Abstract
Myostatin is a secreted protein that normally functions as a negative regulator of muscle growth. Agents capable of blocking the myostatin signaling pathway could have important applications for treating human muscle degenerative diseases as well as for enhancing livestock production. Here we describe a potent myostatin inhibitor, a soluble form of the activin type IIB receptor (ACVR2B), which can cause dramatic increases in muscle mass (up to 60% in 2 weeks) when injected into wild-type mice. Furthermore, we show that the effect of the soluble receptor is attenuated but not eliminated in Mstn-/- mice, suggesting that at least one other ligand in addition to myostatin normally functions to limit muscle growth. Finally, we provide genetic evidence that these ligands signal through both activin type II receptors, ACVR2 and ACVR2B, to regulate muscle growth in vivo.
Keywords: myostatin, TGF-β
Myostatin is a TGF-β family member that plays a key role in regulating skeletal muscle growth (for review, see ref. 1). Mice carrying a targeted mutation in the myostatin gene have muscles that are about twice the normal size as a result of a combination of muscle fiber hyperplasia and hypertrophy (2). Myostatin appears to play a similar role in other species as well; naturally occurring mutations in the myostatin gene have been shown to be responsible for the double-muscling phenotype in cattle (3–6), and recent studies have demonstrated that a human baby with approximately twice the normal muscle mass is also homozygous for a loss-of-function mutation in the MSTN gene (7). These findings have raised the possibility that agents capable of targeting the myostatin signaling pathway may be useful for increasing muscle mass for both agricultural and human therapeutic applications. In this regard, loss of myostatin signaling has been shown to have beneficial effects in mouse models of muscle degenerative (8, 9) and metabolic (10) diseases.
Various myostatin-binding proteins have been identified that are capable of inhibiting myostatin activity in vitro (8, 11–16). Two of these proteins, the JA16 neutralizing monoclonal antibody (Ab) directed against myostatin (8, 15) and a mutant form of the myostatin propeptide resistant to members of the BMP-1/tolloid family of metalloproteases (16), have been shown to be capable of increasing muscle mass by ≈25% when administered to wild-type (WT) mice. To determine whether these increases in muscle growth are the maximal achievable by targeting this signaling pathway, we sought additional myostatin inhibitors that might have a broader specificity in their ability to target additional members of the TGF-β superfamily. Previous studies have demonstrated that myostatin is capable of binding the two activin type II receptors, ACVR2B and, to a lesser extent, ACVR2, in transfected COS cells (11, 17). Moreover, transgenic mice in which a myosin light chain promoter/enhancer was used to express a truncated form of ACVR2B in skeletal muscle were found to have dramatic increases in muscle mass (11). Because the activin type II receptors have been shown to be capable of binding a number of other TGF-β family members in addition to myostatin (for review, see ref. 18), we examined the effect of administering a soluble form of ACVR2B (ACVR2B/Fc) to adult mice.
Materials and Methods
Acvr2 (19) and Acvr2b (20) mutant mice were maintained on a hybrid C57BL/6, 129 Sv/J background. Mstn mutant mice (2) were maintained on a C57BL/6 background. CHO cells expressing the extracellular domain of murine ACVR2B fused to a murine Fc domain were selected as described in ref. 21. The ACVR2B/Fc fusion protein was purified by using a protein A Sepharose column. Myostatin, GDF-11/BMP-11, and activin activities were measured by using the pGL3-(CAGA)12-luciferase reporter assay in A204 rhabdomyosarcoma cells as described in ref. 12. To analyze the effect of administering ACVR2B/Fc to mice, 6-week-old females were injected i.p. on days 1, 4, 8, 15, and 22 either with ACVR2B/Fc or with PBS and killed on day 29 for muscle analysis. For the experiments in which effects of ACVR2B/Fc were assessed after 1, 2, or 3 weeks, animals were injected with ACVR2B/Fc on day 22, days 15 and 22, or days 8, 15, and 22, respectively, and with PBS on each of the remaining time points (for example, on days 1, 4, and 8 for the 2-week experiment). For measurement of muscle weights, individual muscles from both sides of the animal were dissected, and the average weight was used for each muscle. For morphometric analysis, the gastrocnemius and plantaris muscles were sectioned serially to their widest point by using a cryostat, and fiber diameters were measured (as the shortest distance across the fiber passing through the midpoint) from hematoxylin/eosin-stained sections. Quantification of protein and DNA content of the muscles was carried out as described in ref. 2.
