Abstract
Abnormal processing of somatosensory inputs in the central nervous system (central sensitization) is the mechanism accounting for the enhanced pain sensitivity in the skin surrounding tissue injury (secondary hyperalgesia). Secondary hyperalgesia shares clinical characteristics with neurogenic hyperalgesia in patients with neuropathic pain. Abnormal brain responses to somatosensory stimuli have been found in patients with hyperalgesia as well as in normal subjects during experimental central sensitization. The aim of this study was to assess the effects of gabapentin, a drug effective in neuropathic pain patients, on brain processing of nociceptive information in normal and central sensitization states. Using functional magnetic resonance imaging (fMRI) in normal volunteers, we studied the gabapentin-induced modulation of brain activity in response to nociceptive mechanical stimulation of normal skin and capsaicin-induced secondary hyperalgesia. The dose of gabapentin was 1,800 mg per os, in a single administration. We found that (i) gabapentin reduced the activations in the bilateral operculoinsular cortex, independently of the presence of central sensitization; (ii) gabapentin reduced the activation in the brainstem, only during central sensitization; (iii) gabapentin suppressed stimulus-induced deactivations, only during central sensitization; this effect was more robust than the effect on brain activation. The observed drug-induced effects were not due to changes in the baseline fMRI signal. These findings indicate that gabapentin has a measurable antinociceptive effect and a stronger antihyperalgesic effect most evident in the brain areas undergoing deactivation, thus supporting the concept that gabapentin is more effective in modulating nociceptive transmission when central sensitization is present.
Keywords: deactivation, fMRI, hyperalgesia, nociceptive system
After skin injury, an increased sensitivity to mechanical stimuli occurs in a large, uninjured area surrounding the injury site (1, 2). This phenomenon is termed secondary hyperalgesia and is the consequence of neuroplastic changes leading to a state of sensitization of the central nervous system (central sensitization) (3). Secondary hyperalgesia can be experimentally induced by treating the skin with high doses of the vanilloid capsaicin (by intradermal injection or topical application).
Two forms of mechanical hyperalgesia occur in the area of secondary hyperalgesia: hyperalgesia to gentle skin stroking (stroking hyperalgesia or allodynia) and hyperalgesia to punctate stimuli (punctate hyperalgesia). Although both stroking and punctate hyperalgesia are due to central sensitization, they have different psychophysical characteristics (punctate hyperalgesia is easier to establish, it encompasses a larger area and it is longer-lasting than stroking hyperalgesia). They are mediated by different primary afferents (3): stroking hyperalgesia is signaled by low-threshold mechanoreceptors (4), whereas punctate hyperalgesia is signaled by capsaicin-insensitive A-fiber nociceptors (type I A-fiber mechano-heat nociceptors) that project to mechanospecific spinal interneurons sensitized by strong activation of C-fiber nociceptors after capsaicin injection (5-7).
Neuropathic pain is defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system. It is characterized by two different clinical features: neurogenic hyperalgesia in patients with minor sensory impairment and painful hypoalgesia in patients with major sensory deficits (8). Central sensitization of nociceptive neurons in the spinal cord by primary nociceptive afferent input is the mechanism underlying neurogenic hyperalgesia during neuropatic pain. Accordingly, patients with pain and hyperalgesia exhibit a nearly identical shift of stimulus-response function and incidence of hyperalgesia to stroking as normal subjects after capsaicin injection (9). These findings indicate that capsaicin-induced experimental hyperalgesia is a valid human surrogate model for neurogenic hyperalgesia, and support the view that central sensitization in neurogenic hyperalgesia is a feature of one group of neuropathic pain states (8, 10).
Gabapentin is a compound known to bind to the α2δ-type voltage-gated calcium channels (11). It has an analgesic effect documented in several rat models of neuropathic pain (12, 13) and in patients with different neuropathic pain conditions, such as postherpetic neuralgia and painful diabetic neuropathy (14, 15). Experimental studies in animals and human volunteers have shown that, although gabapentin exerts a minimal modulation of normal synaptic transmission in the dorsal horn (i.e., it has a negligible antinociceptive effect) (16, 17), it is clearly able to modulate the development of and synaptic transmission during central sensitization (i.e., it has a stronger antihyperalgesic effect) (16, 18). However, the site and mechanism of action of gabapentin are still undefined (19).
We have demonstrated that pharmacological functional MRI (fMRI) is a robust and reliable technique to detect central effects of pain-relieving drugs (20, 21), and the combination of drug administration with fMRI has been recently recommended in European guidelines for neuropathic pain assessment (22). Therefore, to evaluate the effects of gabapentin on processing of nociceptive inputs during normal and central sensitization state, we used blood oxygen level-dependent (BOLD) fMRI in normal volunteers to investigate the gabapentin-induced modulation of brain responses to noxious punctate mechanical stimulation of normal skin and of an area of capsaicin-induced secondary hyperalgesia.
Materials and Methods
Subjects. Twelve right-handed male volunteers aged 25-30 years (mean, 27 ± 2.1) participated in the study. Inclusion and exclusion criteria are given in the Supporting Text, which is published as supporting information on the PNAS web site. All participants gave written informed consent, and the local ethics committee approved the procedures.
Study Design. The study was double-blind, placebo controlled, four-way crossover with two paired randomized periods. Subjects underwent four fMRI scanning sessions, referred to as periods 1-4, after the administration of placebo (periods 1 and 3) or gabapentin (periods 2 and 4) in the presence and absence of central sensitization. The administration of placebo and gabapentin within experimental condition (i.e., normal state and central sensitization) was randomized. For a scheme of the study design, see Fig. 4, which is published as supporting information on the PNAS web site.
