Abstract
Due to the apparent absence of an agent-specific nucleic acid genome, scrapie strains cannot be classified by genome characterization, which is commonly used for the classification of many viruses. However, scrapie strains can be distinguished to some extent by biological properties such as transmissibility to experimental animals and distribution of neuropathological lesions and by biochemical properties such as the molecular mass and relative protease-resistance of the disease-specific isoform of prion protein (PrPSc). In order to preliminarily characterize the scrapie strains that are prevalent in Japan, we analyzed the transmissibility of sheep scrapie isolates to mice and the relative proteinase K (PK) resistance of the corresponding PrPSc. The results indicate that Japanese scrapie strains can be divided into at least three groups based on biological and biochemical properties. The first group includes isolates which cause disease in mice with an incubation period of ∼400 days and possess PrPSc with relatively high PK resistance. Isolates of the second group contain PrPSc that is highly resistant to PK digestion but transmit poorly to mice. The final group consists of isolates that cause disease in mice with an incubation period of less than 300 days and are associated with PrPSc with reduced PK resistance. Sheep scrapie has occurred sporadically in Japan since1982, with only ∼60 officially reported cases so far. However, the diversity of scrapie strains in the field suggested by our data raises the concern that a scrapie strain similar to the parental agent of bovine spongiform encephalopathy could exist or emerge in Japan. Thus, continuous surveillance for scrapie will be required to prevent the further spread of scrapie, not only among the sheep population but also to other species, and to eliminate any potential risk of sheep scrapie to public health.
Transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative diseases, which include scrapie in sheep and goats, bovine spongiform encephalopathy (BSE), and Creutzfeldt-Jakob disease (CJD) in humans. Scrapie has existed for more than two centuries, while BSE was first recognized in 1985, followed by a BSE epidemic in the United Kingdom (31). Epidemiological studies suggested that BSE was primarily caused by feeding meat and bone meal (MBM) contaminated with scrapie agent to cattle (32). Once BSE appeared, the causative agent spread through the cattle population by the use of BSE agent-contaminated MBM. The appearance of feline spongiform encephalopathy (FSE) in domestic and captive cats (34, 35) and, more recently, variant CJD (vCJD) in humans in 1996 (33), has raised a global concern for the spread of the BSE agent to other species via the food chain.
It is known that there are biologically distinguishable sheep scrapie strains in the United Kingdom (8, 11); however, BSE isolates studied so far appear to have comparatively uniform characteristics (4). The biological and biochemical properties of the BSE agent are very similar to those of the agents of vCJD and FSE, providing strong evidence that cross-species transmission of BSE to humans and felids resulted in vCJD and FSE, respectively (7, 13). Thus, it is now probable that a particular scrapie strain, which possessed enhanced resistance to heat inactivation, survived the rendering process and was transmitted to cattle via contaminated MBM. Thereafter, the agent passed into humans, possibly via the food chain. Alternatively, a particular strain capable of infecting both cattle and humans might have been selected and amplified during the transmission of the scrapie agent to cattle.
One of the characteristics of TSEs is an accumulation of a protease-resistant, abnormal isoform of a host protein, PrPSc, which is specifically detected in the central nervous system and lymphoid tissues of scrapie-affected animals. PrPSc is posttranslationally generated from the host-encoded sialoglycoprotein, prion protein (PrPC). PrPC and PrPSc have the same primary structure (14) but different conformations as detected by analysis of biophysical properties (24, 27) and biochemical properties, such as resistance to protease digestion and solubility in nonionic detergent (23). The presence of PrPSc usually correlates with scrapie infectivity. Thus, PrPSc is thought to be one of the major components of the scrapie agent, and so detection of PrPSc is often considered an indication of the presence of infectivity. Although the exact nature of the scrapie agent is still controversial, the failure to find an agent-specific genome to date prevents the use of nucleic acid sequencing for strain characterization, a method commonly used for strain typing of viruses and bacteria (1, 22). However, TSE agents can be distinguished to some extent by incubation periods and distribution of neuropathological lesions on transmission to experimental animals (5, 6), as well as biochemical properties, such as relative protease resistance and/or molecular mass of PrPSc (2, 3, 25, 30) and differences in the ratio of glycosylated PrPSc bands (10).
There is a sporadic occurrence of scrapie in Japan, and the existence of BSE was disclosed in September 2001. Since BSE is thought to originate from sheep scrapie, surveillance of scrapie-positive sheep and characterization of prevalent scrapie strains in the field are required for the prediction and elimination of a potential risk of scrapie to public health. In order to attempt to characterize scrapie strains present in Japan, we analyzed several isolates for their transmissibility to mice and the biochemical properties of the associated PrPSc. The results showed that at least three different strains of scrapie agent exist in Japan.
MATERIALS AND METHODS
Sheep with scrapie.