Results and Discussion
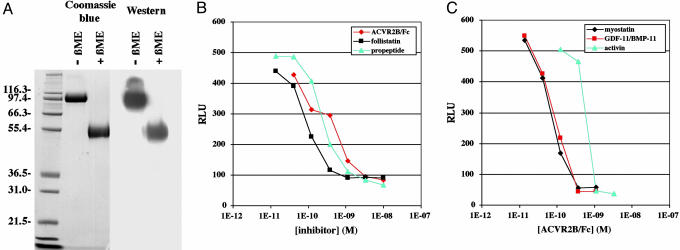
To generate a soluble form of the ACVR2B receptor with enhanced stability in vivo, we fused the extracellular domain of ACVR2B to an Fc domain. We generated CHO cells expressing high levels of ACVR2B/Fc and then purified the protein from the conditioned medium of these CHO cells. As shown in Fig. 1A, the purified ACVR2B protein consisted of a disulfide-linked dimer with a monomeric molecular weight of ≈52,000. We first tested the ability of ACVR2B/Fc to block myostatin signaling in cell culture by using a pGL3-(CAGA)12-luciferase reporter gene as a readout for activation of intracellular Smad proteins. As described previously (12), incubation of myostatin with A204 rhabdomyosarcoma cells transfected with the reporter construct induced expression of luciferase above basal levels. Addition of ACVR2B/Fc to the assay blocked myostatin activity in a dose-dependent manner with an IC50 of ≈180 pM (Fig. 1B). The potency of ACVR2B/Fc in vitro was comparable with that seen for two other myostatin inhibitors, follistatin and the myostatin propeptide. ACVR2B/Fc was also capable of blocking the activity of two other TGF-β-related ligands, GDF-11/BMP-11 and activin (Fig. 1C).
Fig. 1.
Increased muscle mass in mice injected with ACVR2B/Fc. (A) Purified ACVR2B/Fc analyzed by Coomassie blue staining and Western blot using Abs directed against the Fc domain. (B) Effect of ACVR2B/Fc, follistatin, and the myostatin propeptide on luciferase reporter activity induced in A204 cells by 3 ng/ml myostatin. (C) Effect of ACVR2B/Fc on luciferase activity induced in A204 cells by 10 ng/ml myostatin, 10 ng/ml GDF-11/BMP-11, and 20 ng/ml activin. RLU, relative light units.
We then examined the ability of ACVR2B/Fc to enhance muscle growth in vivo. Female C57BL/6 mice beginning at 6 weeks of age were given five i.p. injections of ACVR2B/Fc over a span of 4 weeks at a dose of 10 mg/kg per injection. We compared muscle weights in these mice with those of control mice that had received the same schedule of injections of PBS. In previous experiments, we had shown that an identical injection regimen using two other proteins, a control mouse monoclonal Ab and the myostatin propeptide fused to an Fc domain, had no effect compared with PBS (16). In contrast, injection of ACVR2B/Fc caused increases in muscle growth by 32–40% (Table 1), an effect that was highly significant (P values ranged from 10-7 to 10-8). The effect of ACVR2B/Fc on muscle growth was rapid, with the maximal effect being reached after only two injections spaced 1 week apart (Table 1). The effect was also dose-dependent, and at the highest dose (50 mg/kg), we were able to achieve muscle mass increases of 39–61% after just 2 weeks (Table 1). These findings demonstrate that the capacity for increasing muscle growth by interfering with this signaling pathway is significantly larger than previously appreciated.
Table 1. Muscle weights of mice injected with ACVR2B/Fc.
| Muscle weight, mg
|
|||||
|---|---|---|---|---|---|
| Mice | No. | Pectoralis | Triceps | Quadriceps | Gastrocnemius |
| WT | |||||
| + PBS | (n = 10) | 46.3 ± 1.2 | 70.9 ± 1.8 | 142.1 ± 2.9 | 99.1 ± 1.9 |
| + ACVR2B/Fc | |||||
| 10 mg/kg, 1 wk | (n = 6) | 57.8 ± 2.8* | 89.0 ± 4.5* | 166.7 ± 6.6* | 115.0 ± 5.0† |
| 10 mg/kg, 2 wk | (n = 10) | 68.1 ± 1.8‡ | 96.3 ± 2.3‡ | 185.4 ± 4.1‡ | 124.6 ± 3.1‡ |
| 10 mg/kg, 3 wk | (n = 10) | 64.0 ± 1.7‡ | 96.4 ± 2.2‡ | 182.2 ± 5.1‡ | 121.4 ± 2.9‡ |
| 10 mg/kg, 4 wk | (n = 10) | 63.8 ± 1.5‡ | 99.5 ± 2.5‡ | 188.0 ± 4.8‡ | 131.6 ± 2.8‡ |
| 30 mg/kg, 2 wk | (n = 6) | 67.2 ± 3.8* | 103.7 ± 3.3‡ | 191.7 ± 7.4§ | 125.8 ± 5.0* |
| 50 mg/kg, 2 wk | (n = 7) | 71.0 ± 1.2‡ | 114.4 ± 2.9‡ | 204.7 ± 4.5‡ | 138.1 ± 3.5‡ |
| Mstn-/- | |||||
| + PBS | (n = 10) | 104.6 ± 3.2 | 154.9 ± 4.1 | 267.0 ± 7.6 | 177.2 ± 5.9 |
| + ACVR2B/Fc | |||||
| 10 mg/kg, 4 wk | (n = 10) | 129.3 ± 3.6¶ | 194.7 ± 5.3¶ | 306.7 ± 7.9∥ | 214.8 ± 5.4** |
, P < 0.01 vs. WT + PBS
, P < 0.05 vs. WT + PBS