In each of the 4 scanning periods subjects received 1,800 mg of gabapentin or placebo per os. The order of drug and placebo administration was randomized across subjects.
Two hours after the drug/placebo administration, the areas for mechanical stimulation were defined. For each subject, two concentric rectangles were drawn on the shin area on both legs, at symmetrical sites: an inner rectangle (4 × 4 cm) and an outer rectangle (8 × 8 cm). The area between the borders of the two rectangles defined a 48-cm2 “target” area where mechanical stimuli were delivered during the fMRI scan. Three hours after drug/placebo administration, fMRI was performed. Four hours after the drug/placebo administration, a blood sample was taken to measure drug concentration in the plasma. Timing of actions performed during periods 3 and 4 is summarized in Fig. 4B, and compared to the known time course of the gabapentin plasma concentration after the same administration procedure.
In periods 3 and 4 only, combined thermal and chemical nociceptive stimulation was used to induce secondary hyperalgesia to punctate mechanical stimuli (23): 45°C thermal stimulation was delivered for 5 min in the middle of the inner rectangle (4 × 4 cm) of the right leg, using a contact thermode (3 × 3 cm). Immediately afterward, 0.075% capsaicin cream was applied over the same area as the thermode and left on until the end of the fMRI scanning. The inner rectangle was larger than the size of the thermode (and implicitly the site of capsaicin application) to exclude any spread of the capsaicin cream to the area of punctate stimulation, ensuring that mechanical stimuli were limited to the area of secondary hyperalgesia. Forty-five minutes after capsaicin application (before fMRI scanning) and at the end of fMRI scanning, we checked for the development of secondary hyperalgesia in the “target” area. The presence of secondary hyperalgesia was defined as a clear change in sensation (“increased pain sensation”) after mechanical stimulation of the “target” skin area compared to stimulation at least 15 cm outside the treatment area (24). At the end of fMRI scan, average ratings for spontaneous pain and burning sensations during the fMRI session were collected, using a 0-100 scale. Afterward, capsaicin was removed. To avoid long-lasting effects (i.e., capsaicin-induced desensitization, ref. 5) on subsequent sessions, capsaicin application was always performed in the last two study periods.
fMRI Scanning and Stimulation Paradigm. During each period, fMRI was performed on a 3T system. The timing of experimental events during each scanning session is summarized in Fig. 4 B and C.
A head-only gradient coil was used with a birdcage radio-frequency head coil for pulse transmission and signal reception. A whole-brain gradient-echo, echo-planar imaging sequence was used for functional scans (echo time = 30 ms, 24 contiguous 6-mm-thick axial slices, field of view 256 × 192 mm, matrix 64 × 64) with a repetition time of 3 s over 500 volumes, corresponding to a total scan time of 25 min. During the functional scan, the same researcher delivered 40 identical punctate stimuli to the target area on the right or left leg, using a 60-g von Frey hair (i.d. = 0.8 mm); the duration of each stimulus was ≈1 s; the same number of stimuli (20) was delivered to each leg; the side of the stimulation was randomized, and the stimulus was pseudorandomly jittered in time, with an average interstimulus interval of 30 s (range, 20-40 s). Subjects were instructed to keep their eyes closed throughout the mechanical stimulation. Thirty seconds after the last mechanical stimulus, visual stimulation was delivered to control for a possible confounding effect of global modulation of BOLD signal by gabapentin. Visual stimulation consisted of five 30-s blocks of a black and white checkerboard flickering at 2 Hz, alternated with five 30-s blocks of rest.
Lastly, in period 1 only, a T1-weighted, 1 × 1 × 3 mm structural image was collected for overlay of brain activation and registration.
Analysis of Stimulus-Evoked fMRI Signal Changes. Image analysis to reveal significant brain activity based on changes in BOLD signal was performed on each subject's data by using feat (www.fmrib.ox.ac.uk/fsl). The following processing was applied to each subject's time series of fMRI volumes: motion correction (25), spatial smoothing using a Gaussian kernel of full-width-half-maximum of 5 mm, demeaning of each voxel time course, and nonlinear high-pass temporal filtering (cutoff 50 s). The fMRI signal was then positively and negatively linearly modeled by using a general linear model approach with local autocorrelation correction (26).
Group analysis was carried out by using a mixed-effect approach (27), generating group-representative statistical maps of increased and decreased brain activity in response to mechanical and visual stimulation. Paired comparisons were performed to test for differences between experimental conditions (drug/placebo, capsaicin/control). For the purpose of the group analysis, registration of low-resolution functional images to the corresponding high-resolution structural images was performed (28), followed by registration to a standard brain (MNI template) (29). The Z statistic images from the group analysis were thresholded at Z > 2.3, with a cluster threshold of P = 0.01. This cluster-based significance thresholding procedure provides a correction for multiple comparisons (30).
Analysis of Baseline fMRI Signal. Analysis of baseline BOLD fMRI signal was performed on the preprocessed data. Masks of gray and white brain matter were obtained by segmenting the high-resolution structural scan of each subject. These segmented images were eroded to account for inaccuracies in the segmentation process and transformed into the functional space of the four corresponding functional scans (25). The gray and white matter masks in functional space were subsequently applied to an image obtained by averaging the whole functional scan, and to an image obtained by averaging only the functional images collected between 5 s after the end of the mechanical and the start of the visual stimulation (i.e., 25 s without any expected response to external stimulation). The ratio between mean signal intensities of gray and white matter was calculated to provide a scaled value of baseline fMRI signal comparable between-subject and across the four periods. To control for possible modulations of the baseline fMRI signal in the regions involved in processing nociceptive inputs, the gray matter mask was further masked with eight anatomical regions of interest [pain-matrix regions of interest (ROIs), see below]. Baseline fMRI signal values thus obtained were compared across the four study periods using a two-way repeated measures ANOVA, with “drug treatment” and “capsaicin application” as factors.