Eight naturally occurring sheep scrapie isolates (KH2, KU, SB, Y2, Y5, S1, S2, and S3) collected from 1987 to 1996 and three first-passage isolates from experimental sheep scrapie infections (A1, B3, and G1) were used in this study. As a negative control, one sheep (S4), which was defined as negative for scrapie by the absence of both neuronal vacuolation in histopathological examination and detectable PrPSc in central nervous system and lymphoid tissues in immunoblot analysis, was also used. Sheep S1, S2, S3, and S4 were kept on ranch S. Sheep S1, S2, and S3 had been born from two ewes which showed neurological dysfunction several months after delivery and were diagnosed with scrapie by clinical and/or histopathological methods. The sheep S1, S2, and S3 shared the same sire. However, there were no disease-associated mutations in the PrP genes of these sheep, and thus the sheep S1, S2, and S3 were tentatively grouped as cases of endemic scrapie. Sheep KH2, KU, and SB, each from independent ranches, and sheep Y2 and Y5 from ranch Y, were grouped as sporadic cases because there is no immediate blood relationship among these sheep. Sheep A1 and B3 with experimental scrapie received the same brain homogenate of a scrapie-affected sheep that was not included in the natural scrapie group used here, and the results of experimental transmission of this sheep scrapie isolate to mice were reported elsewhere (29). Sheep G1 was inoculated intravenously with a 10% brain homogenate of scrapie-affected sheep Y4 from the Y ranch. Sheep Y2, Y4, and Y5 were grown on the same farm and developed scrapie within 6 months, but they were born to different ewes.
PrP genotyping of sheep.
PrP genotyping of sheep was performed as described previously (18). Amino acid polymorphisms at codons 112 Met/Thr, 136 Ala/Val, 154 Arg/His, and 171 Gln/Arg/His were basically used for distinction of the PrP genotype.
Bioassay.
Twenty microliters of 10% brain homogenates (in phosphate-buffered saline) from scrapie-affected or scrapie-negative sheep were inoculated intracerebrally into 4-week-old female ICR mice (PrP allotype PrPA/A; PrPA encodes PrP with codons 108 Leu and 189 Thr). In some cases I/LnJ mice (PrP allotype PrPB/B; PrPB encodes PrP with codons 108 Phe and 189 Val) were also used. When mice showed clinical symptoms of the terminal stage of scrapie, mice were sacrificed under anesthesia and brains were removed and processed for the detection of PrPSc. The brains of mice that died of unknown causes were also checked for the presence of PrPSc.
Sample preparation and PK digestion.
Preparation of brain samples for proteinase K (PK) digestion was carried out as described elsewhere with slight modifications (12). Brains were homogenized with 7 volumes (wt/vol) of 10 mM Tris-HCl (pH 7.5) and 7.5 mM MgCl2, and the homogenates were incubated at 37°C for 1 h with DNase I (40 μg/100 mg tissue). After adding 20% Sarkosyl to a final concentration of 5%, the homogenates were kept at room temperature (RT) for 30 min and then centrifuged at 12,000 × g at RT for 5 min. The resulting supernatants were transferred to new tubes, and then solid NaCl was added to give a final concentration of 10%. After a 16-h incubation at 4°C with continuous rotation, the homogenates were centrifuged at 16,000 × g, 4°C and, for 40 min, and the resulting pellet was resuspended in 10 mM Tris-HCl (pH 7.5). This suspension was subjected to PK digestion at various PK concentrations and times as indicated in each experiment. Phenylmethylsulfonyl fluoride (PMSF) was added to stop the digestion (final conc. 1 mM), and then the reaction mixture was adjusted to 4% sodium dodecyl sulfate (SDS) and boiled for 5 min. The proteins were precipitated with 10 volumes of ice-cold methanol, and the final pellet was dissolved by SDS-polyacrylamide gel electrophoresis in sample buffer (4% SDS, 5% 2-mercaptoethanol, 5% glycerol, 0.01% bromophenol blue, 62.5 mM Tris-HCl [pH 6.8]).
Detection of PrPSc. (i) Immunoblot analysis.
Immunoblot analysis was carried out as described previously with B-103 rabbit serum (16). Densitometric analysis of X-ray film was performed with a Lane & Spot Analyzer (Atto, Tokyo, Japan).
(ii) Dot blot analysis.
The 10% brain homogenates of sheep with scrapie were mixed with an equal volume of cold lysis buffer [0.5% Nonidet P-40, 0.5% sodium deoxycholate, 10 mM EDTA, 100 mM NaCl, 50 mM Tris-HCl (pH 7.4)], diluted serially in twofold steps and blotted onto a nitrocellulose membrane. The membrane was dried and then washed with Tris-buffered saline containing 0.1% Tween 20 (TBST). The membrane was treated with PK (25 μg/ml) in TBST at 37°C for 1 h, and the reaction was stopped by adding PMSF to a final concentration of 2 mM. After washing with TBST, the membrane was treated with 3 M guanidinium isothiocyanate for 20 min at RT and washed with TBST again. Immunostaining of the membrane was then performed as described for the immunoblot analysis.
