Abstract
The study of 16S rRNA gene sequences of all isolates of Bartonella henselae obtained in our laboratory and others from human patients or cats has revealed two genotypes according to the sequence of the 16S rRNA gene. Two isolates of these genotypes have previously been related to two different serotypes, and lack of cross-protection of the two serotypes has been demonstrated in cats. We investigated the grouping of eight strains of B. henselae on the basis of 16S ribosomal DNA, 35-kDa protein, Pap 31 protein, and internal transcribed spacer (ITS) gene sequencing; sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles; and monoclonal antibody reactivity studies. Houston-1, 90-615, and SA2 strains showed the same patterns in SDS-PAGE, but they differed from the patterns of B. henselae isolates URBHLLY8, URBHLIE9, Cat6, Fizz, and CAL-1. Nine monoclonal antibodies derived from BALB/c mice immunized with B. henselae Houston-1 strain reacted only with strains Houston-1, 90-615, and SA2, and not with any other Bartonella strains. The two serogroups corresponded with two genotypes based on differences in the sequences of the genes encoding 16S rRNA, 35-kDa protein, and Pap 31 protein. Sequences of ITS genes were highly divergent among strains, as each had a unique sequence and the subdivision was not supported by DNA-DNA relatedness study. Study of 22 additional strains of B. henselae isolated from French bacteremic cats demonstrated that they all belong to one or the other of the proposed serotype or genotype.
Bartonella henselae is a gram-negative, oxidase-negative, fastidious, aerobic, rod-shaped, slow-growing bacterium. Stoler et al. (41) first demonstrated the involvement of a bacterium in the etiology of an AIDS-related syndrome, bacillary angiomatosis (BA). In 1990, a 16S ribosomal gene fragment was amplified by PCR directly from tissue samples from patients with BA. By 16S rRNA gene sequencing, the bacterium was identified as Rochalimaea (Bartonella) spp.-like (33), and the novel species name, B. henselae, was proposed in 1992 (32). Improvements in the techniques used to isolate B. henselae and new methods for the identification and detection of the organism have enabled further clinical manifestations of infections to be determined. These now include cat scratch disease (CSD) (10, 15, 21, 38), BA (33, 40), peliosis hepatitis (40), septicemia (39), endocarditis (13, 31), and neurological disorders (27).
In 1996, Drancourt et al. reported a new serotype of B. henselae named Marseille (10), which was also a new genotype. The authors found that two isolates of B. henselae, from a patient with endocarditis and a patient with CSD, were genetically different from all previously isolated strains by sequence analysis of the 16S rRNA-encoding gene. Investigators in The Netherlands (2) also demonstrated two restriction fragment length polymorphism (RFLP) patterns of B. henselae DNA in samples from CSD patients. This was shown by analysis of the 16S-23S rRNA gene spacer PCR fragments and 16S rRNA gene PCR products digested with AluI. The presence of two genotypes was later confirmed in France and Germany (17, 19, 37, 38) based on sequencing of the 16S rRNA-encoding gene. Based on 16S RNA gene differences, genotypes I and II were proposed. PCR-based RFLP analysis of the 16S-23S rRNA intergenic spacer region using AluI and HaeIII demonstrated seven composite RFLP types in 11 B. henselae isolates from patients with BA, septicemia, and parenchymal bacillary peliosis (26). Rodriguez-Barradas et al. (34) identified five different fingerprint profiles from 17 isolates of B. henselae isolated from cats and from tissue and blood of human immunodeficiency virus-infected patients and patients with CSD. This was demonstrated by repetitive extragenic palindromic PCR and enterobacterial repetitive intergenic consensus PCR. The intergenic spacer region between the 16S and 23S rRNA genes showed that each tested strain (34) had a specific sequence. This method therefore appears to be of limited use in the identification of isolates at the species level. Other tested genes did not produce such diverse results: for example, the sequences of all tested strains of B. henselae based on partial citrate synthase gene were identical (4), and comparison of sequences from the riboflavin synthesis proteins of six isolates of B. henselae found single-nucleotide differences in some strains but no real difference between the representatives of genotype I and II (1).
