Abstract
In 1999 an outbreak involving 188 patients with Legionnaires' disease (LD) occurred among visitors to a flower show in the Netherlands. Two enzyme immunoassays (Binax and Biotest) and one immunochromatographic assay (Binax NOW) were tested, using urine samples from LD patients from the 1999 outbreak. Sensitivity was calculated using positive culture and/or seroconversion as the “gold standard” in outbreak-related patients with radiographically confirmed pneumonia who fulfilled the epidemiological critera. The Binax EIA, Biotest EIA, and Binax NOW assay showed overall sensitivities of 69, 71, and 72%, respectively. When the tests were performed with concentrated urine samples, the overall sensitivities increased to 79, 74, and 81%, respectively. Using multiple logistic regression analysis with backward elimination, a statistically significant association was found between clinical severity and test sensitivity for all tests. For patients with mild LD, the test sensitivities ranged from 40 to 53%, whereas for patients with severe LD who needed immediate special medical care, the sensitivities reached 88 to 100%. These findings have major implications for the diagnostic process in patients with mild pneumonia and suggest that patients with mild pneumonia may go underdiagnosed if urine antigen tests alone are used.
Legionnaires' disease (LD) is an acute pneumonia caused by Legionella, a rod-shaped gram-negative bacillus ubiquitous in (man-made) aquatic reservoirs. Currently 43 Legionella species and 65 serogroups have been described. In the United States, over 90% of Legionnaires' disease cases are caused by Legionella pneumophila, of which 70% of strains belong to serogroup type 1 (16). Legionella spp. are responsible for 1 to 5% of cases of community-acquired pneumonia (CAP) (5). Clinically and radiographically, LD cannot be distinguished from pneumonias caused by other microbial pathogens. Because of the high mortality rate in patients with LD requiring hospitalization, early diagnosis to enable adequate antimicrobial treatment is potentially life-saving. Diagnosis of LD in patients with symptomatic pneumonia is based on culture, serologic testing, or antigen detection in urine. Isolation of Legionella from respiratory secretions is not a very sensitive diagnostic test (25 to 75% sensitivity) (15) and has the disadvantage of delay, because a positive result is not available until at least 3 days of incubation. Seroconversion is a diagnostic test with a high sensitivity and a high (serogroup-dependant) specificity, but it is of limited clinical value since it may take up to 9 weeks for patients to develop detectable antibodies (10, 13).
In contrast to the other tests mentioned above, urinary antigen tests combine reasonable sensitivity and high specificity with rapid results. The reported sensitivities of both enzyme immunoassay (EIA) and immunochromatographic test (ICT) show great variation: 50 to 90% (3, 7, 8, 19). These variations may be explained by differences in patient characteristics, the serogroup with which the patient is infected, the timing of collection the urine sample in the course of illness, and whether the urine is concentrated before testing.
To assess the value of the urinary antigen tests in a large outbreak situation (6), we used three widely used and commercially available tests with urine specimens from patients with outbreak-related LD: the Biotest EIA, the Binax EIA, and the Binax NOW test.
MATERIALS AND METHODS
Patients.
In February 1999 an outbreak involving 188 cases of LD occurred in Bovenkarspel, The Netherlands. The outbreak investigation indicated that a whirlpool displayed at the consumer product division of an annual flower show was the most likely source of infection. Genotyping revealed that isolates from 27 patients were identical to one of the environmental L. pneumophila serogroup 1 strains (6).