, P < 0.0001 vs. WT + PBS
, P < 0.001 vs. WT + PBS
, P < 0.0001 vs. Mstn-/- + PBS
, P < 0.01 vs. Mstn-/- + PBS
, P < 0.001 vs. Mstn-/- + PBS
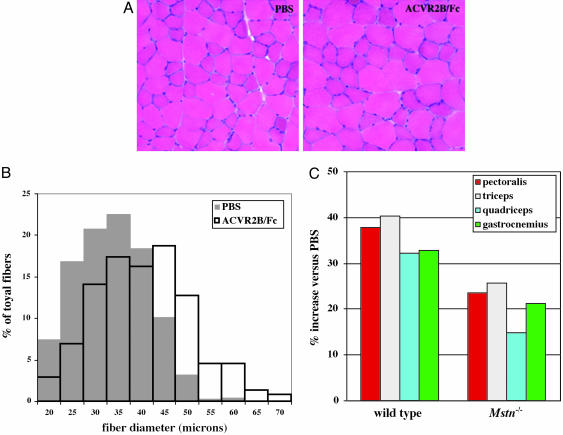
Growth of skeletal muscle postnatally generally occurs by hypertrophy of individual muscle fibers rather than by the formation of new fibers (22). To determine whether administration of ACVR2B/Fc to adult mice caused muscle growth by inducing muscle fiber hypertrophy, we examined the gastrocnemius and plantaris muscles of mice injected with either PBS or ACVR2B/Fc (10 mg/kg for 4 weeks). For these experiments, we analyzed hematoxylin/eosin-stained sections prepared from the widest portion of these muscles. Examination of these sections revealed the overall cross-sectional area of the gastrocnemius and plantaris muscles to be significantly increased in mice injected with ACVR2B/Fc (data not shown). The muscle appeared grossly normal with no evidence of degeneration (Fig. 2A). However, muscle fiber sizes in mice injected with ACVR2B/Fc were clearly increased compared with those of mice injected with PBS (Fig. 2B). Measurement of 250 muscle fibers from each of three mice injected with PBS revealed mean fiber diameters of 31.5, 34.5, and 35.1 μm. In contrast, a similar analysis of three mice injected with ACVR2B/Fc revealed mean fiber diameters of 39.9, 40.7, and 40.8 μm. Hence, administration of ACVR2B/Fc caused an average increase in the mean fiber diameter by 20% compared with PBS (P = 0.02). Given that overall muscle fiber volume would be predicted to be roughly proportional to the square of the fiber diameter, the observed increases in fiber diameters appear to fully account for the overall increase in muscle weights (32–40%) that we observed upon this regimen of administration of ACVR2B/Fc.
Fig. 2.
Muscle fiber hypertrophy induced by ACVR2B/Fc. (A) Hematoxylin/eosin sections prepared from gastrocnemius/plantaris muscles of mice injected with either PBS or ACVR2B/Fc. (B) Distribution of muscle fiber sizes in mice injected with either PBS or ACVR2B/Fc. (C) Percent increase in weights of the pectoralis, triceps, quadriceps, and gastrocnemius/plantaris muscles of WT and Mstn-/- mice injected with ACVR2B/Fc (10 mg/kg for 4 weeks) relative to PBS. Actual weights, standard errors, and P values are shown in Table 1.
In addition to measuring fiber sizes, we also analyzed the composition of the muscle in homogenates prepared from these mice. Analysis of the quadriceps muscle revealed total protein content to be increased by 61% from 17.7 ± 4.3 mg in mice injected with PBS to 28.4 ± 2.5 mg in mice injected with ACVR2B/Fc (P = 0.03). Similarly, total DNA content was increased by 46% from 72.1 ± 7.9 μg to 105.5 ± 6.3 μg in mice injected with ACVR2B/Fc (P = 0.02). Hence, the increases in muscle weights induced by administration of ACVR2B/Fc were accompanied by corresponding increases in total protein and DNA content, consistent with muscle fiber hypertrophy.
In general, the increases in muscle weights that we observed upon administration of ACVR2B/Fc appeared to be considerably larger than those obtained in previous experiments with other myostatin inhibitors, such as the D76A mutant myostatin propeptide/Fc fusion protein (16) and the JA16 neutralizing monoclonal Ab directed against myostatin (8, 15), raising the possibility that ACVR2B/Fc might be inhibiting the activities of other ligands in addition to myostatin. To investigate this possibility, we analyzed the effect of injecting ACVR2B/Fc into Mstn-/- mice. Because we wanted to compare the effects of ACVR2B/Fc in Mstn-/- mice directly with those observed in WT C57BL/6 mice, we used Mstn-/- mice that had been backcrossed at least five times onto a C57BL/6 genetic background. As shown in Table 1 and Fig. 2C, administration of ACVR2B/Fc (at 10 mg/kg for 4 weeks) to Mstn-/- mice led to statistically significant increases (P values ranged from 10-3 to 10-5) of 15–26% in the weights of all four muscle groups examined.