In order to better define the classification of B. henselae isolates, especially serotypes associated within the two genotypes we observed by 16S rRNA gene sequence determination, we studied protein profiles of the organisms and sequenced additional genes. This was achieved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western immunoblotting with monoclonal antibodies (MAbs) against B. henselae Houston-1, and amplification and sequencing of genes that encode for the 35-kDa protein (unpublished data, GenBank access number U21304), the Pap 31 protein of phage 60457 (5) of B. henselae, and the internal transcribed spacer (ITS) (35, 36). The results of these studies are described in this report.
MATERIALS AND METHODS
Bartonella strains and antigen preparations.
The sources of strains used in this study are presented in Table 1. Bartonella isolates were grown on Columbia sheep blood agar (BioMerieux, Marcy l'Etoile, France) at 37°C in a 5% carbon dioxide incubator, except for B. bacilliformis, which was grown at 32°C. Bacteria were harvested after 5 to 7 days of culture and suspended in deionized water for SDS-PAGE or in phosphate-buffered saline (PBS) for microimmunofluorescence (MIF) assay. Twenty-two additional strains isolated from bacteremic cats, cultivated as described above (23), were used in an MIF assay and for PCR amplification.
TABLE 1.
Sources of Bartonella isolates used in this studya
| Species | Strains | Source (reference) |
|---|---|---|
| B. henselae | Houston-1T (ATCC 4988) | Septicemia, United States (32) |
| B. henselae | Cat6 | Blood from bacteremic cat, South Africa |
| B. henselae | Fizz | Blood from bacteremic cat, Switzerland |
| B. henselae | 90-615 (King) | BA, United States (45) |
| B. henselae | SA2 | CSD, United States (9) |
| B. henselae | CAL-1 | Septicemia, United States |
| B. henselae | URBHLLY8-Marseille (CIP 104756) | CSD, France (10) |
| B. henselae | URBHLIE9 | Endocarditis, France (10) |
| B. quintana | URBQTBAAH1 | BA, France (14) |
| B. quintana | URBQMLY15 | Chronic lymphadenopathy, France (11) |
| B. quintana | URBQPBAA7 | BA, France |
| B. quintana | URBQLIEH6 | Endocarditis, France (24) |
| B. quintana | URBQMTF14 | Trench fever, France (7) |
| B. quintana | URBQMTF15 | Trench fever, France (7) |
| B. quintana | FullerT (ATCC VR-358) | Trench fever (42) |
| B. quintana | URBQLY4 | Chronic lymphadenopathy, Franceb |
| B. quintana | Oklahoma | Septicemia, United States |
| B. quintana | URBQPIEH2 | Endocarditis, France (24) |
| B. quintana | SH-PERM | NAc, Russia |
| B. elizabethae | F9251T (ATCC 49927) | Endocarditis (8) |
| B. grahamii | V2T (NTCC 12860) | Blood from Clethrionomys glareolus (3) |
| B. taylorii | M6T (NTCC 12861) | Blood from Apodemus sp. (3) |
| B. doshiae | R18T (NTCC 12862) | Blood from Microtus agrestis (3) |
| B. vinsonii | BakerT (ATCC VR-152) | Spleen from Microtus pennsylvanicus (44) |
| B. bacilliformis | Acoch 812 | Blood from bartonellosis patient, Peru |
| B. bacilliformis | Monzon 269 | Blood from bartonellosis patient, Peru |
| B. weissii | BW137 | Blood from cow, France |
| B. clarridgeiae | URBCMNHC26 | Blood from cat, France |
| B. koehlerae | C-29 (ATCC 700693) | Blood from cat (12) |
| Bartonella sp. | C1-phy | Blood from Phyllotis sp. (4) |
| Bartonella sp. | C4-phy | Blood from Phyllotis sp. (4) |
| Bartonella sp. | C5-rat | Blood from Rattus sp. (4) |
| Bartonella sp. | C7-rat | Blood from Rattus sp. (4) |
| Bartonella sp. | C1-phy1 | Blood from Phyllotis sp. (4) |
| Bartonella sp. | C1-phy2 | Blood from Phyllotis sp. (4) |
| Bartonella sp. | N40 | Blood from Apodemus sylvaticus (4) |
Source is given when isolation of strain was not detailed elsewhere.