ROI Analysis. Eleven ROIs were anatomically defined on the high-resolution structural image of each subject and transformed into the corresponding four functional images of the same subject. The ROIs included left and right thalamus, left and right insula, anterior cingulate cortex, left and right primary and secondary somatosensory cortex, brainstem, and primary visual cortex.
A single summary value was calculated from all of the voxels constituting each ROI by using the following procedure: the β values of all of the voxels in each ROI were extracted, and the mean of the 20% of voxels with the highest positive β values was calculated and taken as the summary. This “top 20%” summary (as we will refer to hereafter) concentrates on the higher β values within each ROI, with the aim of reducing the noise introduced by including all voxels, some of which may have little or no activation. This approach shows several advantages for disclosing condition-specific effects: (i) it takes into account the possible functional heterogeneity within the same anatomical ROI; (ii) it takes into account the functional variability between-subjects; (iii) it avoids the problem of selecting just outlier values; (iv) it allows comparing the same number of voxels for each ROI across different periods. In preliminary analyses, we found that the value of 20% provides the best sensitivity in disclosing a drug-induced effect.¶
A mixed-effect model was used to analyze the ROI data for main effects of gabapentin and central sensitization on stimulus-evoked positive BOLD signal changes. Fixed effects were central sensitization, drug treatment, the interaction between central sensitization and treatment, and a session (order) effect. Random effects were subject and central sensitization within-subjects. The model was fitted by using the Satterthwaite correction for the degrees of freedom.
Results
Psychophysics. All subjects developed hyperalgesia to punctate mechanical stimulation after heat/capsaicin application. Hyperalgesia was still present at the end of the fMRI scanning. Average ratings for spontaneous pain sensation were 4 ± 5.9 and 3 ± 6.2 (of 100) during periods 3 (placebo) and 4 (gabapentin), respectively; this difference was not significant (P > 0.2, paired t test). Average ratings for spontaneous burning sensation were 30 ± 18.7 and 19 ± 19.8 (of 100) during periods 3 (placebo) and 4 (gabapentin), respectively; this difference was not statistically significant (P = 0.066, paired t test, one-tailed).
Gabapentin Plasma Concentrations. In all subjects, gabapentin was detected in the plasma during drug periods only. Plasma concentrations ranged between 3.4 and 10.6 μg/ml (average 6.8), and were not different between drug treatments (periods 2 and 4, paired t test, P > 0.2).
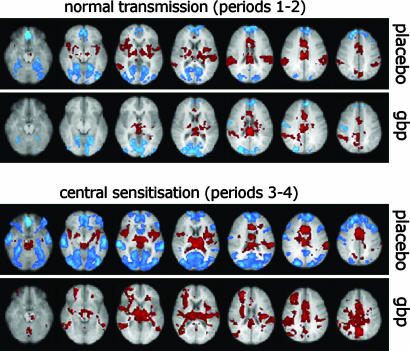
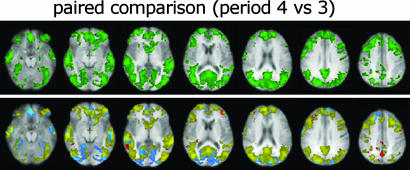
Whole-Brain Analysis. Statistical parametric maps showing brain areas significantly activated and deactivated in response to punctate stimulation of the right leg in the four periods are displayed in Fig. 1. The paired group comparison between placebo and drug during central sensitization (periods 3 vs. 4) was the only comparison that showed significant differences, with increased fMRI signal in response to right leg stimulation during gabapentin (Fig. 2). Because this increase in fMRI signal could have been due to either an increased activation or a decreased deactivation during gabapentin compared to placebo (or both), we performed a retrospective analysis of the voxels significantly different between these two conditions. This analysis revealed that 94% of these voxels (color-coded in blue and yellow) were deactivated during placebo (period 3), but less deactivated or activated during gabapentin (period 4) (Fig. 2). This finding indicates that the observed difference was mostly the result of gabapentin-induced reduction of brain deactivations in response to punctate mechanical stimulation.
Fig. 1.
Group activations in response to punctate mechanical stimulation of the right leg. Brain areas significantly activated (red) or deactivated (blue) by stimulation of normal skin (Upper) or of the area of secondary hyperalgesia (Lower), after placebo (upper row) or a single dose (1,800 mg) of gabapentin (gbp, lower row). Note the drug-induced reduction in activation within the “pain matrix” (e.g., right and left insular cortex, brainstem, thalamus) and in deactivation outside the “pain matrix” (e.g., occipital, frontal and temporal cortex).
Fig. 2.
Paired group comparison of whole-brain activity during central sensitization between placebo (period 3) and gabapentin (period 4). (Upper) Voxels with a significant fMRI signal increase during period 4. (Lower) The same voxels, color-coded according to their response to stimulation in the two periods. Blue voxels showed deactivation in period 3 and reduced deactivation in period 4; yellow voxels showed deactivation in period 3 and activation in period 4; red voxels showed activation in period 3 and increased activation in period 4. Note that most of the voxels (94%) are blue and yellow, indicating that the fMRI signal increase during period 4 was mostly the result of drug-induced reduction of brain deactivations in response to punctate mechanical stimulation.
