-2466 | 22 | 20 | 258 | 13 (L27989) |
| katG | Catalase-peroxidase (INH) | G2922C(S315T); G2922A (S315N) | 2746-2950 | 20 | 20 | 205 | 31 (X68081) |
| embB | Arabinosyl transferase (EMB) | A7868G (M306V); G7870A(M306I) | 7800-7984 | 20 | 19 | 185 | 28 (U68480) |
| rpsL | S12 ribosomal protein (STR) | A128G (K43R); A263G (K88R) | 106-301 | 19 | 20 | 196 | 10 (X80124) |
| pncA | pyrizinamidase (PZA) | A29C(Q10P); A139G (T47A); T398A (I133N); T515C (L172P) | −17-562 | 20 | 20 | 579 | 23 (U59967) |
| oxyR | Oxidative stress regulator | G1031A | 942-1190 | 20 | 20 | 249 | 7 (U16243) |
GenBank accession number.
Primer selection and PCR conditions.
We selected oligonucleotide primers for PCR amplification of genes (Table 1) using the Primer3 software program available at www.genome.wi.mit.edu/cgi-bin/primer/primer3. Primers were chosen to generate products that encompassed regions of the six genes where polymorphisms among isolates used in the study occurred. We analyzed selected primer sequences using WAVEmaker software (Transgenomic, Inc.), a DHPLC analytical program that predicts the effects of temperature on the helicity of the resulting PCR products. The software algorithm calculates the probability that each nucleotide base in a sequence will remain in a helical duplex or nonhelical single-stranded structure at various column temperatures. Primers were evaluated both with and without a 20-nucleotide guanine- and cytosine- (GC)-containing linker (15) on the 5′ terminus of the forward primer. The software calculated experimental HPLC conditions using the optimal analytical temperature. We prepared PCR products (Table 1) in duplicate using 12.5 μl of HotStarTaq polymerase Master Mix (Qiagen, Inc., Chatsworth, Calif.), which includes Taq DNA polymerase and nucleotides; 1 μl of DNA template; and 0.5 μmol of each primer in a total reaction volume of 50 μl. Thermocycling was performed in a Gene-Amp PCR System 2400 Thermocycler (Perkin-Elmer, Inc., Foster City, Calif.) set for the following conditions: 15 min at 95°C, 35 cycles of 30 s at 95°C, 30 s at the proper annealing temperature, and 30 s at 72°C, with a final incubation for 10 min at 72°C. Annealing temperatures were 55°C for pncA and oxyR, 58°C for katG and rpsL, 59°C for rpoB, and 63°C for embB. We evaluated the amplification reactions of these PCR products for specificity through the generation of single chromatographic peaks by injecting them into a temperature-controlled DHPLC column at a temperature (50°C) at which they remain helical.
TMHA.
The matrix of the column (DNASep) is hydrophobic poly(styrene-divinylbenzene) particles (11). Nucleic acids are bound in the presence of an ion-pairing reagent, 0.1 M triethylammonium acetate, pH 7 (buffer A); eluted in the presence of 0.1 M triethylammonium acetate in 25% acetonitrile (buffer B); and detected spectrophotometrically by UV absorption at 260 nm. Three methods, which specify column temperatures and injection programs for samples and gradients of buffers A and B, are produced by the WAVEmaker software on the basis of sequences that are entered into the program. The program plots helicity versus sequence at each of the three optimal temperatures using the Fixman-Friere nearest-neighbor algorithm (9, 15). We combined test samples (5 μl containing 200 to 500 μg of DNA in PCR buffer with no additional purification) in approximately equimolar ratios with a corresponding wild-type reference PCR product (prepared from M. tuberculosis strain H37Rv), heated the mixtures to 95°C for 10 min, and cooled them to 35°C using a 1-h ramping time (i.e., using a thermocycler) to form hetero- and homoduplex molecules prior to applying them to the column at the prescribed analytical temperatures. We then compared chromatographic peaks observed from the mixtures with wild-type homoduplex peaks, which were generated by reannealing reference products with either no additional PCR product or other products known to be wild type.
Disclaimer.
Use of commercial trade names is for descriptive purposes only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention.