All 180 hospitalized patients with a confirmed Legionella pneumonia were included in this study after written consent was obtained from patients or their relatives. A confirmed case of LD (“gold standard”) was defined as a patient who fulfilled the epidemiological criteria (visitor to the 1999 Bovenkarspel flowershow or member of the exhibition staff) and who suffered from symptoms compatible with pneumonia, who showed radiological signs of infiltration, and who showed laboratory evidence of infection with L. pneumophila. Laboratory evidence included (i) isolation of L. pneumophila from a respiratory sample cultured on buffered charcoal yeast extract supplemented with α-ketoglutarate followed by genotyping and subsequent comparison to the environmental strains of the Bovenkarspel outbreak; or (ii) a fourfold rise in the titer of immunoglobulin M (IgM) antibodies to L. pneumophila in paired acute-phase and convalescent-phase sera, with final titers of ≥1:32 in accord with the 99% cutoff values found in a serosurvey of healthy volunteers representative of the Dutch population (4) using a microagglutination IgM, serotype 1, antibody assay (12); or (iii) seroconversion to positive IgM or IgG antibodies to L. pneumophila in paired acute-phase and convalescent-phase sera, with age-specific titers in accord with the 99% cutoff values found in a serosurvey among healthy volunteers representative of the Dutch population (4), using a commercial enzyme-linked immunosorbent assay to detect IgM and IgG serotype 1 to 7 antibodies (Serion ELISA; Institut Virion\Serion GmbH, Würzburg, Germany) (11).
For isolation and genotyping of L. pneumophila from sputum samples, the National Institute for Public Health and the Environment was the reference laboratory; for detection of antibodies against L. pneumophila in serum, the Regional Laboratory of Public Health Tilburg was the reference laboratory.
Classification of severity of disease.
To investigate the relation between test sensitivity and severity of disease, the patients were divided into three clinical categories for CAP. Clinical data were collected from the hospital chart by using a standardized case record form. Severity of pneumonia was scored according to the minor criteria for severity of CAP advised by the American Thoracic Society (17), using the following clinical criteria at hospital admission: (i) respiratory frequency above 30 breaths per minute, (ii) PaO2 below 60 mm Hg or O2 saturation below 92%, (iii) bilateral or multilobar infiltration on chest X-ray, and (iv) systolic blood pressure below 90 mm Hg or diastolic blood pressure below 60 mm Hg.
Patients with a radiographically proven unilateral unilobar pneumonia, but without signs or symptoms according to the above-mentioned criteria, were classified as CAP category 1 (mild pneumonia). CAP category 2 consisted of patients with a proven pneumonia who fulfilled only one of the criteria; according to our definition, these patients were suffering from a moderately severe pneumonia. CAP category 3 consisted of patients who presented with two or more criteria; these patients were considered to be suffering from severe pneumonia.
Collection of urine samples.
All medical microbiologists who had assisted in the diagnosis and treatment of the pneumonia patients in to this outbreak were asked to send available urine samples from the patients to the Regional Laboratory of Public Health in Haarlem, The Netherlands. After collection, the urine samples were stored in portions at −70°C. All but eight available urine samples had been collected during the hospital stay. In four cases, the urine samples had been obtained shortly before admission, and in four cases, they had been obtained after discharge from hospital.
Urinary antigen tests.
The presence of L. pneumophila antigens in urine samples was investigated by using the Binax (Portland, Maine) and Biotest (Biotest AG, Dreieich, Germany) Legionella urinary antigen tests, both EIAs, and with the Binax NOW test, a qualitative ICT. All tests were used as specified by the manufacturers. However, to ensure maximum specificity for the ICT (14), samples giving positive tests were reexamined after 60 min. Urine samples were tested nonconcentrated and, to enhance the intensity of the reaction, after concentration by selective ultrafiltration (Minicon B15; Millipore Corp. Bed Ford, Mass.). This selective ultrafiltration system consists of a permeable membrane that permits the passage of water and substances with molecular weights less than 15,000.
Statistics.
Statistical analysis was performed with the statistical program SPSS version 10.0 (Statistical Product and Service Solutions, Chicago, Ill.). Univariate analysis (chi-square test for dichotomous and ordinal variables; independent t test for discrete variables) was used to calculate the association with positive urinary antigen test results for the following variables: age, gender, clinical severity, number of days between onset of symptoms and collection of first urine sample, and number of times a urine sample was collected. Variables that were (borderline) significant were entered in a multiple logistic regression model. Using backward elimination, independent predictors for test positivity were established. Variables were retained in the model if the likelihood ratio test was significant (P < 0.1).
RESULTS
Patient selection and classification of disease severity.