Reference: D. Raoult, M. Drancourt, A. Carta, and J. A. Gastaut, Letter, Lancet 343:977, 1994.
NA, not available.
Production of MAbs (18).
Six-week-old female BALB/c mice were inoculated three times intraperitoneally with 2 × 104 B. henselae Houston-1 organisms suspended in 0.5 ml of PBS at 7-day intervals. One week after the third injection, the mice were given intravenous boosters with 4 × 103 organisms suspended in 0.1 ml of PBS. Three days later, spleen cells from immunized mice were subjected to fusion with SP2/0-Ag14 myeloma cells (10:1) using 50% polyethylene glycol (molecular weight, 1,300 to 1,600; Sigma Chemical Co., St. Louis, Mo.) Fusion cells were grown in hybridoma medium (Seromed, Berlin, Germany) with 20% fetal bovine serum (GibcoBRL, Gaithersburg, Md.) and hypoxanthine aminopterin-thymidine (HAT) selective medium (Sigma Chemical Co., St. Louis, Mo.) at 37°C in a humidified atmosphere supplemented with 5% CO2. The supernatants were screened for antibodies to B. henselae by MIF assay. Representative hybridomas were subcloned twice by limiting dilution. Isotypes of MAbs were determined with an ImmunoType Mouse Monoclonal Antibody Isotyping Kit with antisera to mouse immunoglobulin M (IgM), IgA, IgG1, IgG2a, IgG2b, and IgG3 (Sigma). Specificities of MAbs were tested by Western immunoblotting (see below). Ascitic fluids were produced by injecting about 3 × 106 hybridoma cells suspended in 0.5 ml of PBS into mice, 1 week after an intraperitoneal injection of 0.5 ml of 2,6,10,14-tetramethylpentadecane (Pristane; Sigma).
MIF assay.
MIF (30) was used to screen hybridoma clones and to determine the specificity of MAbs. Antigens, including all Bartonella species listed in Table 1, were placed on 24-well microscope slides with a pen nib. The antigens were fixed in methanol for 10 min at room temperature before MAbs were added at a 1/32 dilution and the slides were incubated in a humidified chamber at 37°C for 30 min. Following two washes in PBS (5 min each) and rinsing with distilled water, the slides were air dried at 37°C. After incubation at 37°C for 30 min with fluorescein (dechlorotriazinyl amino fluorescein)-conjugated goat anti-mouse IgG+IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), diluted 1:200 in PBS containing 0.2% Evans blue (BioMerieux), the slides were washed (as above), mounted with coverslips using Fluoprep (BioMerieux) and read on a Zeiss epifluorescent microscope (Axioskop20; Carl Zeiss, Gottingen, Germany) at a magnification of ×400. For MAbs that were positive at the 1/32 dilution, MIF titers were determined by end point dilution. Sera from immunized mice were used as positive controls, and sera from healthy mice were used as negative controls. A MAb obtained against B. henselae Houston-1 was later used to test 22 B. henselae strains isolated from cat blood. MIF assay using a MAb (Bh1H8) diluted 1:200 was performed as described above.
SDS-PAGE and Western immunoblotting.