RESULTS
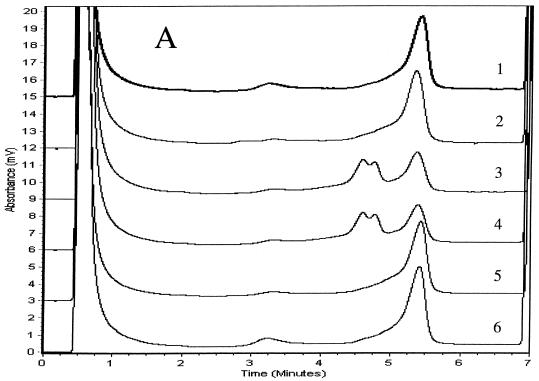
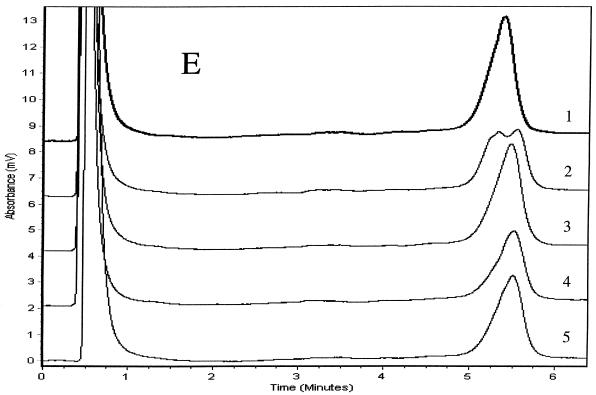
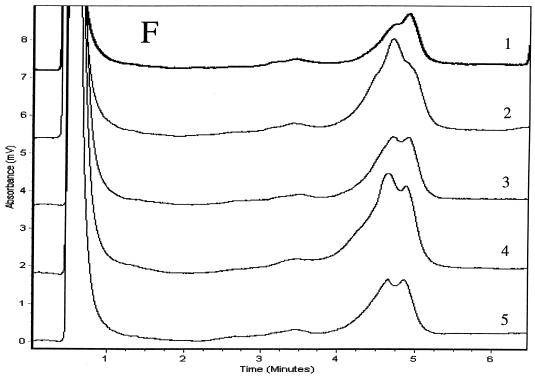
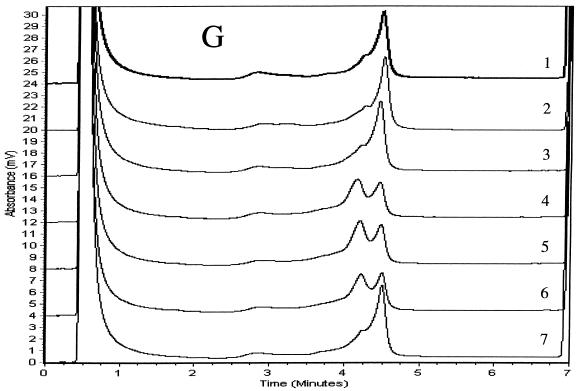
We determined PCR products for the six genes to be specific if single chromatographic peaks appeared when they were injected into the DHPLC system at a column temperature of 50°C. We analyzed duplexes formed between the strands of the reference product and either wild-type or mutant products using three temperatures and other accompanying variables in the DHPLC method that were recommended by the analytical software. We considered the TMHA conditions to be optimal if wild-type homoduplexes eluted as a single peak and if all mixtures of mutant and wild-type amplicons produced patterns that were clearly distinguishable from the reference patterns. Elution times for diagnostic peaks ranged from 3.5 to 6 min. Mixtures of wild-type (H37Rv) products with at least three wild-type PCR products for each of the six genes examined produced single peaks that matched one another when examined at the optimal analytical column temperatures. Mixtures of mutant and wild-type amplicons (i.e., mixtures of homoduplexes and heteroduplexes) eluted as two peaks for rpoB, katG, embB, rpsL, and oxyR amplicons (Fig. 1). Because chromatographic peaks for mixtures of wild-type PCR products and the four pncA mutants were not distinguishable from the wild-type reference peak at any of the three temperatures initially tested (65, 66, or 67°C), we analyzed pncA heteroduplexes at column temperatures between 64 and 67°C in 0.1°C increments. Although we could not identify the four mutants at a single column temperature, the Q10P polymorphism (which results from an adenine-to-cytosine substitution at nucleotide 29 located 46 nucleotides from the start of the forward primer) produced two peaks optimally at 65.3°C (Fig. 1E). The three remaining pncA mutations in codons 47, 133, and 172 were identifiable only at 66.9°C (Fig. 1F). These three mutants (Fig. 1F, patterns 3 to 5) produced double peaks compared with H37Rv and the A29C mutant (patterns 1 and 2), for which single peaks with shoulders appeared. The resolution of heteroduplex peaks from homoduplexes was not improved by the addition of the 20-nucleotide GC-containing linker to forward primers for any of the six genes (data not shown). The duplicate PCR products for mutants and the reference (H37Rv) for the six genes matched the original samples in column elution times and in the peak shapes of homo- and heteroduplexes, but their relative peak heights varied slightly.