This ability of ACVR2B/Fc to increase muscle mass in Mstn-/- mice contrasts with what we have observed using JA16, a neutralizing monoclonal Ab directed against myostatin. In previous experiments, we showed that administration of JA16 to WT mice can cause an up to 25% increase in muscle weights (15, 16). In further experiments, however, we did not observe any effect on muscle growth when this Ab was administered to Mstn-/- mice, even after 9 weekly injections at a dose of 60 mg/kg (data not shown). These data suggest that the muscle-enhancing effect of the JA16 Ab in WT mice results solely or predominantly from inhibition of myostatin activity. The fact that ACVR2B/Fc had a proportionately reduced effect in Mstn-/- mice compared with WT mice suggests that at least part of the effect of ACVR2B/Fc in WT mice resulted from its antagonism of myostatin activity. However, the fact that effects of ACVR2B/Fc were observed at all in Mstn-/- mice suggests that ACVR2B/Fc is capable of antagonizing at least one other ligand that also functions to suppress muscle growth.
We presume that this other ligand (or ligands) is also a member of the TGF-β superfamily, but at present, we can only speculate as to its identity. In addition to myostatin, a number of other TGF-β family members have been shown to be capable of binding activin type II receptors (for review, see ref. 18), and any of these could conceivably play a similar role to myostatin in muscle. Perhaps the most likely candidate is GDF-11/BMP-11, which is highly related to myostatin in the mature region of the protein (2, 23, 24) and is also expressed in skeletal muscle. Genetic studies in mice have demonstrated clear roles for Gdf11 in regulating anterior/posterior patterning (25), kidney development (25, 26), neuronal development (27, 28), and pancreas development (29). Because mice completely lacking GDF-11/BMP-11 die during the perinatal period, however, elucidation of the role, if any, that GDF-11/BMP-11 plays in regulating muscle growth will require the generation of mice in which the Gdf11 gene can be deleted in either a muscle-specific or inducible manner. Whatever the identity of this other ligand may be, the broader specificity of ACVR2B/Fc compared with other myostatin inhibitors likely explains its ability to enhance greater muscle growth than has been observed previously with these other inhibitors.
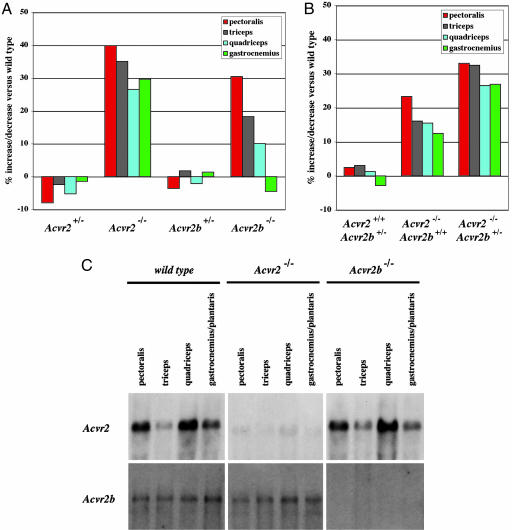
To determine whether the effects of myostatin and the other ligand in regulating muscle growth are mediated by ACVR2, ACVR2B, or both, we examined the muscles of mice carrying mutations in one or both activin type II receptor genes. Acvr2 mutant mice have been shown previously to have craniofacial defects as well as abnormalities in reproductive function (19). Although some Acvr2-/- mice die during the early perinatal period, many homozygous mutants survive to adulthood. To assess whether ACVR2 may be involved in regulating muscle growth, we compared the weights of the pectoralis, triceps, quadriceps, and gastrocnemious/plantaris muscles isolated from 4-month-old WT, heterozygous, and homozygous mutant offspring obtained from crosses of heterozygous mice. As shown in Table 2 and Fig. 3A, weights of individual muscles of female Acvr2-/- mice showed statistically significant (P < 0.001) increases of 27–40% compared with those of WT control mice. These increases were observed in each of the muscles that were examined, and statistically significant increases also were seen in the same muscle groups in male mice, although to a lesser degree (16–23%; data not shown).