SDS-PAGE (22) and Western immunoblotting were performed using the methods described previously. Equal volumes of antigen (titrated to 4 mg of protein/ml) and sample buffer (0.0625 M Tris hydrochloride [pH 8.0], 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue) were separated electrophoretically in a 12% resolving gel and a 5% stacking gel at a constant current (8 to 10 mA) at room temperature for 4 h in running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS) in an electrophoretic cell (Mini Protein II; Bio-Rad, Richmond, Calif.). Prestained SDS-PAGE standards (Low-Range Standard; Bio-Rad) were used as a reference. The separated antigens on the gels were transferred to a 0.45-μm-pore-size nitrocellulose membrane (Hybond-C; Amersham, Little Chalfont, United Kingdom) at 50 V for 1 h at 4°C in an electrophoretic transfer cell (Mini Trans-Blot; Bio-Rad) and incubated overnight in PBS with 5% nonfat dry milk to block nonspecific binding sites. The membranes were washed three times with PBS, air dried, and cut into strips, which were incubated with MAbs, diluted 1:10 in PBS containing 3% milk, at room temperature for 1 h and washed as described above. Bound antibodies were detected with peroxidase-conjugated F(ab′)2 fragment goat anti-mouse IgG (heavy and light chains; AffiniPure; Jackson ImmunoResearch) and diluted at 1:200 in PBS containing 3% nonfat dry milk at room temperature for 1 h. After washing in PBS (as above), color was developed by the addition of a solution containing 0.015% 4-chloro-1 naphthol (Sigma, St. Louis, Mo.), 0.015% hydrogen peroxide, and 16.7% methanol in Tris-buffered saline.
DNA-DNA relatedness.
DNA was extracted and purified as previously described (6). The procedure for labeling DNA with tritium-labeled nucleotides and for hybridization experiments (S1 nuclease treatment, trichloroacetic procedure) have been detailed elsewhere (16, 20).
Gene amplification.
PCR was carried out in a Peltier model PTC-200 thermal cycler (MJ Research, Inc.). Five microliters of the purified genomic DNA was amplified in 50-μl reaction volumes with the different amplification primers (Table 2) (43). Genes encoding 16S rRNA, 35-kDa protein, Pap 31 protein, and ITS were amplified by using, respectively, fD1-rP2, 35KD1f-35KD1r, PAP1f-PAP4r, and QHVE1-QHVE2 primer pairs. Reaction mixture contained 25 pmol of each primer, 2 U of Taq DNA polymerase (GibcoBRL), a 0.2 mM concentration of each deoxynucleoside triphosphate (dATP, dCTP, dGTP, and dTTP), 1.6 μl of MgCl2 (50 mM), and 5 μl of template DNA, with a final volume of 50 μl. For amplification, a 10-min predenaturation step at 95°C was followed by 40 cycles consisting of denaturation at 95°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 1 min 30 s. Amplification was completed by incubation for 3 min at 72°C to allow complete extension of the PCR products. For study of the gene encoding 16S rRNA of the 22 additional B. henselae strains isolated from bacteremic cats, PCR incorporated the fD1-357f primer pair under conditions described above.
TABLE 2.
List of primers used in this study
| Protein encoded by gene | Primer | Nucleotide sequence (5′-3′) |
|---|---|---|
| 16S rRNA | fD1a | AGAGTTGATCCTGGCTCAG |
| Rp2a | ACGGCTACCTTGTTAGGACTT | |
| 357f | TACGGGAGGCAGCAG | |
| 357ra | CTGCTGCCTCCCGTA | |
| 536F | CAGCAGCCGCGGTAATAC | |
| 536R | GTATTACCGCGGCTGCTG | |
| 800F | ATTAGATACCCTGGTAG | |
| 800R | CTACCAGGGTATCTAAT | |
| 1050F | TGTCGTCAGCTCGTG | |
| 1050R | CACGAGCTGACGACA | |
| 35 kDa | 35KD1fa | GTCGCTAAAGGCTGATGA |
| 35KD2ra | GACTGATATCGTGCGTGTG | |
| 35KDs1f | GGTACGACGACAGTAATTGTT | |
| 35KDs2r | GATTTAAGAGATACCAACCA | |
| Pap 31 | PAP1fa | CTTTAATGACGACTTCTGTT |
| PAP4ra | CCGAAATCTGAGTAACGGTA | |
| PAP2r | CCCTAAATGTTTCAAGTTCA | |
| PAP3f | GCTGACAGAGAAGACGCAA | |
| ITS | 16SFa | AGAGGCAGGCAACCACGGTA |
| 23S1a | GCCAAGGCATCCACC | |
| QHVE1 | TTGGGATCATCATCTGAA | |
| QHVE2 | TTGGGATCATCATCTGAA | |
| QHVE3 | GATATATTCAGACATGTT | |
| QHVE4 | AACATGTCTGAATATATC |
Primer used for PCR amplification and sequencing.