RESULTS
Sheep with scrapie.
Characteristics of the sheep used in this study are summarized in Table 1. The major symptoms of all scrapie cases used here were ataxia and, at the terminal stage, astasia. No sheep except for KU showed obvious loss of fleece, which is thought to be caused by pruritus. The sheep S1, S2, and S3, which were tentatively grouped as cases of endemic scrapie (see Materials and Methods), differed from other sheep with scrapie. They showed hypersensitivity at the onset of disease and died of scrapie at a considerably young age (average, 16.3 months old) compared to sheep with scrapie grouped as sporadic cases here (average, 42.0 months old) or those described by others (2 to 6 years old [11]).
TABLE 1.
Sheep used in this study
| Sheep | Breeda (age [mo])b | PrP genotypec | Symptom(s) |
|---|---|---|---|
| With scrapie | |||
| Sporadic | |||
| KH2 | S (48) | MARQ/MARR | NAd |
| KU | S (52) | MARQ/TARQ | Ataxia, subtle loss of fleece |
| SB | S (34) | MARQ/MARQ | Ataxia, debilitation |
| Y2 | S (35) | MARQ/MARQ | Ataxia, debilitation |
| Y5 | S (41) | MARQ/MARQ | Ataxia, debilitation |
| Endemic | |||
| S1 | S × C (12) | MARQ/MARH | Ataxia, hypersensitivity |
| S2 | S × C (21) | MARQ/MARH | Ataxia, hypersensitivity |
| S3 | S × C (16) | MARQ/MVRQ | Ataxia, hypersensitivity |
| Exptl | |||
| A1 | S (27) | MARQ/MARQ | Ataxia |
| B3 | S (21) | MARQ/TARQ | Ataxia |
| G1 | S (26) | MARQ/MARQ | Ataxia, debilitation |
| Healthy (S4) | S × C (20) | MARQ/MARQ |
Abbreviations: S, Suffolk; C, Corriedale; S × C, F1 of Suffolk and Corriedale.
Age of the sheep with scrapie grouped as sporadic or endemic indicates the age at death (months old), while that of the experimental group indicates time to death after inoculation (months postinoculation).
The nomenclature of PrP genotypes was described elsewhere (18).
NA, no record of clinical symptoms was available.
Transmissibility of sheep scrapie to mice.
Transmission of 11 sheep scrapie samples to mice is shown in Table 2. Eight of 11 successfully transmitted to ICR mice (PrP allotype, PrPA/A), with average incubation periods from 229 to 451 days postinfection (dpi). These eight sheep scrapie samples seem to be divided into two groups based on the incubation periods; one includes A1 and B3, which transmitted to mice with incubation periods of around 230 dpi, and the other includes KH2, KU, SB, Y2, Y5, and G1, which caused disease in mice with incubation periods of ∼386 to 451 dpi. There is no significant difference among the incubation periods of KH2-, KU-, SB-, Y2-, and Y5-inoculated mice (P > 0.05 in t test). However, the incubation period of G1-inoculated mice was somewhat shorter than that of the others, with a statistically significant difference between the incubation period of G1-inoculated mice and that of KH2-, SB-, and Y2-inoculated mice (P < 0.05 in t test). The sheep A1 and B3 received the same brain homogenate from a naturally occurring sheep scrapie isolate by intravenous injection. The transmission of this parental isolate of A1 and B3 directly to mice was achieved within 271 to 307 dpi (29), consistent with the incubation periods observed in A1- and B3-inoculated mice. Thus, the first-pass scrapie samples A1 and B3 and their parental isolate all possessed the ability to transmit to mice with relatively short incubation periods.
TABLE 2.
Transmissibility of sheep scrapie to mice
| Sheep | Mouse strain | Dead mice (n/N)a | PrPSc-positive mice (n′/N′)b | Mean time to death ± SD (day) |
|---|---|---|---|---|
| Scrapie | ||||
| Sporadic | ||||
| KH2 | ICR | 6/6 | 5/5 | 417 ± 29 |
| KH2 | I/LnJ | 5/5 | 3/3 | 274, 275, 275, 275, 307d |
| KU | ICR | 7/7 | 7/7 | 394 ± 672 |
| SB | ICR | 9/9 | 6/7 | 231, 451 ± 39e |
| Y2 | ICR | 5/5 | 4/4 | 418 ± 42 |
| Y5 | ICR | 5/5 | 5/5 | 427 ± 15 |
| Endemic | ||||
| S1 | ICR | 1/6 | 1/6 | 359, >462f |
| S2 | ICR | 5/6 | 0/6 | 301, 400, 430, 478, 536, >620 |
| S3 | ICR | 2/7 | 0/7 | 222, 413, >620 |
| S3 | I/LnJ | 5/5 | 0/5 | 188, 200, 270, 363, 603g |
| Exptl | ||||
| A1 | ICR | 6/6 | 6/6 | 229 ± 12 |
| B3 | ICR | 7/7 | 7/7 | 236 ± 14 |
| G1 | ICR | 9/9 | 8/9 | 190, 386 ± 20h |
| Healthy (S4) | ICR | 0/4 | NDc | >620 |
Abbreviations: n, number of animals which died of any cause during the observation period; N, number of animals which received brain homogenates.