Table 2. Muscle weights of individual Acvr2 and Acvr2b mutant mice.
| Muscle weight, mg
|
|||||
|---|---|---|---|---|---|
| Mice | No. | Pectoralis | Triceps | Quadriceps | Gastrocnemius |
| Acvr2+/+ | (n = 11) | 64.3 ± 3.5 | 86.6 ± 3.2 | 167.0 ± 8.0 | 103.7 ± 4.6 |
| Acvr2+/- | (n = 13) | 59.8 ± 2.7 | 84.5 ± 3.1 | 158.3 ± 5.0 | 102.2 ± 3.3 |
| Acvr2-/- | (n = 11) | 89.9 ± 2.9* | 117.1 ± 4.2* | 211.5 ± 7.3† | 134.5 ± 4.5* |
| Acvr2b+/+ | (n = 17) | 56.9 ± 1.3 | 82.5 ± 2.0 | 154.9 ± 3.8 | 96.3 ± 1.9 |
| Acvr2b+/- | (n = 12) | 54.8 ± 1.8 | 83.9 ± 2.2 | 151.7 ± 4.2 | 97.7 ± 2.3 |
| Acvr2b-/- | (n = 7) | 71.6 ± 2.8† | 99.3 ± 3.9‡ | 167.0 ± 6.8 | 93.3 ± 4.1 |
, P < 0.0001 vs. WT
, P < 0.001 vs. WT
, P < 0.01 vs. WT
Fig. 3.
Increased muscle mass in Acvr2 and Acvr2b mutant mice. (A) Percent increase/decrease in weights of the pectoralis, triceps, quadriceps, and gastrocnemius/plantaris muscles of Acvr2+/-, Acvr2-/-, Acvr2b+/-, and Acvr2b-/- mice relative to WT mice. Calculations for Acvr2 or Acvr2b mutant mice were made relative to WT offspring obtained from matings of Acvr2+/- or Acvr2b+/- mice, respectively. Actual weights, standard errors, and P values are shown in Table 2. (B) Percent increase/decrease in weights of the pectoralis, triceps, quadriceps, and gastrocnemius/plantaris muscles of Acvr2+/+Acvr2b+/-, Acvr2-/--Acvr2b+/+, and Acvr2-/-Acvr2b+/- mice relative to Acvr2+/+Acvr2b+/+ mice. Actual weights, standard errors, and P values are shown in Table 3. (C) Northern blot analysis of 20 μg total RNA isolated from adult WT, Acvr2-/-, and Acvr2b-/- mice.
We also carried out a similar analysis of Acvr2b mutant mice. Acvr2b-/- mice have been shown to have defects in both axial and left–right patterning as well as in kidney formation (20). Despite these abnormalities, ≈30% of Acvr2b-/- mice survive to adulthood. As shown in Table 2 and Fig. 3A, analysis of the pectoralis and triceps muscles of surviving female Acvr2b-/- mice at 4 months of age revealed statistically significant (P < 0.01) increases in muscle weights of 26% and 20%, respectively, compared with WT mice. However, no statistically significant increases were observed in the quadriceps and gastrocnemious/plantaris muscles. Virtually identical results were obtained from analysis of male Acvr2b-/- mice, which showed increases of 27% and 24% in the weights of the pectoralis and triceps muscles, respectively, and no statistically significant increases in the weights of the quadriceps and gastrocnemious/plantaris muscles (data not shown).
Based on these data, both ACVR2 and ACVR2B appear to be involved in regulating muscle mass in vivo. All four muscle groups examined were increased in size in mice lacking ACVR2, whereas two of the four muscle groups were affected in mice lacking ACVR2B. In neither case, however, were the increases comparable with those seen in Mstn-/- mice, which have muscles about twice the size of WT mice (2). Therefore, we generated compound mutants to investigate the possibility that the functions of these two receptors in regulating muscle mass may be redundant with one another. Previous studies using this same Acvr2b mutant allele in combination with a different Acvr2 mutant allele showed that Acvr2-/-Acvr2b-/- and Acvr2-/--Acvr2b+/- embryos die early during gestation and that Acvr2+/--Acvr2b-/- mice die either late during gestation or during the perinatal period (30). Consistent with these findings, we did not obtain any Acvr2-/-Acvr2b-/- or Acvr2+/-Acvr2b-/- mice at weaning among 359 offspring born from crosses of Acvr2+/--Acvr2b+/- mice. Unexpectedly, we also obtained just a single male adult mouse that was Acvr2+/+Acvr2b-/- even though we readily obtained Acvr2b-/- offspring from crosses of Acvr2b+/- mice. We do not know the reason for this discrepancy, although potential explanations would include differences in genetic background or maternal effects resulting from loss of one Acvr2 allele.
From these crosses of Acvr2+/-Acvr2b+/- mice, we did obtain the other genetic combinations, and we analyzed the muscles of these offspring at 4 months of age. Analysis of Acvr2-/-Acvr2b+/+ progeny from this cross generally confirmed the findings described above, although we did find some quantitative differences presumably as a result of either maternal effects or different genetic backgrounds introduced by the interbreeding. In particular, Acvr2-/-Acvr2b+/+ progeny of crosses of double heterozygotes did have statistically significant increases in muscle mass compared with Acvr2+/+Acvr2b+/+ mice (Table 3 and Fig. 3B), but the magnitude of the increases was smaller (13–23% in females; 9–19% in males) than that observed in Acvr2-/- progeny obtained from matings of Acvr2+/- mice.