Purification and sequence analysis of PCR products.
The PCR products were purified on a QIAquick PCR product purification spin column (QIAGEN, Hilden, Germany). For sequencing, we used sequencing primers (Table 2) in a commercially available sequencing kit (dRhodamine Terminator cycle Sequencing ready reaction with AmpliTaq Polymerase FS; PE Applied Biosystems, Warrington, United Kingdom) was used. For PCR products obtained using the fD1-357r primer pair, sequencing was performed using the primers fD1 and 357r. Sequencing products were purified by ethanol-magnesium precipitation, resolved on a 5% polyacrylamide gel (Long Ranger Singel packs, type 377-36cm WTR; Tebu, Le Perray en Yvelines, France) by electrophoresis with an ABI PRISM 377 DNA sequencer (Perkin-Elmer). For the 16S rRNA gene, partial sequences obtained were compared with that of Escherichia coli (43) to determine the position of base 170, which is the beginning of the 16S rRNA gene. For genes that encode for the 35-kDa protein and the Pap 31 protein of phage 60457, positions of signature sequences were determined according to the position of the open reading frame of the gene of B. henselae Houston-1 (GenBank accession numbers U21304 and AF001274, respectively). Thereafter, the gene sequences were compared for each strain.
Cell wall fatty acid analysis.
Colonies of all the B. henselae strains described in this study were grown on Trypticase soy agar with 5% sheep blood (Becton Dickinson) at 37°C for 48 h and then saponified. Cell wall fatty acids were then extracted and analyzed by gas chromatography as previously reported (L. Miller and T. Berger, Hewlett-Packard company publication, 1985).
RESULTS
SDS-PAGE profiles of B. henselae strains.
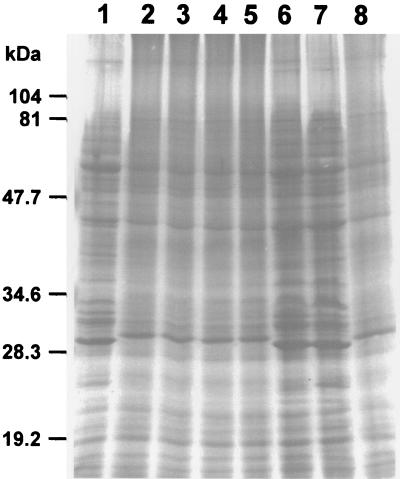
The Coomassie brilliant blue staining profiles of the eight strains of B. henselae examined by SDS-PAGE revealed dominant polypeptide bands of 60, 43, 29/30 (dual band at 29 and 30), and 32 kDa and a number of minor bands when solubilized at room temperature (Fig. 1). Two PAGE patterns could be determined based on the 29- and 30-kDa band (Fig. 1). PAGE pattern I, obtained for Houston-1, 90-615, and SA2 strains, showed a 29-kDa protein band, and PAGE pattern II, obtained for the URBHLLY8, URBHLIE9, Cat6, Fizz, and CAL-1 strains, consisted of a 30-kDa protein band (Fig. 1). The 32-kDa band was more intense in pattern I strains.
FIG. 1.
Coomassie brilliant blue staining profiles of B. henselae strains following SDS-PAGE. Lanes: 1, Houston-1; 2, URBHLLY8; 3, URBHLIE9; 4, Cat6; 5, Fizz; 6, 90-615; 7, SA2; 8, CAL-1.
MIF reactivity and MAb isotype.