FIG. 1.
TMHAs of PCR products for six mycobacterial genes using DHPLC. Reference PCR product was prepared from M. tuberculosis strain H37Rv. PCR products examined are shown in Table 1. Genes and optimum column temperatures were katG, 67°C (A); rpoB, 66°C (B); rpsL, 67°C (C); embB, 67°C (D); pncA, 65.3°C (E); pncA, 66.9°C (F); and oxyR, 68°C (G). These genes and temperatures were used to examine the relevant genotypes of samples. (A) Patterns: 1, wild type (reference); 2, wild type; 3, G2922C; 4, G2922A; 5 and 6, wild type. (B) Patterns: 1, wild type (reference); 2, CA2415-2416TG; 3, C2431T; 4, A2385T; 5, C2415T; 6, A2377T; 7, AAC2390-2392 deletion; 8, TTC2379-2381 insertion; 9, T2437C; 10, C2415G; 11, wild type. (C) Patterns: 1, wild type (reference); 2, wild type; 3, A128G; 4, A263G; 5 and 6, wild type. (D) Patterns: 1, wild type (reference); 2, A7868G; 3, wild type; 4, G7870A; 5 to 7, wild type. (E) Patterns: 1, wild type (reference); 2, A29C; 3, A139G; 4, T398A; 5, T515C. (F) Patterns: 1, wild type (reference); 2, A29C; 3, T515C; 4, T398A; 5, A139G. (G) Patterns: 1, M. tuberculosis (reference); 2 and 3, M. tuberculosis; 4, M. caprae; 5, M. bovis BCG; 6, M. bovis; 7, M. tuberculosis.
DISCUSSION
Using the Wave DHPLC system, we were able to identify polymorphisms in six mycobacterial genes by TMHA. These polymorphisms included 18 nucleotide substitutions (missense mutations), a three-base deletion, and a three-base insertion. Among the missense mutations were transversions (those involving purine-to-pyrimidine substitutions or vice versa) and transitions (those involving purine-to-purine or pyrimidine-to-pyrimidine substitutions) at the same nucleotide. These transitions and transversions included C2415T and C2415G in rpoB and G2922C and G2922A in katG. Although we were able to distinguish these and the other amplicons with mutations from wild-type amplicons using the TMHA method, we could not distinguish or identify individual mutations by our approach. We also have not established that all known or novel mutations within specific amplicons can be identified. The accuracy of nucleotide incorporation during PCR and the specificity of the resulting amplicon can adversely affect the results of genotypic assays, including TMHA, that identify mutations in PCR products. Although TMHA may be a useful genotypic screen for mutations associated with antimicrobial resistance, phenotypic drug susceptibility assays are still required, because some strains of M. tuberculosis are resistant to drugs because of unknown mechanisms for which genotypic assays are unavailable.
We encountered substantial difficulty with pncA (579-bp amplicon), for which we found regions of highly stable helicity at all temperatures evaluated by the WAVEmaker software. To overcome this difficulty, we precisely identified the optimal column temperature experimentally by injecting the products at various temperatures in increments of 0.1°C. This solution would not be possible using column ovens that are incapable of maintaining precise temperatures at fractions of degrees. Diagnostic peaks for mutants within each of the two pncA regions were less pronounced than those for mutations in the remaining five genes even at the optimal temperatures. Although the pncA amplicon was considerably larger than those for the other genes, size has been reported to influence results less than other considerations such as GC content, especially in DNA regions within 10 to 20 bases of the mismatch (17). For example, an adenine-thymine-rich region, in which a mismatch may be located, may undergo complete denaturation at a given temperature, thus making it impossible for analysts to distinguish the mismatch from the corresponding homoduplex. With the WAVEmaker software, however, domains within an amplicon that are divergent from other regions in terms of stability are typically identified during initial evaluation. We are nonetheless currently performing TMHA evaluations using smaller overlapping PCR products designed according to the helicity of the various pncA regions.