Abbreviations: n′, number of PrPSc-positive mice; N′, number of mice used for the examination of PrPSc.
ND, not determined.
All the mice died of wounds received in violent fights without any symptoms of scrapie. The three mice which died at 275 dpi were found to be positive for PrPSc.
One mouse which died at 231 dpi was negative for PrPSc. The mean and SD for time to death were calculated from data for the remaining eight mice.
One mouse which died at 359 dpi did not show scrapie symptoms but was found to be positive for PrPSc.
Four mice died at 188, 200, 270, and 363 dpi due to fights.
One mouse which died at 190 dpi was negative for PrPSc. The mean and SD for time to death was calculated from data for the remaining eight mice.
In contrast, the S1, S2, and S3 scrapie isolates were virtually nontransmissible to ICR mice (Table 2). One mouse inoculated with the brain homogenate of sheep S1 died at 359 dpi without typical symptoms of scrapie but was positive for PrPSc in the brain. We cannot confirm whether this one case is due to actual transmission or contamination with a mouse-adapted scrapie agent. Several ICR mice inoculated with the brain homogenates of S2 or S3 sheep died without typical symptoms of scrapie during the observation period but were also negative for PrPSc. Therefore, it is obvious that S1, S2, and S3 differ from the other scrapie isolates used here. Furthermore, isolate S3 did not transmit to I/LnJ mice (PrP allotype, PrPB/B), whereas transmission of isolate KH2 to these mice was confirmed by the detection of PrPSc at 275 dpi. The sheep S1, S2, and S3 were born on the same farm and in the same parturient season from two ewes that developed scrapie several months after delivery, and thus the sheep were potentially infected with the same agent. One explanation for the failure of the transmission of these isolates to mice is that the brain homogenates may possess a relatively low degree of infectivity. However, dot blot analysis of serial dilutions of brain homogenates revealed that variations in the amount of PrPSc in the homogenates appear to be less than fourfold among the samples tested (Fig. 1), suggesting that the lack of transmission was not due to reduced degrees of infectivity. Therefore, we conclude that the sheep scrapie isolates S1, S2, and S3 have an extremely low, if any, transmissibility to mice.
FIG. 1.
Dot blot analysis of sheep brain homogenates for the presence of PrPSc. Serial twofold dilutions of sheep brain homogenates used for inoculation into mice were dotted onto a nitrocellulose membrane and stained with B-103 anti-PrP synthetic peptide rabbit serum and ECL Western blot detection reagent (Amersham). The sheep are indicated at the bottom and dilutions are on the right. In this dot blot analysis, S1 appears to be negative for PrPSc; however, PrPSc was detected in the immunoblot analysis (data not shown).
PK resistance of sheep PrPSc.