The fusion of spleen cells from BALB/c mice immunized with B. henselae Houston-1 with myeloma cells enabled the selection of 9 MAbs of the IgG3 subclass which could be used to differentiate strains of B. henselae (Table 3). All reacted to B. henselae Houston-1, 90-615, and SA2, but none reacted against B. henselae URBHLLY8, URBHLIE9, Cat6, Fizz, and CAL-1 or with strains of other Bartonella species presented in Table 1. The titers of the MAbs against Houston-1 were higher than those against the 90-615 and SA2 strains (Table 3). The titers of the MAbs against strains 90-615 and SA2 were, however, identical. In an MIF assay using Bh1H8 and the 22 B. henselae strains isolated from cats, 15 gave a positive reaction. Despite repeated attempts, we never obtained MAbs specific for strains URBHLLY8, URBHLIE9, Cat6, Fizz, and CAL-1.
TABLE 3.
Reciprocal titers of MAbs to B. henselae Houston-1 against different isolates of B. henselae by MIF
| MAb | Reciprocal titer of MAb to B. henselae isolate
|
||
|---|---|---|---|
| Houston-1 | 90-615 | SA2 | |
| Bh1E10 | 2,048 | 512 | 512 |
| Bh1H8 | 1,024 | 512 | 512 |
| Bh2F7 | 1,024 | 512 | 512 |
| Bh3H3 | 8,192 | 2,048 | 2,048 |
| Bh8D4 | 4,096 | 1,024 | 1,024 |
| Bh10E5 | 1,024 | 512 | 512 |
| Bh12B8 | 8,192 | 1,024 | 1,024 |
| Bh15B10 | 2,048 | 1,024 | 1,024 |
| Bh18C2 | 2,048 | 1,024 | 1,024 |
Western immunoblotting.
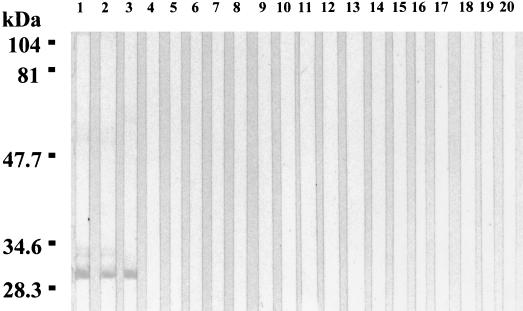
The nine MAbs reacted with the 29-kDa protein of the Houston-1, 90-615, and SA2 B. henselae strains but not with those of other Bartonella species tested. A weak reactivity was also observed with the 32-kDa protein of 90-615 and SA2 (Fig. 2). In Western blots where the antigens had been heated to 100°C for 5 min or digested in proteinase K, no MAb reactivity could be detected.
FIG. 2.
Western immunoblotting of MAb Bh1E10 with Bartonella antigens. Lanes: 1, B. henselae 90-615; 2, B. henselae SA2; 3, B. henselae Houston-1; 4, B. henselae URBHLLY8; 5, B. henselae URBHLIE9; 6, B. henselae Cat6; 7, B. henselae Fizz; 8, B. henselae CAL-1; 9, B. quintana URBQLY4; 10, B. quintana Oklahoma; 11, B. quintana URBQPIEH2; 12, B. quintana Fuller 13, B. elizabethae F9251; 14, B. grahamii V2; 15, B. taylorii M6; 16, B. doshiae R18; 17, B. vinsonii Baker; 18, Bartonella sp. strain C1-phy; 19, Bartonella sp. strain C5-rat; 20, B. bacilliformis Monzon 812.
DNA-DNA relatedness.
Relative reassociation with labeled DNA from strain URBHLLY8 was as follows: URBHLLY8, 100%; URBHLIE9, 92%; Fizz, 98%; CAL-1, 100%; Houston-1T, 94%; 90-615, 89%; SA2, 98%.
Sequence analysis of amplified genes.