In the 1999 outbreak, 188 LD patients were diagnosed, of whom 133 fulfilled the criteria for a confirmed case. Of the patients with confirmed cases, 132 had been hospitalized and were enrolled in this study. A large number of patients in this outbreak (51 patients) were diagnosed by a urinary antigen test alone, leaving 81 patients for evaluation. For 58 (72%) of these 81 patients, urine samples were available. The microbiological diagnosis in these cases was established by culture alone in 11 cases, by culture and serologic testing in 14 cases, and by serologic testing alone in 33 cases. For 55 of the 58 confirmed cases with available urine samples, data on clinical severity could be collected. Women were overrepresented in CAP category 1 (67%), in contrast to CAP categories 2 (25%) and 3 (39%). The median age for women was lower then for men in categories 1 and 2 (64 and 73 years and 57 and 63 years, respectively), in contrast to category 3 (70 and 62 years, respectively). Mean age did not differ significantly between CAP categories, but the age difference between male and female subgroups in the lowest CAP category was significant (independent t test; P = 0.04). All patients who were classified in CAP category 3 needed medical attention in a specialized unit.
Nonconcentrated urine samples.
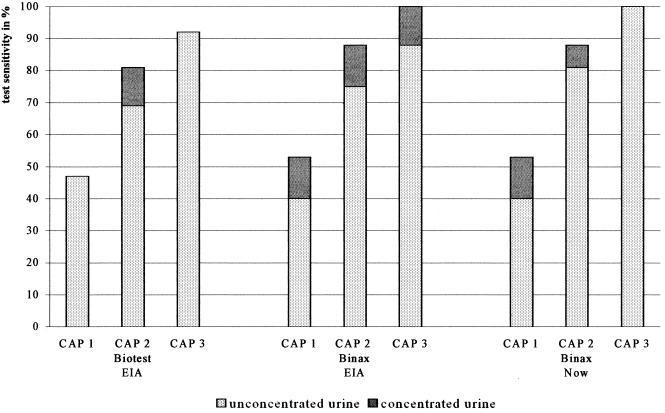
Using nonconcentrated urine, the sensitivities calculated for the three tests were 71, 69, and 72% for the Biotest EIA, Binax EIA, and Binax NOW test, respectively. The differences between the test sensitivities were not significant. When the LD patients were divided in three groups according to their CAP category, it appeared that the average sensitivities for the three urinary antigen tests increased from 42% for patients in the lowest CAP category to 75% for those in CAP category 2 and 93% for those in CAP category 3. The individual sensitivities of the three tests when used with nonconcentrated urine samples are shown in Fig. 1. Table 1 shows variables which in univariate analysis were significantly associated with test positivity. Age was not associated with test positivity.
FIG. 1.
Sensitivity for three urinary L. pneumophila antigen tests in different categories of clinical severity, using concentrated and unconcentrated urine.
TABLE 1.
Odds ratios and mean differences with confidence interval for variables associated with urinary L. pneumophila antigen test positivity
| Sample and test | Odds ratio (confidence interval) for:
|
Mean difference (confidence interval) for:
|
|||
|---|---|---|---|---|---|
| CAP category 2 versus 1 | CAP category 3 versus 1 | Male gender | Days before first urine sample | No. of urine samples | |
| Nonconcentrated urine | |||||
| Biotest EIA | 2.5 (0.6-10.9) | 12.6 (2.1-74) | 2.4 (0.8-7.9) | 4.5 (1.3-7.6) | 0.7 (0.0-1.6) |
| Binax EIA | 4.5 (1.0-21.0) | 10.5 (2.1-52) | 4.2 (1.3-13.6) | 3.8 (0.7-6.9) | 0.4 (0.0-1.3) |
| Binax NOW | 6.5 (1.3-33.0) | 16.5 (2.8-98) | 3.0 (0.9-9.9) | 4.9 (1.2-8.5) | 0.7 (0.0-1.6) |
| Concentrated urine | |||||
| Biotest EIA | 5.0 (1.0-25.0) | 12.6 (2.1-74) | 2.5 (0.8-8.4) | 3.8 (0.4-7.2) | 0.6 (0.0-1.5) |
| Binax EIA | 6.1 (1.0-36.9) | 20.1 (2.1-190) | 3.4 (0.9-13.0) | 4.9 (1.2-8.5) | 0.8 (0.0-1.8) |
| Binax NOW | 6.1 (1.0-36.9) | 4.0 (2.2-7.3) | 1.8 (0.5-6.6) | 6.7 (3.1-10.3) | 1.0 (0.3-1.6) |
Multiple logistic regression analysis showed that the CAP category remained associated with test positivity in all tests used (Table 2). Separate analysis for male and female patients gave identical results, indicating that the association was not modified by gender.