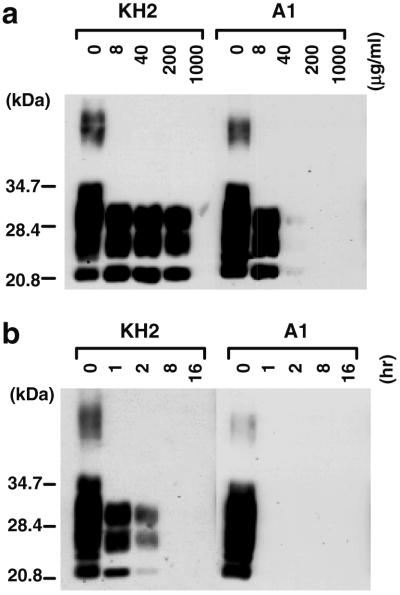
Since some TSE strains are known to differ in the relative PK resistance of PrPSc (3), we analyzed the PK resistance of PrPSc in the brains of sheep with scrapie in order to identify any biochemical differences among sheep scrapie isolates. Partially purified PrPSc-containing fractions without PK treatment were first analyzed by immunoblot, and following densitometric analysis to allow normalization of the amount of PrPSc among the samples, the fractions were digested with PK at 0, 8, 40, 200, and 1,000 μg/ml for 2 h (Fig. 2a). The bands of PrPSc were clearly detected after PK treatment at 40 μg/ml in KH2 and S2, while PrPSc in A1 was undetectable under the same digest conditions. To confirm this difference, the same fractions were treated with PK at 40 μg/ml for various time periods (Fig. 2b). Consistent with the result in Fig. 2a, the PrPSc bands in the A1 fraction were virtually eliminated by a 2-h treatment with 40-μg/ml of PK, while the PrPSc bands in the KH2 and S2 fractions were readily detected after the same treatment. The PrPSc of B3 exhibited the same PK resistance as A1, while KU, SB, Y2, Y5, S1, and S3 showed levels of PK resistance similar to KH2 and S2 (data not shown). Thus, the sheep scrapie isolates used here appeared to be divided into two groups based on the relative PK resistance of PrPSc. One possesses PrPSc that is highly resistant to PK digestion, as defined by resistance to treatment with PK at 40 μg/ml for 2 h, and the other possesses PrPSc of relatively low PK resistance and is completely degraded under these conditions. Together, the mouse transmission and PrPSc PK resistance data can be used to further divide the sheep scrapie isolates into three groups (Table 3). The first group includes KH2, KU, SB, Y2, Y5, and G1, which are transmissible to ICR mice with incubation periods of ∼400 days and contain PrPSc with relatively high PK resistance. The second group includes S1, S2, and S3, which transmit poorly to mice but possess PrPSc with a relative PK resistance similar to the first group. The last group includes A1, B3, and probably their parental isolate, which are transmissible to ICR mice with incubation periods of ∼230 days and possess PrPSc with relatively low PK resistance.
FIG. 2.
Relative PK resistance of sheep PrPSc. (a) PrPSc-containing fractions prepared from sheep brains were treated with various concentrations of PK (0 to 1,000 μg/ml, indicated above the photo) at 37°C for 2 h. Molecular mass markers are indicated. PrP was detected by immunoblotting with PrP-specific antibody and ECL. (b) The same PrPSc-containing fractions as used in panel a were treated with PK (200 μg/ml) at 37°C for various times (0 to 16 h, indicated above the photo).
TABLE 3.
Grouping of sheep scrapie samples based on transmissibility to mice and relative PK resistance of PrPSc
| Sheep | PK resistancea (Sheep PrPSc) | Transmissibility to ICR mice (mean ± SD [day]) | PK resistanceb (Mouse PrPSc) |
|---|---|---|---|
| Group 1 | |||
| KH2 | H | Yes ( 417 ± 29) | H |
| KU | H | Yes ( 394 ± 67) | H |
| SB | H | Yes ( 451 ± 39) | NDc |
| Y2 | H | Yes ( 418 ± 42) | ND |
| Y5 | H | Yes ( 427 ± 15) | H |
| G1 | H | Yes ( 386 ± 20) | ND |
| Group 2 | |||
| S1 | H | No | NAd |
| S2 | H | No | NA |
| S3 | H | No | NA |
| Group 3 | |||
| A1 | L | Yes ( 229 ± 12) | L |
| B3 | L | Yes ( 236 ± 14) | L |
H indicates PrPSc that is resistant to a treatment with PK (40 μg/ml) for 2 h, while L indicates PrPSc that is sensitive to the same treatment.
H indicates PrPSc that is resistant to a treatment with PK (200 μg/ml) for 1 h, while L indicates PrPSc that is sensitive to the same treatment.
ND, not determined.
NA, not available.
PK resistance of PrPSc in mice inoculated with sheep scrapie.
Sheep scrapie isolates which transmitted to ICR mice segregated into two groups based on incubation periods (Table 2), and interestingly, the grouping of sheep scrapie isolates by relative PK resistance of PrPSc in sheep brains appears to coincide with the grouping by incubation periods (Table 3). To address the question of whether the phenotype of relative PK resistance of PrPSc in sheep brain is conserved in infected mice, the relative PK resistance of PrPSc in the mouse brains was also examined (Fig. 3). PrPSc in the brain of a mouse inoculated with KH2 was resistant to treatment with PK at 200 μg/ml for 1 h, while PrPSc in the brain of a mouse inoculated with A1 was sensitive to this condition (Fig. 3a). This difference in the relative PK resistance was also confirmed by the experiments in Fig. 3b, in which the samples were treated with PK at 200 μg/ml for the various time periods indicated. PrPSc in the brains of mice inoculated with KU and Y5 showed the same PK resistance as that of a KH2-inoculated mouse, whereas PrPSc in the brain of a mouse inoculated with B3 showed the same PK resistance as that of an A1-inoculated mouse (data not shown). Therefore, the relative PK resistance of PrPSc generated in the brains of mice was similar to that of PrPSc in the inoculum of the corresponding sheep.
FIG. 3.