Table 3. Muscle weights of compound Act RIIA, Act RIIB mutant mice.
| Muscle weight, mg
|
|||||
|---|---|---|---|---|---|
| Mice | No. | Pectoralis | Triceps | Quadriceps | Gastrocnemius |
| Acvr2+/+Acvr2b+/+ | (n = 8) | 65.4 ± 3.1 | 89.0 ± 3.0 | 172.1 ± 6.6 | 111.9 ± 3.7 |
| Acvr2+/+Acvr2b+/- | (n = 17) | 67.0 ± 2.1 | 91.8 ± 2.3 | 174.3 ± 4.4 | 108.8 ± 3.1 |
| Acvr2-/-Acvr2b+/+ | (n = 10) | 80.6 ± 3.8* | 103.3 ± 4.6† | 198.8 ± 7.6† | 125.9 ± 4.9† |
| Acvr2-/-Acvr2b+/- | (n = 9) | 87.0 ± 3.2‡ | 117.9 ± 2.8§¶ | 217.7 ± 3.7§¶ | 142.0 ± 3.1§¶ |
, P < 0.01 vs. WT
, P < 0.05 vs. WT
, P < 0.001 vs. WT
, P < 0.0001 vs. WT
, P < 0.05 vs. Acvr2-/- Acvr2b+/+
In contrast to previously reported studies, we did obtain viable Acvr2-/-Acvr2b+/- offspring from crosses of doubly heterozygous mice. Analysis of muscle weights in Acvr2-/-Acvr2b+/- mice compared with Acvr2+/+Acvr2b+/- and Acvr2-/-Acvr2b+/+ mice showed that although the presence of one mutant Acvr2b allele had no effect on an Acvr2+/+ background, loss of a single copy of Acvr2b caused further increases in muscle mass in the complete absence of Acvr2 (Table 3 and Fig. 3B). For example, in female mice, the weight of the triceps was increased by 16% in mice lacking both copies of Acvr2 and by 32% in mice additionally lacking one copy of Acvr2b. Similar trends were observed in the other muscle groups that were examined. For most of the muscle groups analyzed in female mice, there was a statistically significant difference in comparing Acvr2+/+-Acvr2b+/+ mice with Acvr2-/-Acvr2b+/+ mice as well as in comparing Acvr2-/-Acvr2b+/+ mice with Acvr2-/-Acvr2b+/- mice. We also observed the same trends in male mice, although all of the differences were somewhat reduced compared with those observed in female mice (data not shown); additional experiments will be required to determine the significance, if any, of the gender differences in the magnitude of these effects. Nevertheless, these data constitute strong evidence that the two receptors are functionally redundant with respect to regulating muscle mass.
Because we could not analyze mice completely lacking both receptor genes and because these genetic studies could not distinguish developmental from postnatal effects on muscle mass, we examined the effects of injecting ACVR2B/Fc into mice lacking either ACVR2 or ACVR2B. In these experiments, we analyzed the effects on both WT and homozygous mutant offspring obtained from crosses of heterozygous mice to control for differences in genetic backgrounds. As shown in Table 4, administration of ACVR2B/Fc (at a dose of 10 mg/kg for 4 weeks) to Acvr2+/+ mice resulted in increases in muscle growth by 41–44% in the various muscle groups examined. Significantly, these increases in muscle growth were somewhat attenuated (29–37%), but not eliminated, in Acvr2-/- mice. Similarly, administration of ACVR2B/Fc to Acvr2b+/+ mice caused increases in muscle growth by 35–49%, and similar effects were also observed in Acvr2b-/- mice (28–49%). The finding that ACVR2B/Fc caused muscle growth in mice deficient for either receptor suggests that the effect of ACVR2B/Fc in WT mice does not result from inhibition of signaling solely through ACVR2 or solely through ACVR2B. The simplest interpretation of these data is that both ACVR2 and ACVR2B regulate muscle growth in adult mice. Consistent with these findings, we were able to detect expression of both Acvr2 and Acvr2b transcripts in adult muscle by Northern blot analysis (Fig. 3C). Although levels of Acvr2 expression varied considerably from muscle to muscle, the relative expression levels did not appear to correlate with the relative magnitude of muscle weight increases induced by ACVR2B/Fc in these muscles (Table 4).
Table 4. Muscle weights of mice injected with ACVR2B/Fc.