TABLE 2.
Multiple logistic regression models showing odds ratios with confidence interval for variables associated with urinary L. pneumophila antigen test positivity
| Sample and test | Odds ratio (confidence interval) for:
|
|||
|---|---|---|---|---|
| CAP category 2 versus 1 | CAP category 3 versus 1 | Male gender | Days before first urine sample | |
| Nonconcentrated urine | ||||
| Biotest EIA | 2.0 (0.4-9.7) | 10.0 (1.6-63) | 0.9 (0.7-1.0) | |
| Binax EIA | 2.9 (0.5-15) | 9.1 (1.8-47) | 3.9 (0.9-15.6) | 0.9 (0.8-1.0) |
| Binax NOW | 6.0 (1.0-35) | 14 (2.2-90) | 0.8 (0.7-1.0) | |
| Concentrated urine | ||||
| Biotest EIA | 4.2 (0.8-22) | 10.3 (1.7-62) | 0.9 (0.8-1.1) | |
| Binax EIA | 6.1 (1.0-37) | 20.1 (2.1-190) | ||
| Binax NOW | 6.1 (1.0-37) | |||
Concentrated urine samples.
After concentration of the urine samples for all three tests, a clear but not statistically significant increase in sensitivities was found: to 74, 79, and 81% for the Biotest EIA, Binax EIA, and Binax NOW test, respectively. This increase in sensitivity was small in the Biotest EIA (3%) and more prominent in the Binax EIA (10%) and Binax NOW assay (9%). When test sensitivity results were compared for categories with increasing clinical severity, concentration of urine samples yielded higher sensitivities predominantly for patients in CAP categories 1 and 2 (Fig. 1).
CAP category, male gender, number of urine samples, and shorter period between onset of symptoms and collection of the first urine sample were associated with a positive test result, but age was not (individual test results are given in Table 1). Multiple logistic regression analysis showed that the CAP category was the only factor associated with test positivity in all tests used (see Table 2). Separate analysis for male and female patients gave identical results.
DISCUSSION
The first urinary antigen tests, based on an ELISA, were described in 1979 (2, 21). Since then, numerous publications have followed that confirmed the value of urinary antigen detection for the diagnosis of Legionnaires' disease, regardless of the technical configuration of the test (1, 3, 7, 8, 18-20). Based on prospective and retrospective studies using data from solitary cases, moderate to high urinary antigen test sensitivities have been described. All reported test sensitivities are based on studies using sporadic LD cases. Most of these studies used a selection of patients or were retrospective (Table 3); they are thereby subject to selection bias. Some of them include patients with LD caused by other serogroups than serogroup 1, which leads to underestimation of the test sensitivity. Furthermore, the clinical conditions of the patients described in these studies were not taken into account, which may explain the range of sensitivity values found by different authors.
TABLE 3.