Relative PK resistance of PrPSc generated in mouse brain. (a) PrPSc-containing fractions prepared from mouse brains were treated with various concentrations of PK (0 to 1,000 μg/ml, indicated above the photo) at 37°C for 2 h. Molecular mass markers are indicated. PrP was detected by immunoblotting as in Fig. 2. (b) The same PrPSc-containing fractions as used in panel a were treated with PK (200 μg/ml) at 37°C for various times (0 to 16 h, indicated above the photo).
DISCUSSION
We were interested in characterizing the scrapie strains prevalent in Japan. In the absence of known biological clones (which would take many years to develop), we attempted to gain information about the nature of these strains by examining the biological and biochemical properties of a panel of isolates and showed that at least three types of scrapie strains exist in Japan. It is thought that the scrapie agent entered into Japan along with imported sheep in the1970s (17, 29). Since the recognition of the first scrapie case early in the1980s, ∼60 scrapie cases have been officially reported so far. Thus, our data indicate the possibility that the primary scrapie strain has already exhibited considerable variation during the past quarter century. However, it is unknown if the current repertoire of scrapie strains is derived from a single parental strain that has been changing during its spread through the sheep population, similar to the mutation-like change found on serial transmission of the scrapie agent in experimental animals (20). Alternatively, multiple infection is likely to occur in natural scrapie (19), so that it is also possible that multiple strains have entered into Japan with imported sheep. The diversity of sheep scrapie strains in Japan seems similar to that described in the United Kingdom based on transmissibility to mice (8, 11), because some isolates were essentially nontransmissible whereas others were readily transmissible to PrPA/A mice with incubation periods of >350 days. However, to our knowledge, sheep scrapie which successfully transmits to mice with an average incubation period of ∼230 days, like A1 and B3, has an extremely short incubation period in primary transmission.
TSE agents have been characterized by transmissibility to experimental animals and distribution of neuropathological lesions (5, 11). In addition, biochemical characterization of PrPSc seems to be useful for discriminating between TSE strains. For example, two hamster-adapted transmissible mink encephalopathy strains, “Hyper” and “Drowsy,” can be distinguished from each other not only by incubation period and neuropathology but also by the relative PK resistance and molecular mass of PrPSc (2, 3). Here we found that relative PK resistance of PrPSc is likely to be useful for the distinction of sheep scrapie strains to some extent, as the samples we analyzed could be divided into two groups based on relative PK resistance. Recently, the ratio of glycosylated PrPSc bands was used to distinguish vCJD from other types of CJD (10), and sheep scrapie can be divided into several groups by using this glycoform typing of PrPSc (15). In general, strain typing approaches using a combination of different properties provide a more reliable means of strain differentiation. Analyses of biochemical properties of PrPSc are less time-consuming than bioassays in mice, and therefore, it is advantageous to use a combination of several biochemical properties such as relative PK resistance, molecular mass, and glycoform typing of PrPSc, to achieve more definitive strain typing.
A recent study using 10 sheep with scrapie in the United States showed a perfect correlation between the detection of PrPSc in sheep brain and transmissibility to mice (26). In contrast, it is well-known that some sheep scrapie strains are difficult to transmit to mice (4, 8, 11). In this study, we showed that scrapie strains classified into groups 1 and 2 differed in their transmissibility to mice but were indistinguishable by the relative PK resistance of PrPSc (Table 3). The discrepancy between the presence of PrPSc and the transmissibility to mice may imply that factors other than PrPSc are involved in determining the infectivity or that PrPSc is not the molecule responsible for infectivity (21). Alternatively, strain-specific conformations of PrPSc have been shown (9, 28), and so it is also conceivable that subtle differences in biochemical and/or biophysical properties of PrPSc which cannot be detected by PK digestion may influence the transmissibility to mice. It is also possible that the sheep PrP genotype may explain the discrepancy. It has not yet been clarified whether amino acid sequences of sheep PrPSc influence transmission to mice. Here we showed that scrapie occurring in sheep homozygous for PrPMARQ transmitted to mice (e.g., SB, Y2, Y5, A1, and G1). This indicates that sheep PrPSc composed of the product of the PrPMARQ allele could initiate the accumulation of PrPSc in mice by using mouse PrPC as a substrate. However, there are differences in the incubation periods for A1 between these sheep and other sheep possessing the PrPMARQ/MARQ genotype (Table 2), and more recently, it was reported that one scrapie case occurring in PrPARQ/ARQ sheep (amino acid polymorphisms at codon 112 was unavailable) was virtually nontransmissible to mice (8), suggesting the amino acid sequence of sheep PrPSc is not the sole determinant of the transmissibility to mice.