An advantage of the DHPLC system is that multiple methods may be programmed into the DHPLC system, thus enabling researchers to analyze different products in a walk-away fashion using the instrument's autosampler and programmable column heater. Samples may also be recovered by using an optional fraction collector. Although the initial cost of the DHPLC system components is higher that that of comparable HPLC systems, the DHPLC system allows users to analyze samples from a variety of organisms as well as eucaryotic samples on the same instrument, which may be shared among laboratories or placed in a multiuser location such as a core facility within an institution. The samples that we analyzed in this study were standard PCR products, which did not require additional and expensive detection labels or commercial kits. Additional cost savings may be achieved by reducing the volume of reagents used in PCRs, since only 5 μl is typically required for the DHPLC analysis. It is also possible to discontinue the evaluation of PCR products by standard agarose gel analysis once amplification conditions are established, since injecting and analyzing the PCR products in the DHPLC system at 50°C enables users to confirm product specificity and relative quantity. Because the elution of double-stranded DNA at lower column temperatures is related to the size of the product, the DHPLC system will also allow users to analyze restriction-fragment length polymorphisms of PCR products (11).
REFERENCES
- 1.Abbadi, S., H. G. Rasheed, G. P. Morlock, C. L. Woodley, O. El Shanawy, and R. C. Cooksey. 2001. Characterization of IS6110 restriction fragment length polymorphisms patterns and mechanisms of antimicrobial resistance for multidrug-resistant isolates of Mycobacterium tuberculosis from a major reference hospital in Assiut, Egypt. J. Clin. Microbiol. 39:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooksey, R. C., G. P. Morlock, A. McQueen, S. E. Glickman, and J. T. Crawford. 1996. Characterization of streptomycin resistance mechanisms among Mycobacterium tuberculosis isolates from patients in New York City. Antimicrob. Agents Chemother. 40:1150-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooksey, R. C., G. P. Morlock, S. Glickman, and J. T. Crawford. 1997. Evaluation of a line probe assay kit for characterization of rpoB mutations in rifampin-resistant Mycobacterium tuberculosis isolates from New York City. J. Clin. Microbiol. 35:1281-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooksey, R. C., G. P. Morlock, B. P. Holloway, G. H. Mazurek, S. Abaddi, L. K. Jackson, G. S. Buzard, and J. T. Crawford. 1998. Comparison of two nonradioactive, single-strand conformation polymorphism electrophoretic methods for identification of rpoB mutations in rifampin-resistant isolates of Mycobacterium tuberculosis. Mol. Diagn. 3:73-80. [DOI] [PubMed] [Google Scholar]
- 5.Cooksey, R. C., B. P. Holloway, M. C. Oldenburg, S. Listenbee, and C. W. Miller. 2000. Evaluation of the Invader assay, a linear signal amplification method for the identification of mutations associated with antimicrobial resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:1296-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Beenhouwer, H., Z. Lhiang, G. Jannes, W. Mijs, L. Machtelinckz, R. Rossau, H. Traore, and F. Portaels. 1995. Rapid detection of rifampin resistance in sputum and biopsy specimens from tuberculosis patients by PCR and line probe assay. Tuber. Lung Dis. 76:425-430. [DOI] [PubMed] [Google Scholar]
- 7.Deretic, V., W. Philipp, S. Dhandayuthapani, M. H. Mudd, R. Curcic, T. Garbe, B. Heym, L. E. Via, and S. T. Cole. 1995. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol. Microbiol. 17:889-900. [DOI] [PubMed] [Google Scholar]
- 8.Desmond, E. P., and K. Loretz. 2001. Use of the Gen-Probe amplified mycobacterium tuberculosis direct test for early detection of Mycobacterium tuberculosis in BACTEC 12B Medium. J. Clin. Microbiol. 39:1993-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fixman, M., and J. J. Freire. 1977. Theory of DNA melting curves. Biopolymers 16:2693-2704. [DOI] [PubMed] [Google Scholar]