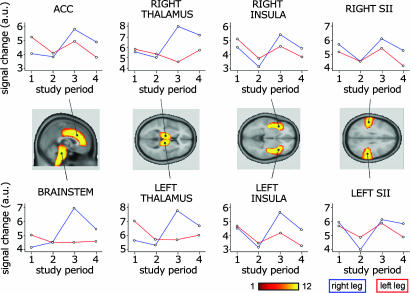
ROI Analysis. The analysis on the top 20% ROI data showed the following results (summarized in Table 1, which is published as supporting information on the PNAS web site). After right leg stimulation: (i) Main effect of central sensitization, with increased fMRI signal during the capsaicin periods in the contralateral (left, P = 0.031) and ipsilateral (right, P = 0.058) thalamus, the anterior cingulate cortex (P = 0.054), and brainstem (P = 0.019). (ii) Main effect of drug, with reduced fMRI signal during the drug periods in the contralateral (left, P = 0.019) and ipsilateral (right, P = 0.007) insula, and in the contralateral (left, P = 0.070) and ipsilateral (right, P = 0.047) secondary somatosensory cortex (SII). (iii) Interaction between the effects of drug and central sensitization in the brainstem, where, in the presence of central sensitization, the fMRI signal was increased more during placebo period (P = 0.013) compared to gabapentin period (P = 0.401).
After left leg stimulation: (i) Lack of any effect of central sensitization. (ii) Main effect of drug, with reduced fMRI signal during the drug periods in the contralateral (right, P = 0.009) and ipsilateral (left, P = 0.001) insular cortex, the anterior cingulate cortex (P = 0.004) and the contralateral (right, P = 0.062) and ipsilateral (left, P = 0.011) SII.
Baseline BOLD fMRI Signal. Because baseline BOLD fMRI signal values obtained by averaging the whole functional scan and those obtained by averaging the 25 s without external stimulation were nearly identical in all of the explored conditions (P > 0.8, paired t test), only the results based on baseline signal obtained averaging the whole functional scan are reported. Baseline fMRI signal was not affected by the factor “drug treatment” (P > 0.5), both in the total gray matter and in the pain-matrix ROIs. In contrast, baseline fMRI signal was significantly reduced in periods 3 and 4, when capsaicin was applied; this effect was more significant when baseline signal was obtained from the total gray matter (P < 0.02) than from the pain-matrix ROIs (P < 0.05, repeated measures ANOVA). Baseline fMRI signal values for the four study periods are represented in Fig. 5, which is published as supporting information on the PNAS web site.
Discussion
Our results show that gabapentin has a major effect on human brain responses to nociceptive mechanical stimulation when neural transmission of nociceptive inputs is enhanced, i.e., when central sensitization is present. A smaller but measurable antinociceptive effect of gabapentin was also observed, independently of the presence of central sensitization. The most robust gabapentin effect during central sensitization was a reduction of stimulus-induced brain deactivations. Baseline analysis of BOLD fMRI signal showed that the observed drug effects were not caused by drug-induced global changes of brain activity, indicating a true gabapentin modulation of neural transmission. These findings support the concept that gabapentin is more effective in preventing the establishment of a central sensitization state and/or in reducing the neural transmission when central sensitization is present.
Afferent Input, Central Sensitization, and Brain Activations. Stimulation of the skin using von Frey filaments allows us to apply a reproducible and controlled force. In this study we delivered mechanical stimuli using a von Frey filament exerting a force of 588 mN over a round surface of ≈0.5 mm2 (i.d. = 0.8 mm). Although this rather large diameter also deforms the deep cutaneous tissues and activates non-nociceptive mechanoreceptors (31), the somato-sensory input was largely nociceptive, because the force exerted was suprathreshold for type-I A-fiber mechano-heat nociceptors (32). This kind of nociceptor signals pain to punctate stimuli in normal skin (5), as well as punctate hyperalgesia during capsaicin-induced central sensitization (7).
Topical application of capsaicin over preheated skin resulted in the development of secondary hyperalgesia to punctate stimuli in all subjects, confirming the validity of this model to induce central sensitization (23, 24, 33), although the contribution of preliminary skin heating has been recently questioned and is probably negligible (34).
Although central sensitization has been described mainly as a spinal cord phenomenon (3), supraspinal structures contribute to its development and maintenance (35). In addition, descending facilitatory influences have been suggested to underlie chronic pain states (36, 37). Some studies have recently investigated the brain responses to somatosensory stimuli delivered to an area of secondary hyperalgesia during central sensitization, in normal subjects and in patients (24, 33, 38-40). The results of these studies are somewhat contrasting, mainly because of the different kind of somato-sensory stimulation delivered (innocuous brushing, punctate stimulation of different intensities) and the investigation technique chosen (fMRI, PET, MEG). We found that brain activity in the brainstem, bilateral thalamus, and the anterior cingulate cortex was significantly increased when punctate stimuli were delivered to the area of secondary hyperalgesia (Figs. 1 and 3). These structures are part of the larger network of brain areas reported in the only study investigating secondary hyperalgesia to punctate stimuli using whole-brain fMRI (24). This difference can be explained by the stronger somatosensory input used in the present study, which produces a more robust activation of pain-related areas in the normal state, thus making the size of the increased activity during central sensitization comparatively smaller.
Fig. 3.
ROI analysis. Plots showing fMRI signal changes for the four study periods (1, normal state/placebo; 2, normal state/drug; 3, central sensitization/placebo; 4, central sensitization/drug) in a priori selected ROIs. Blue and red lines indicate the stimulation of the right and left leg, respectively. ROIs were anatomically defined on the high-resolution structural image of each subject. For each ROI, the number of subjects showing spatial overlap is color coded from red to yellow. Note the main effect of gabapentin in the right and left insula (after stimulation of both legs, P < 0.02), the main effect of central sensitization in the anterior cingulate cortex (P = 0.054), right (P = 0.058) and left (P = 0.031) thalamus (after right leg stimulation), and the interaction between central sensitization and drug effect in the brainstem after right leg stimulation, i.e., greater evidence of central sensitization effect during placebo (P = 0.013) than during drug (P = 0.401). A systematic description of the ROI analysis results is shown in Table 1.