Among the sheep with scrapie used here, S1, S2, and S3 differed in clinical course from other sheep by death at a young age and hypersensitivity. The simple explanation for this is that the strain(s) infecting these sheep differs from those of the other scrapie cases, and indeed, these agents obviously differed from the others in transmissibility to mice. Scrapie strains adapted to rodents exhibit strain-specific clinical symptoms, neuropathological lesions, and incubation periods (3, 5, 6). However, it is unclear whether a given sheep scrapie strain determines strain-specific clinical and/or clinico-pathological features. As described in Materials and Methods, these sheep have an immediate blood relationship and were kept on the same ranch, and so other factors such as genetic background, breeds, route of infection, and environment may be involved in defining the type of disease in these particular cases.
A particular type of scrapie agent prevalent in the United Kingdom is believed to have initially caused BSE, and later on, this agent passed to felids and human beings through cattle (4, 7, 13). The diversity of field scrapie isolates in Japan is similar to that reported in the United Kingdom at least in terms of primary transmission to mice (11). Therefore, at present, we cannot exclude the possibility of the presence of a scrapie strain in Japan that possesses properties similar to the BSE agent. Strain typing using two mouse strains, RIII and C57BL, carrying the PrPA/A allotype appears to be one of the methods to distinguish BSE and BSE-related TSE agents from sheep scrapie (4, 8). Further analysis will be required to investigate whether sheep scrapie strains in Japan have the potential risk to create new epidemics like the BSE agent.
Acknowledgments
We thank Gerald S. Baron, Rocky Mountain Laboratories, NIAID, NIH, for critical reading of the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports, and Culture of Japan (grant 09660312) and a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (grant 2120).
REFERENCES
- 1.Aiken, J. M., and R. F. Marsh. 1990. The search for scrapie agent nucleic acid. Microbiol. Rev. 54:242-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessen, R. A., and R. F. Marsh. 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 66:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessen, R. A., and R. F. Marsh. 1992. Identification of two biologically distinct strains of transmissible spongiform encephalopathy agent. J. Gen. Virol. 73:329-334. [DOI] [PubMed] [Google Scholar]
- 4.Bruce, M., A., Chree, I. McConnell, J. Foster, G. Pearson, and H. Fraser. 1994. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Phil. Trans. R. Soc. Lond. B 343:405-411. [DOI] [PubMed] [Google Scholar]
- 5.Bruce, M. E., and A. G. Dickinson. 1987. Biological evidence that scrapie agent has an independent genome. J. Gen. Virol. 68:79-89. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, M. E. 1993. Scrapie strain variation and mutation. Br. Med. Bull. 49:822-838. [DOI] [PubMed] [Google Scholar]
- 7.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 8.Bruce, M. E., A. Boyle, S. Cousens, I. McConnell, J. Foster, W. Goldmann, and H. Fraser. 2002. Strain characterization of natural sheep scrapie and comparison with BSW. J. Gen. Virol. 83:695-704. [DOI] [PubMed] [Google Scholar]
- 9.Caughey, B., G. J. Raymond, and R. A. Bessen. 1998. Strain-dependent differences in β-sheet conformations of abnormal prion protein. J. Biol. Chem. 273:32230-32235. [DOI] [PubMed] [Google Scholar]
- 10.Collinge, J., K. C. L. Sidle, J. Meads, J. Ironside, and A. F. Hill. 1996. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383:685-690. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson, G. A. 1976. Scrapie in sheep and goats, p. 209-241. In R. H. Kimberlin (ed.), Slow virus diseases of animals and man. North-Holland Publishing Company, Amsterdam, The Netherlands.
- 12.Doi, S., M. Ito, M. Shinagawa, G. Sato, H. Isomura, and H. Goto. 1988. Western blot detection of scrapie-associated fibril protein in tissues outside the central nervous system from preclinical scrapie-infected mice. J. Gen. Virol. 69:955-960. [DOI] [PubMed] [Google Scholar]
- 13.Hill, A. F., M. Desbruslais, S. Joiner, K. C. L. Sidle, I. Gowland, J. Collinge, L. J. Doey, and P. Lantos. 1997. The same prion strain causes vCJD and BSE. Nature 389:448-450. [DOI] [PubMed] [Google Scholar]
- 14.Hope, J., L. J. D. Morton, C. F. Farquhar, G. Multhaup, K. Beyreuther, and R. H. Kimberlin. 1986. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 5:2591-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hope, J., S. C. E. R. Wood, C. R. Birkett, A. Chong, M. E. Bruce, D. Cairns, W. Goldmann, N. Hunter, and C. J. Bostock. 1999. Molecular analysis of ovine prion protein identifies similarities between BSE and an experimental isolate of natural scrapie, CH1641. J. Gen. Virol. 80:1-4. [DOI] [PubMed] [Google Scholar]
- 16.Horiuchi, M., N. Yamazaki, T. Ikeda, N. Ishiguro, and M. Shinagawa. 1995. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J. Gen. Virol. 76:2583-2587. [DOI] [PubMed] [Google Scholar]
- 17.Ichijo, S., I. Inada, T. Sarashina, T. Ono, and H. Taniyama. 1984. Clinical findings on ovine scrapie in Japan. Jpn. J. Vet. Med. Assoc. 37:720-725. (In Japanese.)