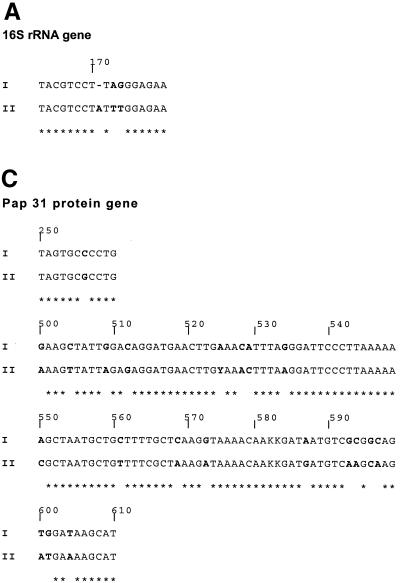
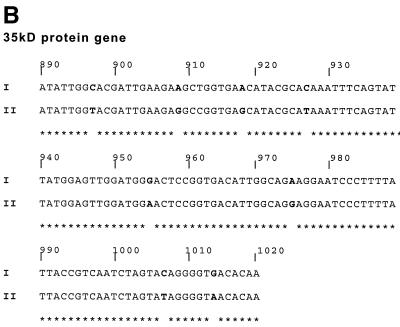
The genotypes of our B. henselae strains were first defined according to the sequences of a small fragment of the 5′ end of the gene coding for 16S rRNA (Fig. 3). The beginning of the gene (base 170) was determined for each strain by comparing the sequence we obtained with that previously reported for E. coli in which the sequence of the gene is clearly defined. For genes that encode for the 35-kDa protein and the Pap 31 protein of phage 60457, positions of signature sequences were determined according to the position of the open reading frame. The sequences obtained for Houston-1, 90-615, and SA2 strains (genotype I in Fig. 3) were identical to one another but different from those of the URBHLLY8, URBHLIE9, Cat6, Fizz, and CAL-1 strains (genotype II in Fig. 3). ITS sequences revealed DNA similarity values from 91.3 to 99.7%, and each isolate had a different sequence. The PCR assays that allow amplification of a short fragment that contains signature sequences from genes encoding 16S rRNA, 35-kDa protein, Pap 31 protein allowed us to determine, after sequencing, that among the 22 B. henselae strains isolated from bacteremic cats, 15 belong to genotype I and 7 belong to genotype II. The 15 strains classified in genotype I were the 15 that gave positive reactions in the MIF assay using the Bh1H8 MAb.
FIG. 3.
Comparison of partial sequences of the 16S rRNA gene (A), the 35-kDa protein gene (B), and the Pap 31 protein (C) of Houston-1, 90-615, and SA2 strains (genotype I) and URBHLLY8, URBHLIE9, Cat6, Fizz, and CAL-1 strains (genotype II).
Cell wall fatty acid analysis.
All the B. henselae isolates were indistinguishable by using cell wall fatty acid analysis (data not shown).