Functional Meaning of Stimulus-Induced Decreases of the fMRI Signal. PET and fMRI studies have shown that the activity in a large and specific set of brain regions is consistently reduced in response to a range of sensory stimuli. These regions include large areas of the medial posterior cortices (the posterior cingulate, precuneus and retrosplenial cortices), the lateral posterior cortices (especially the parieto-temporo-occipital junction) and the ventral and dorsal medial prefrontal cortices, as revealed by two large metaanalyses (41, 42). Signal decreases in these regions are task-independent (i.e., they are present during a large spectrum of experimental paradigms), and are pronounced when the task of interest is compared with a truly passive state, i.e., eyes closed or fixation (for review, see ref. 43). Because the stimulus-evoked decrease of fMRI signal reflects, at least in part, an actual reduction in neural activity (44, 45), when a true passive baseline is present these diffuse signal decreases can be considered to represent deactivations. The leading hypothesis regarding the functional meaning of this phenomenon suggests that the network of regions showing these task-independent, stimulus-induced deactivations underlies a “default mode” of brain activity, which is mainly present at rest and strongly reduced during various goal-directed tasks (46). In other words, during a novel and specific task, processing resources are moved from the areas normally engaged in the “default mode” to the areas relevant for the presented task. Accordingly, in the present study we show that significant brain deactivations are induced by nociceptive stimulation in the normal state (Fig. 1, period 1). During central sensitization, nociceptive stimulation of the area of secondary hyperalgesia induced stronger deactivations in a much larger set of brain regions (Fig. 1, period 3), namely the inferior and medial temporal cortices and the fusiform gyrus bilaterally, the orbito-frontal cortex bilaterally, the perigenual cingulate cortex, large sections of the occipital cortex, the medial prefrontal cortex, large sections of the frontal cortex (medial and superior), and the medial parietal cortex (including posterior cingulate and precuneus); these areas include the majority of structures commonly deactivated across a wide range of tasks and stimulus modalities (43). The increased magnitude and the larger extent of deactivated areas in the central sensitization state suggest a greater shift of processing resources toward a stronger and more attentionally demanding stimulus. Accordingly, an increase in magnitude of deactivation has been demonstrated with a cognitive task of increasing difficulty (47), and also reported with increased perception of different kinds of experimental pain, in the medial posterior cortices and the parieto-temporo-occipital junction (48), as well as in the medial prefrontal and perigenual cingulate cortex (49), i.e., all regions consistently deactivated also in the present study.
Gabapentin Modulation of fMRI Brain Responses: Antihyperalgesic Effects. The main finding of this study is that gabapentin has a state-specific action, i.e., it has a major modulatory effect on fMRI brain responses to nociceptive inputs only during central sensitization (Figs. 3 and 4). A dissociation between the size of antinociceptive and antihyperalgesic effects of gabapentin is also supported by other studies. Behavioral and electrophysiological experiments in animals show that gabapentin has minor modulatory effects on acute nociception, but it produces a robust dose-dependent inhibition of allodynia and hyperalgesia in different models of central sensitization (12, 13, 16, 50). In humans, gabapentin does not increase thermal and mechanical pain thresholds (51) or tolerance to cold pressure test (17), but it reduces stroking and punctate hyperalgesia in experimental central sensitization (18, 52). Gabapentin also reduced pain intensity in patients with postherpetic neuralgia and diabetic neuropathy, as shown in two large, placebo-controlled studies (14, 15), although it must be noted that the modality of gabapentin administration in patients (by slowly increasing the dose over weeks) is considerably different from the single-dose administration we adopted in this study (because of the time constraints of the fMRI experimental design). The fact that our results have been obtained in a time window of 1-2 h after hyperalgesia induction confirms the concept that gabapentin is also effective in counteracting acute hyperalgesia, and that the up-regulation of the α2δ subunit of the voltage-dependent calcium channel, occurring in animal models of hyperalgesia with a time course of several days (53), is not a prerequisite for its action.
Contrasting results of antihyperalgesic effect have been reported when gabapentin is administered after hyperalgesia is induced, suggesting a stronger effect in preventing the induction of central sensitization (18, 54). For this reason we applied the capsaicin when the plasma concentration of the drug was already significant (Fig. 4).
Despite all of the evidence of a robust antihyperalgesic effect of gabapentin in animals, normal subjects and neuropathic pain patients, its site and mechanisms of action remain unclear. Our results address these issues, indicating that the antihyperalgesic effects of gabapentin are specifically reflected in (i) an especially strong reduction of stimulus-evoked deactivation in a large set of brain areas and (ii) a reduction of stimulus-evoked activation in the brainstem (Figs. 3 and 4).
Gabapentin effect on deactivations. Because the gabapentin-induced reduction in brain deactivations encompassed several brain structures, it could have been merely due to a global change in brain metabolism. However, its specificity for somatosensory processing during central sensitization is indicated by two factors: first, the absence of a similar modulation during the normal state (study period 1 vs. 2), second, the lack of gabapentin-induced changes in the baseline fMRI signal (see Fig. 5 and Supporting Text). fMRI signal changes are detected by comparing a task of interest (in this case the somatosensory stimulation) with a baseline condition. Consequently, changes in baseline can produce misleading results, because the total energy rather than the energy increment is needed for neural function. For example, stimulus-evoked fMRI changes have increased magnitude in anaesthetized animals (55, 56). Similarly, the large reduction of stimulus-evoked deactivations in period 4 when compared to period 3 (Fig. 2) could have been simply a result of reduction of baseline fMRI signal induced by the drug. However there was no evidence for an fMRI baseline signal difference in periods 3 and 4, thus excluding an important global effect of gabapentin on cerebral metabolism, and indicating a specific effect on the brain processing of facilitated nociceptive input.