| Muscle weight, mg
|
|||||
|---|---|---|---|---|---|
| Mice | No. | Pectoralis | Triceps | Quadriceps | Gastrocnemius |
| Acvr2+/+ | |||||
| + PBS | (n = 10) | 58.2 ± 2.3 | 81.7 ± 2.2 | 150.8 ± 4.1 | 97.4 ± 2.8 |
| + ACVR2B/Fc | (n = 5) | 81.8 ± 4.1* | 118.0 ± 0.5† | 214.4 ± 5.4† | 139.6 ± 5.7† |
| Change, % | +40.5 | +44.4 | +42.2 | +43.4 | |
| Acvr2-/- | |||||
| + PBS | (n = 4) | 66.3 ± 8.1 | 92.0 ± 11.7 | 170.0 ± 12.6 | 116.3 ± 11.0 |
| + ACVR2B/Fc | (n = 5) | 85.6 ± 5.2 | 125.6 ± 7.6‡ | 227.0 ± 14.9‡ | 154.6 ± 12.3‡ |
| Change, % | - | +29.2 | +36.5 | +33.5 | +33.0 |
| Acvr2b+/+ | |||||
| + PBS | (n = 15) | 53.6 ± 1.2 | 76.2 ± 1.8 | 133.6 ± 4.3 | 87.1 ± 2.5 |
| + ACVR2B/Fc | (n = 9) | 79.2 ± 3.3§ | 113.6 ± 5.9§ | 186.2 ± 11.4¶ | 117.9 ± 6.2¶ |
| Change, % | +47.9 | +49.0 | +39.4 | +35.4 | |
| Acvr2b-/- | |||||
| + PBS | (n = 5) | 66.6 ± 3.6 | 95.4 ± 7.2 | 149.0 ± 11.3 | 88.6 ± 5.3 |
| + ACVR2B/Fc | (n = 4) | 99.5 ± 5.0‖ | 138.0 ± 10.7** | 200.0 ± 13.2** | 113.3 ± 7.7** |
| Change, % | +49.4 | +44.7 | +34.2 | +27.8 | |
, P < 0.001 vs. Acvr2+/+ + PBS
, P < 0.0001 vs. Acvr2+/+ + PBS
, P < 0.05 vs. Acvr2-/- + PBS
, P < 0.0001 vs. Acvr2b+/+ + PBS
, P < 0.001 vs. Acvr2b+/+ + PBS
, P < 0.001 vs. Acvr2b-/- + PBS
, P < 0.05 vs. Acvr2b-/- + PBS
Together, the data presented here demonstrate that multiple ligands, including myostatin, signal through ACVR2 and ACVR2B to limit muscle growth, with perhaps ACVR2 playing a more prominent role. Additional experiments will be required to determine whether each ligand regulating muscle growth signals through both receptors or whether each receptor is specific for a given ligand in vivo. It also will be important to identify all of the components of this signaling pathway in muscle, because agents capable of blocking this pathway could have widespread applications for the treatment of human conditions in which increasing muscle growth may be beneficial as well as for livestock production.
Acknowledgments
We thank Alexandra McPherron and Jeremy Nathans for helpful discussions. This work was supported by National Institutes of Health Grants R01HD35887 (to S.-J.L.), R01CA88866 (to S.-J.L.), and R01HD32067 (to M.M.M.) and by funds from Wyeth Research and MetaMorphix, Inc.
Author contributions: S.-J.L., L.A.R., M.V.D., S.G., M.E.P.G., K.N.T., J.F.W., L.-f.L., E.Q., R.L.S., and N.M.W. designed research; S.-J.L., L.A.R., M.V.D., S.G., M.E.P.G., K.N.T., J.F.W., C.B., G.E., J.H., B.T., B.G., M.-S.J., S.M.S., and L.-f.L. performed research; L.A.R., M.V.D., S.G., M.E.P.G., K.N.T., J.F.W., C.B., G.E., J.H., B.T., B.G., M.-S.J., M.M., E.L., and L.-f.L. contributed new reagents/analytic tools; S.-J.L. analyzed data; and S.-J.L. and N.M.W. wrote the paper.
Conflict of interest statement: Myostatin was licensed by The Johns Hopkins University to MetaMorphix and sublicensed to Wyeth. S.-J.L. is entitled to a share of sales royalty received by The Johns Hopkins University from sales of this factor. The Johns Hopkins University and S.-J.L. also own MetaMorphix stock, which is subject to certain restrictions under university policy. S.-J.L. is a paid consultant to MetaMorphix. The terms of these arrangements are being managed by the university in accordance with its conflict of interest policies.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lee, S.-J. (2004) Annu. Rev. Cell Dev. Biol. 20, 61-86. [DOI] [PubMed] [Google Scholar]
- 2.McPherron, A. C., Lawler, A. M. & Lee, S.-J. (1997) Nature 387, 83-90. [DOI] [PubMed] [Google Scholar]
- 3.Grobet, L., Martin, L. J. R., Poncelet, D., Pirottin, D., Brouwers, B., Riquet, J., Schoeberlein, A., Dunner, S., Ménissier, F., Massabanda, J., et al. (1997) Nat. Genet. 17, 71-74. [DOI] [PubMed] [Google Scholar]
- 4.Kambadur, R., Sharma, M., Smith, T. P. L. & Bass, J. J. (1997) Genome Res. 7, 910-915. [DOI] [PubMed] [Google Scholar]