Overview of urinary antigen test sensitivity for sporadic cases of LD
| Test | Gold standard | Serogroup(s) | No. of patients | Study population | Sensitivity (%) | Yr, country | Reference |
|---|---|---|---|---|---|---|---|
| RIAa | Culture | 1, 4, 9b | 23 | Retrospective sample of hospitalized LD patients | 57 | 1988, USA | 1 |
| EIA | Culture or 4-fold rise in IFAT titer | 1, 3, 8, 12 | 120 | Selected sample of LD patients | 77 | 1990, Britan | 3 |
| Culture | 1 | 51 | Selected sample of LD patients | 84 | 1990, Britan | 3 | |
| EIA | Culture or 4-fold rise in IFAT titer or single high titer | 1, 4, 6 | 27 | Prospective inclusion of hospitalized LD patients | 70 | 1990, Germany | 19 |
| Culture or 4-fold rise in IFAT titer or single high titer | 1 | 20 | Prospective inclusion of hospitalized LD patients | 84 | 1990, Germany | 19 | |
| RIA | Culture or 4-fold rise in IFAT titer | 1, 3, 7b | 68 | Prospective inclusion of hospitalized LD patients | 56 | 1995, USA | 18 |
| Culture | 1 | 35 | Prospective inclusion of hospitalized LD patients | 80 | 1995, USA | 18 | |
| EIA | Culture or 4-fold rise in IFAT titer | Unknown | 65 | Selected sample of LD patients | 67 (87)c | 1998, Spain | 8 |
| EIA | Culture or 4-fold rise in IFAT titer | Unknown | 65 | Selected sample of LD patients | 64 (89)c | 1998, Spain | 8 |
| ICT | Culture or 4-fold rise in IFAT titer | 1, 2, 3, 4, 5, 6, 10 | 187 | Selected sample of LD patients | 80 | 2001, Germany | 14 |
| EIA | Culture or 4-fold rise in IFAT titer | 1, 2, 3, 4, 5, 6, 10 | 187 | Selected sample of LD patients | 79 | 2001, Germany | 14 |
| EIA | Culture or 4-fold rise in IFAT titer | 1, 2, 3, 4, 5, 6, 10 | 187 | Selected sample of LD patients | 83 | 2001, Germany | 14 |
RIA, radioimmunoassay; IFAT, immunofluorescence antibody test.
including cases of nonpneumophila LD.
After concentration of urine.
To our knowledge, there are no publications on test sensitivities in an outbreak situation. The 1999 outbreak in The Netherlands provided a unique opportunity to evaluate urinary antigen tests in an outbreak caused by an identified L. pneumophila serogroup 1 strain. A nationwide alert for LD cases ensured optimal case finding, thereby decreasing patient selection bias. Furthermore, the conditions for a gold standard were favorable: two national reference laboratories performed all microbiological tests, and Dutch reference titers were calculated using the distribution of antibodies against L. pneumophila serogroup 1 in a large sample from a national serum bank (4). In addition, all available clinical data for the LD patients in this outbreak were recorded centrally.
Assuming that patient inclusion in an outbreak situation resembles a prospective study design, our data are best compared with the published results of two prospective studies on urinary antigen test sensitivity in LD. One study (19) included a single high titer in the gold standard, inherently lowering the test sensitivity to be measured on the basis of misclassification. In this study, however, a higher sensitivity (84%) was found for patients with LD caused by L. pneumophila serogroup 1. The other prospective study (18) included patients with LD caused by L. pneumophila serogroups 1, 3, and 7, making a lower sensitivity more likely on the basis of a low urine antigen detection capacity for other serogroups than serogroup 1. Indeed, the reported sensitivity in this study was lower (57%). Since the 1999 outbreak in The Netherlands was caused by an L. pneumophila strain of serogroup 1, high sensitivities for the three tests were to be expected. However, overall test sensitivities found in this study were lower than those reported by Ruf et al. (19) and ranged from 69 to 72% for nonconcentrated urine samples. This lower sensitivity may be explained by a difference in the study populations. Active case finding for LD in this outbreak may have resulted in hospitalization of a higher proportion of patients with relatively mild LD compared to a nonoutbreak situation. As our results show, the sensitivity of urinary antigen tests is relatively low for cases in CAP categories 1 and 2.
We do not know of a published study in which an association between the severity of disease and the test sensitivity for LD has been demonstrated, although one study hinted at such an association (22). The association between sensitivity and clinical severity demonstrated in the present study has clinical and diagnostic consequences. Because of the high sensitivity in patients with a severe pneumonia, the early recognition of patients with life-threatening LD can prevent delay in initiating adequate antibiotic therapy. However, the urinary antigen test is less reliable in milder cases of LD, indicating that this diagnostic test, despite its rapid interpretation, cannot replace culture and serologic testing. Therefore, in the setting of persistent clinical or epidemiological suspicion of LD and a negative urinary antigen test result in patients in whom no other microorganism is identified, culture and serologic testing are recommended and treatment must include antibiotic coverage for Legionella.