The gabapentin-induced decrease of stimulus-evoked brain deactivations could be interpreted as an epiphenomenon of the reduction of stimulus-evoked brain activations (Fig. 3), because positive and negative BOLD fMRI responses are functionally closely coupled (45), and the magnitude of stimulus-evoked brain deactivations parallels that of activations (47-49). This hypothesis seems unlikely, because the gabapentin effect we observed on brain deactivations was much more robust than that on brain activations (the latter being only detected using an ROI-based approach) (Figs. 2 and 4). This finding could challenge the notion that task-independent, diffuse stimulus-evoked brain deactivations are simply the counterpart of brain activations, and maybe suggest a more complex functional meaning than a sudden reallocation of processing resources toward areas engaged in elaborating a novel incoming stimulus. Alternatively, the physiological mechanisms mediating the stimulus-evoked reductions in neural activity (which are still largely unknown) could be differentially sensitive to drug modulation from those mediating functional activations, thus making drug effects more evident on negative than positive BOLD fMRI responses.
Gabapentin effect on brainstem activations. The brainstem was the only region to show a clear interaction between central sensitization and drug effect, with central sensitization increasing brainstem activity during placebo (P = 0.01) but not during gabapentin (P = 0.4) (Fig. 3, lower left graph). The increase in brainstem activity during central sensitization under placebo was the most significant among all of the explored ROIs; this finding confirms the important role of this structure in developing and maintaining central sensitization and secondary hyperalgesia, as shown in animal (35) and human studies (24). The present finding demonstrates that the increased brainstem activity during central sensitization is specifically reduced by gabapentin administration. However, the question of whether this effect is due to a direct action of gabapentin on brainstem structures or is secondary to a spinal or a peripheral action of gabapentin remains unanswered. Although an antihyperalgesic action of gabapentin has only been demonstrated at the level of the dorsal horn or the subnucleus caudalis (its functional equivalent in the trigeminal system) (57-59), the hypothesis of a direct gabapentin effect on brainstem structures is reasonable, because spino-bulbo-spinal circuits are contributing to experimental central sensitization. Accordingly, gabapentin has been demonstrated to have ubiquitous effects in the central nervous system (e.g., refs. 60 and 61), and, more importantly, systemic administration of gabapentin reduces the descending facilitatory influences from the reticular formation on the trigeminal sensory neurons (62). The observed interaction between central sensitization and drug effect at brainstem level in humans is in good agreement with a recent study showing that (i) nerve ligation in rodents specifically activates a spinal-bulbo-spinal loop that drives serotoninergic pathways from the brainstem, which, in turn, enhance nociception via a facilitatory action at presynaptic 5HT3 receptors of primary nociceptive afferents, and (ii) that gabapentin is effective only when central sensitization is mediated by this specific brainstem pathway (63). Studies exploring a possible modulation of electrophysiological responses from brainstem structures known to be involved in maintaining hyperalgesia in animal models of central sensitization are needed to address this issue further.
Gabapentin Modulation of fMRI Brain Responses: Antinociceptive Effects. Gabapentin reduced the brain responses to nociceptive inputs independently of the presence of the central sensitization and of the stimulated leg, i.e., it had a measurable antinociceptive effect. This antinociceptive effect was found to be significant only after restricting the analysis to selected and a priori defined brain areas, indicating that it was less robust than the observed antihyperalgesic effect. Consistently, an absent or minimal antinociceptive effect of gabapentin has been reported in electrophysiological and behavioral experiments in animals (e.g., ref. 16) and behavioral experiments in normal subjects (17, 52) and neuropathic pain patients (51), although in some cases the low dose used could have made it difficult to detect an effect (600 mg in ref. 17). The strongest gabapentin antinociceptive effect was detected in the bilateral insula and, less significantly, in the bilateral SII, after the stimulation of both right and left leg (Fig. 3 and Table 1). These regions constitute the main part of the lateral pain system, are largely involved in processing the sensory components of pain perception, and show clear intensity-dependent activations across different nociceptive inputs and techniques used (e.g., refs. 64 and 65). In contrast with the antihyperalgesic effect of gabapentin, its observed antinociceptive action probably takes place directly at cerebral level, because the physiological spinal processing of nociceptive input is not inhibited by systemic gabapentin administration (16, 66).
Supplementary Material
Acknowledgments
We thank Drs. H. Schlitt and R. Buck for helpful advice. This study was partly supported by Pfizer Ltd. (G.D.I., T.S.S., J.P.H., T.J.B., and W.V.), the Wellcome Trust (R.G.W.), and Higher Education Funding Council for England (I.T.).
Author contributions: G.D.I., T.J.B., J.P.H., W.V., and I.T. designed research; G.D.I. and L.Z. performed research; R.G.W. and T.S.S. contributed new reagents/analytic tools; G.D.I., R.G.W., and T.S.S. analyzed data; and G.D.I. wrote the paper.
Conflict of interest statement: This work has been partly supported by Pfizer, Ltd. All funding sources have been acknowledged in the text.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BOLD, blood oxygen level-dependent; ROI, region of interest; fMRI, functional MRI.
See Commentary on page 17885.
Footnotes
Buck, R., Smart, T., Schliett, A., Iannetti, G. D., Wise, R. & Tracey, I. (2005) 11th Annual Meeting of the Organisation for Human Brain Mapping, June 12-16, 2005, Toronto, Canada, abstr. 1290.