- 10.Honore, N., and S. T. Cole. 1994. Streptomycin resistance in mycobacteria. Antimicrob. Agents Chemother. 38:238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber, C. G., P. J. Oefner, and G. K. Bond. 1993. High-resolution liquid chromatography of oligonucleotides on nonporous alkylated styrene divinylbenzene copolymers. Anal. Biochem. 212:351-358. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen, V. N., D. Kelefiotis, T. Kristensen, and A. Børresen-Dale. 2001. High-throughput methods for detection of genetic variation. BioTechniques 30:318-332. [DOI] [PubMed] [Google Scholar]
- 13.Miller, L. P., J. T. Crawford, and T. M. Shinnick. 1994. The rpoB gene of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morlock, G. P., J. T. Crawford, W. R. Butler, G. H. Mazurek, S. E. Brim, C. L. Woodley, and R. C. Cooksey. 1999. Phenotypic characterization of pncA mutants of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2291-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanaswami, G., and P. D. Taylor. 2001. Improved efficiency of mutation detection by denaturing high-performance liquid chromatography using modified primers and hybridization procedure. Genet. Test. 5:9-16. [DOI] [PubMed] [Google Scholar]
- 16.Nash, K. A., A. Gaytan, and C. B. Inderlied. 1997. Detection of rifampin resistance in Mycobacterium tuberculosis by use of a rapid, simple, and specific RNA/RNA mismatch assay. J. Infect. Dis. 176:533-536. [DOI] [PubMed] [Google Scholar]
- 17.Oefner, P. J., and P. A. Underhill. 1998. DNA mutation detection using denaturing high-performance liquid chromatography (DHPLC). Curr. Protocols Hum. Genet. 19(Suppl.):7.10.1-7.10.12. [DOI] [PubMed] [Google Scholar]
- 18.Plikaytis, B. B., R. H. Gelber, and T. M. Shinnick. 1990. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J. Clin. Microbiol. 28:1913-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3-29. [DOI] [PubMed] [Google Scholar]
- 20.Rattan, A., A. Kalia, and N. Ahmad. 1998. Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives. Emerg. Infect. Dis. 4:195-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reischl, U., N. Lehn, H. Wolf, and L. Naumann. 1998. Clinical evaluation of the automated COBAS AMPLICOR MTB assay for testing respiratory and nonrespiratory specimens. J. Clin. Microbiol. 36:2853-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarparo, C., P. Piccoli, A. Rigon, G. Ruggiero, M. Scagnelli, and C. Piersimoni. 2000. Comparison of enhanced Mycobacterium tuberculosis amplified direct test with COBAS AMPLICOR Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens. J. Clin. Microbiol. 38:1559-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 24.Sreevatsan, S., X. Pan, Y. Zhang, B. N. Kreiswirth, and J. Musser. 1997. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob. Agents Chemother. 41:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreevatsan, S., J. Bookout, F. Ringpis, S. Mogazeh, B. Kreiswirth, R. Pottathil, and R. Barathur. 1998. Comparative evaluation of Cleavase fragment length polymorphism with PCR-SSCP and PCR-RFLP to detect antimicrobial agent resistance in Mycobacterium tuberculosis. Mol. Diagn. 3:81-91. [DOI] [PubMed] [Google Scholar]
- 26.Sreevatsan, S., K. E. Stockbauer, X. Pan, B. N. Kreiswirth, S. L. Moghazeh, W. R. Jacobs, Jr., A. Telenti, and J. M. Musser. 1997. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob. Agents Chemother. 41:1677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telenti, A., P. Imboden, P. Marchesi, D. Lowrie, S. Cole, M. J. Colston, L. Matter, K. Schopfer, and T. Bodmer. 1993. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647-650. [DOI] [PubMed] [Google Scholar]
- 28.Telenti, A., W. J. Philipp, S. Sreevatsan, C. Bernasconi, K. E. Stockbauer, B. Wieles, J. M. Musser, and W. R. Jacobs. 1997. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 3:567-570. [DOI] [PubMed] [Google Scholar]
- 29.Williams, D. L., L. Spring, T. P. Gillis, M. Salfinger, and D. H. Persing. 1998. Evaluation of a polymerase chain reaction-based universal heteroduplex generator assay for direct detection of rifampin susceptibility of Mycobacterium tuberculosis from sputum specimens. Clin. Infect. Dis. 26:446-450. [DOI] [PubMed] [Google Scholar]
- 30.Wobeser, W. L., M. Krajden, J. Conly, H. Simpson, B. Yim, M. D'Costa, M. Fuksa, C. Hian-Cheong, A. Patterson, A. Phillips, R. Bannatyne, A. Haddad, J. L. Brunton, and S. Krajden. 1996. Evaluation of Roche Amplicor PCR assay for Mycobacterium tuberculosis. J. Clin. Microbiol. 34:134-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y., B. Heym, B. Allen, D. Young, and S. T. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]