When the detection of antigens in urine during an outbreak is used for epidemiological purposes, one has to keep in mind that 50 to 60% of cases of mild pneumonia will stay undiscovered, depending on whether urine is concentrated (concentration improves the chance of detection). This implies that, due to undiagnosed cases, the size of an outbreak will be underestimated unless complementary diagnostic serologic tests using paired sera are performed in all suspected cases. Because seroconversion can take up to 9 weeks after onset of the disease, a prolonged interval between collection of the two sera is advisable for reliable interpretation of serologic results.
Like other researchers (7, 14), we were unable to demonstrate a significant difference in sensitivity between the two EIAs that were tested and the ICT. Since the latter is very easy to perform without special laboratory equipment and the results are available at short notice even after concentration of the urine samples, this test may be preferable in outbreak situations if serogroup 1 is involved. Previous studies (8, 9) have also demonstrated that a higher sensitivity of urinary antigen detection was found using concentrated urine, regardless of the test used. Concentration by ultrafiltration is easy to perform and can facilitate an early diagnosis, especially in milder cases.
In conclusion, in outbreak situations the urinary antigen tests are a useful tool for early diagnosis of LD, especially in patients with severe cases. The ICT scored at least equal to the EIAs and has the advantage of ease of performance combined with rapid test results. Concentration of the urine samples increases the sensitivity, particularly in patients with less severe illness, and is therefore recommended. In outbreak situations, the use of urinary antigen tests alone for evaluation of the incidence rate will lead to underestimation of the actual incidence. Therefore, culture and serologic testing remain necessary diagnostic tools.
Acknowledgments
We thank all hospital clinicians and microbiologists in requesting patients' permission and allowing us to collect clinical data and urine samples. Special thanks to Yvonne Boelens and Jacob P. Bruin, who performed the urinary antigen tests.
References
- 1.Aguero-Rosenfeld, M. E., and P. H. Edelstein. 1998. Retrospective evaluation of the Du Pont radioimmunoassay kit for detection of Legionella pneumophila serogroup 1 antigenuria in humans. J. Clin. Microbiol. 26:1775-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berdal, B. P., C. E. Farshy, and J. C. Feely. 1979. Detection of Legionella pneumophila in urine by enzyme-linked immunospecific assay. J. Clin. Microbiol. 9:575-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birtles, R. J., T. G. Harrison, D. Samuel, and A. G. Taylor. 1990. Evaluation of urinary antigen ELISA for diagnosing Legionella pneumophila serogroup 1 infection. J. Clin. Pathol. 43:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boshuizen, H. C., S. E. Neppelenbroek, H. van Vliet, J. F. P. Schellekens, J. W. Den Boer, M. F. Peeters, and M. A. E. Conyn-van Spaendonck. 2001. Subclinical Legionella infection in workers near the source of a large outbreak of Legionnaires disease. J. Infect. Dis. 184:515-518. [DOI] [PubMed]
- 5.Breiman, F. R., and J. C. Butler. 1998. Legionnaire's disease: clinical, epidemiological, and public health perspectives. Semin. Respir. Infect. 26:8-11. [PubMed]
- 6.Den Boer, J. W., E. P. F. Yzerman, J. F. P. Schellekens, K. D. Lettinga, H. C. Boshuizen, J. E. Van Steenbergen, A. Bosman, S. Van den Hof, H. A. Van Vliet, M. F. Peeters, R. J. Van Ketel, P. Speelman, J. L. Kool, and M. A. E. Conyn-Van Spaendonck. 2002. A large outbreak of Legionnaires' disease at a flower show, the Netherlands, 1999. Emerg. Infect. Dis. 8:37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez, J. A., N. Gali, L. Matas, P. Pedroso, A. Hernandez, E. Padilla, and V. Ausina. 1999. Evaluation of a rapid immunochromatographic assay for the detection of Legionella antigen in urine samples. Eur. J. Clin. Microbiol. Infect. Dis. 18:896-898. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez, J. A., N. Gali, P. Pedroso, A. Fargas, E. Padilla, J. M. Manterola, and L. Matas. 1998. Comparison of the Binax Legionella urinary antigen enzyme immunoassay (EIA) with the Biotest Legionella urinary antigen EIA for detection of Legionella antigen in both concentrated and nonconcentrated urine samples. J. Clin. Microbiol. 36:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez, J. A., J. M. Manterola, R. Blavia, N. Sopena, F. J. Belda, E. Padilla, M. Gimenez, M. Sabria, J. Morera, and V. Ausina. 1996. Detection of Legionella pneumophila serogroup 1 antigen in nonconcentrated urine and urine concentrated by selective ultrafiltration. J. Clin. Microbiol. 34:2334-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelstein, P. H. 1987. Laboratory diagnosis of infections caused by Legionellae. Eur. J. Clin. Microbiol. 6:4-10. [DOI] [PubMed] [Google Scholar]
- 11.Etienne, J., F. Vandenesch, G. Lina, and S. Jarraud. 1999. Evaluation de Serion ELISA classic Legionella pneumophila 1-7 (IgG, IgM). Centre National de Reference des Legionelles, Lyon, France.