References
- 1.Lewis, T. (1936) Clin. Sci. 2, 373-421. [Google Scholar]
- 2.Hardy, J. D., Woolf, H. G. & Goodell, H. (1952) J. Clin. Invest. 31, 115-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer, R. A. & Treede, R. D. (2004) in Hyperalgesia: Molecular Mechanisms and Clinical Implications, eds. Brune, K. & Handwerker, H. O. (IASP Press, Seattle), Vol. 30, pp. 143-155. [Google Scholar]
- 4.Torebjork, H. E., Lundberg, L. E. & LaMotte, R. H. (1992) J. Physiol. 448, 765-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magerl, W., Fuchs, P. N., Meyer, R. A. & Treede, R. D. (2001) Brain 124, 1754-1764. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs, P. N., Campbell, J. N. & Meyer, R. A. (2000) Pain 84, 141-149. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler, E. A., Magerl, W., Meyer, R. A. & Treede, R. D. (1999) Brain 122, 2245-2257. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner, U., Magerl, W., Klein, T., Hopf, H. C. & Treede, R. D. (2002) Pain 96, 141-151. [DOI] [PubMed] [Google Scholar]
- 9.Magerl, W., Wilk, S. H. & Treede, R. D. (1998) Pain 74, 257-268. [DOI] [PubMed] [Google Scholar]
- 10.Klein, T., Magerl, W., Rolke, R. & Treede, R. D. (2005) Pain 115, 227-233. [DOI] [PubMed] [Google Scholar]
- 11.Gee, N. S., Brown, J. P., Dissanayake, V. U., Offord, J., Thurlow, R. & Woodruff, G. N. (1996) J. Biol. Chem. 271, 5768-5776. [DOI] [PubMed] [Google Scholar]
- 12.Jun, J. H. & Yaksh, T. L. (1998) Anesth. Analg. 86, 348-354. [DOI] [PubMed] [Google Scholar]
- 13.Hwang, J. H. & Yaksh, T. L. (1997) Reg. Anesth. 22, 249-256. [DOI] [PubMed] [Google Scholar]
- 14.Backonja, M., Beydoun, A., Edwards, K. R., Schwartz, S. L., Fonseca, V., Hes, M., LaMoreaux, L. & Garofalo, E. (1998) J. Am. Med. Assoc. 280, 1831-1836. [DOI] [PubMed] [Google Scholar]
- 15.Rowbotham, M., Harden, N., Stacey, B., Bernstein, P. & Magnus-Miller, L. (1998) J. Am. Med. Assoc. 280, 1837-1842. [DOI] [PubMed] [Google Scholar]
- 16.Stanfa, L. C., Singh, L., Williams, R. G. & Dickenson, A. H. (1997) NeuroReport 8, 587-590. [DOI] [PubMed] [Google Scholar]
- 17.Eckhardt, K., Ammon, S., Hofmann, U., Riebe, A., Gugeler, N. & Mikus, G. (2000) Anesth. Analg. 91, 185-191. [DOI] [PubMed] [Google Scholar]
- 18.Dirks, J., Petersen, K. L., Rowbotham, M. C. & Dahl, J. B. (2002) Anesthesiology 97, 102-107. [DOI] [PubMed] [Google Scholar]
- 19.Moore, K. A., Baba, H. & Woolf, C. J. (2002) Neuropharmacology 43, 1077-1081. [DOI] [PubMed] [Google Scholar]
- 20.Rogers, R., Wise, R. G., Painter, D. J., Longe, S. E. & Tracey, I. (2004) Anesthesiology 100, 292-301. [DOI] [PubMed] [Google Scholar]
- 21.Wise, R. G., Rogers, R., Painter, D., Bantick, S., Ploghaus, A., Williams, P., Rapeport, G. & Tracey, I. (2002) NeuroImage 16, 999-1014. [DOI] [PubMed] [Google Scholar]
- 22.Cruccu, G., Anand, P., Attal, N., Garcia-Larrea, L., Haanpaa, M., Jorum, E., Serra, J. & Jensen, T. S. (2004) Eur. J. Neurol. 11, 153-162. [DOI] [PubMed] [Google Scholar]
- 23.Petersen, K. L. & Rowbotham, M. C. (1999) NeuroReport 10, 1511-1516. [DOI] [PubMed] [Google Scholar]
- 24.Zambreanu, L., Wise, R. G., Brooks, J. C., Iannetti, G. D. & Tracey, I. (2005) Pain 114, 397-407. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson, M., Bannister, P., Brady, M. & Smith, S. (2002) NeuroImage 17, 825-841. [DOI] [PubMed] [Google Scholar]
- 26.Woolrich, M. W., Ripley, B. D., Brady, M. & Smith, S. M. (2001) NeuroImage 14, 1370-1386. [DOI] [PubMed] [Google Scholar]
- 27.Beckmann, C. F., Jenkinson, M. & Smith, S. M. (2003) NeuroImage 20, 1052-1063. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson, M. & Smith, S. (2001) Med. Image Anal. 5, 143-156. [DOI] [PubMed] [Google Scholar]
- 29.Collins, D. L., Neelin, P., Peters, T. M. & Evans, A. C. (1994) J. Comput. Assist. Tomogr. 18, 192-205. [PubMed] [Google Scholar]
- 30.Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 31.Treede, R. D., Rolke, R., Andrews, K. & Magerl, W. (2002) Pain 98, 235-240. [DOI] [PubMed] [Google Scholar]
- 32.Treede, R. D., Meyer, R. A. & Campbell, J. N. (1998) J. Neurophysiol. 80, 1082-1093. [DOI] [PubMed] [Google Scholar]
- 33.Maihofner, C., Schmelz, M., Forster, C., Neundorfer, B. & Handwerker, H. O. (2004) Eur. J. Neurosci. 19, 3211-3218. [DOI] [PubMed] [Google Scholar]