- 18.Ikeda, T., M. Horiuchi, N. Ishiguro, Y. Muramatsu, D. K. Grathwohl, and M. Shinagawa. 1995. Amino acid polymorphisms of PrP with reference to onset of scrapie in Suffolk and Corriedale sheep in Japan. J. Gen. Virol. 76:2577-2581. [DOI] [PubMed] [Google Scholar]
- 19.Kimberlin, R. H., and C. A. Walker. 1978. Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J. Gen. Virol. 39:487-496. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin, R. H., C. A. Walker, and H. Fraser. 1989. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J. Gen. Virol. 70:2017-2025. [DOI] [PubMed] [Google Scholar]
- 21.Lasmézas, C. I., J.-P. Deslys, O. Robain, A. Jaegly, V. Berinque, J.-M. Peyrin, J.-G. Fournier, J.-J. Hauw, J. Rossier, and D. Dormont. 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402-405. [DOI] [PubMed] [Google Scholar]
- 22.Meyer, N., V. Rosenbaum, B. Schmidt, K. Gilles, C. Mirenda, D. Groth, S. B. Prusiner, and D. Riesner. 1991. Search for a putative scrapie genome in purified prion fractions reveals a paucity of nucleic acids. J. Gen. Virol. 72:37-49. [DOI] [PubMed] [Google Scholar]
- 23.Meyer, R. K., M. P. McKinley, K. A. Bowman, M. B. Braunfeld, R. A. Barry, and S. B. Prusiner. 1986. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. USA 83:2310-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan, K.-M., M. Baldwin, J. Nguyen, M. Gasset, A. Serban, D. Groth, I. Mehlhorn, Z. Huang, R. J. Fletterick, F. E. Cohen, and S. B. Prusiner. 1993. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 90:10962-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parchi, P., S. Capellari, S. G. Chen, R. B. Petersen, P. Gambetti, N. Kopp, P. Brown, T. Kitamoto, J. Tateishi, A. Giese, and H. Kretzschmar. 1997. Typing prion isoforms. Nature 386:232-234. [DOI] [PubMed] [Google Scholar]
- 26.Race, R., A. Jenny, and D. Sutton. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: Implication for transmission and antemortem diagnosis. J. Infect. Dis. 178:949-953. [DOI] [PubMed] [Google Scholar]
- 27.Safar, J., P. P. Roller, D. C. Gajdusek, and C. J. Gibbs, Jr. 1993. Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J. Biol. Chem. 268:20276-20284. [PubMed] [Google Scholar]
- 28.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 29.Shinagawa, M., A. Matsuda, G. Sato, M. Takeuchi, S. Ichijo, and T. Ono. 1984. Occurrence of ovine scrapie in Japan: Clinical and histological findings in mice inoculated with brain homogenates of an affected sheep. Jpn. J. Vet. Sci. 46:913-916. [DOI] [PubMed] [Google Scholar]
- 30.Telling, G. C., P. Parchi, S. J. DeArmond, P. Cortelli, P. Montagna, R. Gabizon, J. Mastrianni, E. Lugaresi, P. Gambetti, and S. B. Prusiner. 1996. Evidence for the conformation of the pathogenic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079-2082. [DOI] [PubMed] [Google Scholar]
- 31.Wells, G. A. H., A. C. Scott, C. T. Johnson, R. F. Gunning, R. D. Hancock, M. Jeffrey, M. Dawson, and R. Bradley. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121:419-420. [DOI] [PubMed] [Google Scholar]
- 32.Wilesmith, J. W., G. A. H. Wells, M. P. Cranwell, and J. B. M. Ryan. 1988. Bovine spongiform encephalopathy: epidemiological studies. Vet. Rec. 123:638-644. [PubMed] [Google Scholar]
- 33.Will, R. G., J. W. Ironside, M. Zeidler, S. N. Cousens, K. Estibeiro, A. Alperovitch, S. Poser, M. Pocchiari, A. Hofman, and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 34.Willoughby, K., D. F. Kelly, D. G. Lyon, and G. A. H. Wells. 1992. Spongiform encephalopathy in a captive puma (Felis concolor). Vet. Rec. 131:431-434. [DOI] [PubMed] [Google Scholar]
- 35.Wyatt, J. M., G. R. Pearson, T. N. Smerdon, T. J. Gruffydd-Jones, G. A. H. Wells, and J. W. Wilesmith. 1991. Naturally occurring scrapie-like spongiform encephalopathy in five domestic cats. Vet. Rec. 129:233-236. [DOI] [PubMed] [Google Scholar]