- 5.McPherron, A. C. & Lee, S.-J. (1997) Proc. Natl. Acad. Sci. USA 94, 12457-12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grobet, L., Poncelet, D., Royo, L. J., Brouwers, B., Pirottin, D., Michaux, C., Ménissier, F., Zanotti, M., Dunner, S. & Georges, M. (1998) Mamm. Genome 9, 210-213. [DOI] [PubMed] [Google Scholar]
- 7.Schuelke, M., Wagner, K. R., Stolz, L. E., Hübner, C., Riebel, T., Kömen, W., Braun, T., Tobin, J. F. & Lee, S.-J. (2004) N. Engl. J. Med. 350, 2682-2688. [DOI] [PubMed] [Google Scholar]
- 8.Bogdanovich, S., Krag, T. O. B., Barton, E. R., Morris, L. D., Whittemore, L.-A., Ahima, R. S. & Khurana, T. S. (2002) Nature 420, 418-421. [DOI] [PubMed] [Google Scholar]
- 9.Wagner, K. R., McPherron, A. C., Winik, N. & Lee, S.-J. (2002) Ann. Neurol. 52, 832-836. [DOI] [PubMed] [Google Scholar]
- 10.McPherron, A. C. & Lee, S.-J. (2002) J. Clin. Invest. 109, 595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, S.-J. & McPherron, A. C. (2001) Proc. Natl. Acad. Sci. USA 98, 9306-9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thies, R. S., Chen, T., Davies, M. V., Tomkinson, K. N., Pearson, A. A., Shakey, Q. A. & Wolfman, N. M. (2001) Growth Factors 18, 251-259. [DOI] [PubMed] [Google Scholar]
- 13.Hill, J. J., Davies, M. V., Pearson, A. A., Wang, J. H., Hewick, R. M., Wolfman, N. M. & Qiu, Y. (2002) J. Biol. Chem. 277, 40735-40741. [DOI] [PubMed] [Google Scholar]
- 14.Hill, J. J., Qiu, Y., Hewick, R. M. & Wolfman, N. M. (2003) Mol. Endocrinol. 17, 1144-1154. [DOI] [PubMed] [Google Scholar]
- 15.Whittemore, L.-A., Song, K., Li, X., Aghajanian, J., Davies, M. V., Girgenrath, S., Hill, J. J., Jalenak, M., Kelley, P., Knight, A., et al. (2003) Biochem. Biophys. Res. Commun. 300, 965-971. [DOI] [PubMed] [Google Scholar]
- 16.Wolfman, N. M., McPherron, A. C., Pappano, W. N., Davies, M. V., Song, K., Tomkinson, K. N., Wright, J. F., Zhao, L., Sebald, S. M., Greenspan, D. S. & Lee, S.-J. (2003) Proc. Natl. Acad. Sci. USA 100, 15842-15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebbapragada, A., Benchabane, H., Wrana, J., Celeste, A. J. & Attisano, L. (2003) Mol. Cell. Biol. 23, 7230-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Caestecker, M. (2004) Cytokine Growth Factor Rev. 15, 1-11. [DOI] [PubMed] [Google Scholar]
- 19.Matzuk, M., Kumar, T. & Bradley, A. (1995) Nature 374, 356-360. [DOI] [PubMed] [Google Scholar]
- 20.Oh, S. P. & Li, E. (1997) Genes Dev. 11, 1812-1826. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman, R. J. (1990) Methods Enzymol. 185, 487-511. [DOI] [PubMed] [Google Scholar]
- 22.Campion, D. R. (1984) Int. Rev. Cytol. 87, 225-251. [DOI] [PubMed] [Google Scholar]
- 23.Gamer, L. W., Wolfman, N. M., Celeste, A. J., Hattersley, G., Hewick, R. & Rosen, V. (1999) Dev. Biol. 208, 222-232. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima, M., Toyono, T., Akamine, A. & Joyner, A. (1999) Mech. Dev. 80, 185-189. [DOI] [PubMed] [Google Scholar]
- 25.McPherron, A. C., Lawler, A. M. & Lee, S.-J. (1999) Nat. Genet. 22, 260-264. [DOI] [PubMed] [Google Scholar]
- 26.Esquela, A. F. & Lee, S.-J. (2003) Dev. Biol. 257, 356-370. [DOI] [PubMed] [Google Scholar]
- 27.Wu, H.-H., Ivkovic, S., Murray, R. C., Jaramillo, S., Lyons, K. M., Johnson, J. E. & Calof, A. L. (2003) Neuron 37, 197-207. [DOI] [PubMed] [Google Scholar]
- 28.Kim, J., Wu, H.-H., Lander, A. D., Lyons, K. M., Matzuk, M. M. & Calof, A. L. (2005) Science 308, 1927-1930. [DOI] [PubMed] [Google Scholar]
- 29.Harmon, E. B., Apelqvist, A. A., Smart, N. G., Gu, X., Osborne, D. H. & Kim, S. K. (2004) Development (Cambridge, U.K.) 131, 6163-6174. [DOI] [PubMed] [Google Scholar]
- 30.Song, J., Oh, S. P., Schrewe, H., Nomura, M., Lei, H., Okano, M., Gridley, T. & Li, E. (1999) Dev. Biol. 213, 157-169. [DOI] [PubMed] [Google Scholar]