- 34.Dirks, J., Petersen, K. L. & Dahl, J. B. (2003) J. Pain 4, 122-128. [DOI] [PubMed] [Google Scholar]
- 35.Urban, M. O. & Gebhart, G. F. (1999) Proc. Natl. Acad. Sci. USA 96, 7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porreca, F., Ossipov, M. H. & Gebhart, G. F. (2002) Trends Neurosci. 25, 319-325. [DOI] [PubMed] [Google Scholar]
- 37.Gebhart, G. F. (2004) Neurosci. Biobehav. Rev. 27, 729-737. [DOI] [PubMed] [Google Scholar]
- 38.Witting, N., Kupers, R. C., Svensson, P., Arendt-Nielsen, L., Gjedde, A. & Jensen, T. S. (2001) Neurology 57, 1817-1824. [DOI] [PubMed] [Google Scholar]
- 39.Maihofner, C., Neundorfer, B., Stefan, H. & Handwerker, H. O. (2003) NeuroReport 14, 785-789. [DOI] [PubMed] [Google Scholar]
- 40.Baron, R., Baron, Y., Disbrow, E. & Roberts, T. P. (1999) Neurology 53, 548-557. [DOI] [PubMed] [Google Scholar]
- 41.Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M. & Raichle, M. E. (1997) J. Cognit. Neurosci. 9, 648-663. [DOI] [PubMed] [Google Scholar]
- 42.Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houde, O., Crivello, F., Joliot, M., Petit, L. & Tzourio-Mazoyer, N. (2001) Brain Res. Bull. 54, 287-298. [DOI] [PubMed] [Google Scholar]
- 43.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 44.Stefanovic, B., Warnking, J. M. & Pike, G. B. (2004) NeuroImage 22, 771-778. [DOI] [PubMed] [Google Scholar]
- 45.Shmuel, A., Yacoub, E., Pfeuffer, J., Van de Moortele, P. F., Adriany, G., Hu, X. & Ugurbil, K. (2002) Neuron 36, 1195-1210. [DOI] [PubMed] [Google Scholar]
- 46.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKiernan, K. A., Kaufman, J. N., Kucera-Thompson, J. & Binder, J. R. (2003) J. Cognit. Neurosci. 15, 394-408. [DOI] [PubMed] [Google Scholar]
- 48.Coghill, R. C., Sang, C. N., Maisog, J. M. & Iadarola, M. J. (1999) J. Neurophysiol. 82, 1934-1943. [DOI] [PubMed] [Google Scholar]
- 49.Porro, C. A., Cettolo, V., Francescato, M. P. & Baraldi, P. (1998) J. Neurophysiol. 80, 3312-3320. [DOI] [PubMed] [Google Scholar]
- 50.Jones, D. L. & Sorkin, L. S. (1998) Brain Res. 810, 93-99. [DOI] [PubMed] [Google Scholar]
- 51.Attal, N., Brasseur, L., Parker, F., Chauvin, M. & Bouhassira, D. (1998) Eur. Neurol. 40, 191-200. [DOI] [PubMed] [Google Scholar]
- 52.Werner, M. U., Perkins, F. M., Holte, K., Pedersen, J. L. & Kehlet, H. (2001) Reg. Anesth. Pain Med. 26, 322-328. [DOI] [PubMed] [Google Scholar]
- 53.Luo, Z. D., Chaplan, S. R., Higuera, E. S., Sorkin, L. S., Stauderman, K. A., Williams, M. E. & Yaksh, T. L. (2001) J. Neurosci. 21, 1868-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gustorff, B., Hoechtl, K., Sycha, T., Felouzis, E., Lehr, S. & Kress, H. G. (2004) Anesth. Analg. 98, 401-407. [DOI] [PubMed] [Google Scholar]
- 55.Hyder, F., Rothman, D. L. & Shulman, R. G. (2002) Proc. Natl. Acad. Sci. USA 99, 10771-10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shulman, R. G., Rothman, D. L. & Hyder, F. (1999) Proc. Natl. Acad. Sci. USA 96, 3245-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donovan-Rodriguez, T., Dickenson, A. H. & Urch, C. E. (2005) Anesthesiology 102, 132-140. [DOI] [PubMed] [Google Scholar]
- 58.Chapman, V., Suzuki, R., Chamarette, H. L., Rygh, L. J. & Dickenson, A. H. (1998) Pain 75, 261-272. [DOI] [PubMed] [Google Scholar]
- 59.Maneuf, Y. P. & McKnight, A. T. (2001) Br. J. Pharmacol. 134, 237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petroff, O. A., Rothman, D. L., Behar, K. L., Lamoureux, D. & Mattson, R. H. (1996) Ann. Neurol. 39, 95-99. [DOI] [PubMed] [Google Scholar]
- 61.Loscher, W., Honack, D. & Taylor, C. P. (1991) Neurosci. Lett. 128, 150-154. [DOI] [PubMed] [Google Scholar]
- 62.Kondo, T., Fromm, G. H. & Schmidt, B. (1991) Epilepsy Res. 8, 226-231. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki, R., Rahman, W., Rygh, L. J., Webber, M., Hunt, S. P. & Dickenson, A. H. (2005) Pain 117, 292-303. [DOI] [PubMed] [Google Scholar]
- 64.Iannetti, G. D., Zambreanu, L., Cruccu, G. & Tracey, I. (2005) Neuroscience 131, 199-208. [DOI] [PubMed] [Google Scholar]
- 65.Porro, C. A. (2003) Neuroscientist 9, 354-369. [DOI] [PubMed] [Google Scholar]
- 66.Matthews, E. A. & Dickenson, A. H. (2002) Anesthesiology 96, 633-640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.