- 12.Farshy, C. E., G. C. Klein, and J. C. Feeley. 1978. Detection of antibodies to Legionnaires' disease organism by microagglutination and micro-enzyme-linked immunosorbent assay tests. J. Clin. Microbiol. 7:327-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison, T. G. and A. G. Taylor. 1988. The diagnosis of Legionnaires' disease by estimation of antibody levels, p. 130-136. In T. G. Harrison and A. G. Taylor (ed.), A laboratory manual for Legionella. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 14.Helbig, J. H., S. A. Uldum, P. C. Luck, and T. G. Harrison. 2001. Detection of Legionella pneumophila antigen in urine samples by the BinaxNOW immunochromato-graphic assay and comparison with both Binax Legionella urinary enzyme immunoassay (EIA) and Biotest Legionella urinary antigen EIA. J. Med. Microbiol. 50:509-516. [DOI] [PubMed] [Google Scholar]
- 15.Hoge, C. W., and R. F. Breiman. 1991. Advances in the epidemiology and control of Legionella infections. Am. J. Epidemiol. Suppl. 113:329-340. [DOI] [PubMed] [Google Scholar]
- 16.Marston, B. J., H. B. Lipman, and R. F. Breiman. 1994. Surveilance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch. Intern. Med. 154:2417-2422. [PubMed] [Google Scholar]
- 17.Niederman, M. S., J. B. Bass, Jr., G. D. Campbell, A. M. Fein, R. F. Grossman, L. A. Mandell, T. J. Marrie, G. A. Sarosi, A. Torres, and V. L. Yu. 1993. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. American Thoracic Society. Medical Section of the American Lung Association. Am. Rev. Respir. Dis. 148:1418-1426. [DOI] [PubMed] [Google Scholar]
- 18.Plouffe, J. F., T. M. File, R. F. Breiman, B. A. Hackman, S. J. Salstrom, B. J. Marston, and B. S. Fields. 1995. Reevaluation of the definition of Legionnaires' disease: use of the urinary antigen assay. Clin. Infect. Dis. 20:1286-1291. [DOI] [PubMed] [Google Scholar]
- 19.Ruf, B., D. Schurmann, I. Horbach, F. J. Fehrenbach, and H. D. Pohle. 1990. Prevalence and diagnosis of Legionella pneumonia: a 3-year prospective study with emphasis on application of urinary antigen detection. J. Infect. Dis. 162:1341-1348. [DOI] [PubMed] [Google Scholar]
- 20.Tang, P. W. and S. Toma. 1986. Broad-spectrum enzyme-linked immunosorbent assay for detection of Legionella soluble antigens. J. Clin. Microbiol. 24:556-558. [DOI] [PMC free article] [PubMed]
- 21.Tilton, R. C. 1979. Legionnaires' disease antigen detected by enzyme-linked immunosorbent assay. Ann. Intern. Medicine 90:697-698. [DOI] [PubMed] [Google Scholar]
- 22.Wever, P. C., E. P. F. Yzerman, E. J. Kuijper, P. Speelman, and J. Dankert. 2000. Rapid diagnosis of Legionnaires' disease using an immunochromatographic assay for Legionella pneumophila serogroup 1 antigen in urine during an outbreak in The Netherlands. J. Clin. Microbiol. 38:2738-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]