DISCUSSION
The existence of genotypic variation among B. henselae strains has previously been demonstrated using different DNA-based techniques (2, 26, 28, 29, 35, 37, 38, 47). There is little information, however, on the antigenic variation between B. henselae strains, which might be important in the development of improved diagnostic tests for B. henselae infections, the development of effective vaccines, and the discovery of new animal hosts and vectors. We tested eight strains of B. henselae from various places and from different hosts and clinical situations in order to evaluate whether the heterogeneity of the species was clustered into subspecies. The reactivity patterns of the MAbs we produced indicated that the B. henselae strains we had studied could be separated into two distinct groups, based on their antigenic properties, which is consistent with findings we reported previously (10). The first group, the Houston serotype, consisted of the Houston-1, 90-615, and SA2 strains, which could be detected by all the MAbs we produced. The second group, the Marseille serotype, against which none of the MAbs reacted, consisted of the URBHLLY8, URBHLIE9, CAL-1, Fizz, and Cat6 strains. Our findings that the titers of the MAbs to the Houston-1 strain (against which they were produced) were consistently higher than those against the 90-615 and SA2 strains indicate that there are different levels of epitope expression among strains within the serotypes we propose. Further evidence for a distinct phenotypic difference between the two groups was provided by the results of SDS-PAGE study. The 29-kDa protein demonstrated in the Houston serotype strains was not present in the serotype Marseille and was replaced by a 30-kDa protein band (25). The 29-kDa protein was the antigen recognized by the Houston serotype-specific MAbs. Moreover, this heterogeneity can result in false-negative serology in patients infected with B. henselae serotype Marseille, for which antibody reactivity is tested against B. henselae serotype Houston, as previously reported (J. L. Mainardi, C. Figliolini, F. W. Goldstein, P. Blanche, M. Baret-Rigoulet, N. Galezowski, P. E. Fournier, and D. Raoult, Letter, J. Clin. Microbiol. 36:2800, 1998). Curiously, in spite of repeated attempts of inoculation of strain URBHLLY8 to mice, we never succeeded in obtaining a MAb(s) directed only against the group including URBHLLY8, URBHLIE9, CAL-1, Fizz, and Cat6 strains (unpublished data), indicating that the 29-kDa protein could be a modification of the 30-kDa protein in some strains.
This phenotypic clustering was consistent with genotypes determined by 16S rRNA type-specific PCR in The Netherlands (2) and Germany (37, 38). The strains we identified as belonging to genotype I were the same as those identified as belonging to the Houston serotype, based on the phenotypic data we obtained. Similarly, the strains in genotype II were the same as those belonging to the Marseille serotype. Moreover, these two serotypes have been previously shown to be two different pathotypes in cats, as they were unable to provide cross-protection (46). In this study, on the basis of consistent differences in three tested genes (the 16S rRNA gene, the 35-kDa protein gene, and the Pap 31 protein gene), for which the same variations were described in all tested strains, we confirmed that indeed two distinct genotypes exist. However, some genes, such as the ITS gene, showed a wide heterogeneity within each group. Moreover, DNA-DNA relatedness study did not divide the strains into the two clusters observed by serotyping with MAb or sequencing of selected genes. The division into two clusters seems to be based on a limited polymorphism that affects only a limited part of the genome that codes for the major protein epitope recognized by our MAbs and the 35-kDa and Pap 31 proteins. The future publication of the complete genome of the B. henselae Houston-1T strain, which is now undergoing sequencing (http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/bact.html), may help to better the understanding of epitopes involved in reactions with our MAbs. Nevertheless, our results present both phenotypic and genotypic evidence that there are two distinct groups within the B. henselae species. The existence of these two groups may be responsible for possible reinfection in cats, such as that described by Yamamoto et al., who demonstrated a lack of heterologous protection among each of two genotypes or serotypes of B. henselae (46).
Acknowledgments
We are grateful to Armand Tasmadjian for help in the preparation of the experiments, to Elisabeth Ajeron for help in DNA-DNA relatedness study, and to Anne-Marie Pretorius and Sarah Droz for providing some of the cat isolates used in this study.
REFERENCES
- 1.Bereswill, S., S. Hinkelmann, M. Kist, and A. Sander. 1999. Molecular analysis of riboflavin synthesis genes in Bartonella henselae and use of the ribC gene for differentiation of Bartonella species by PCR. J. Clin. Microbiol. 37:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmans, A. M. C., J. F. P. Schellekens, J. D. A. van Embden, and L. M. Schouls. 1996. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J. Clin. Microbiol. 34:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birtles, R. J., T. G. Harrison, N. A. Saunders, and D. H. Molyneux. 1995. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int. J. Syst. Bacteriol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Birtles, R. J., and D. Raoult. 1996. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int. J. Syst. Bacteriol. 46:891-897. [DOI] [PubMed] [Google Scholar]
- 5.Bowers, T. J., D. Sweger, D. Jue, and B. Anderson. 1998. Isolation, sequencing and expression of the gene encoding a major protein from the bacteriophage associated with Bartonella henselae. Gene 206:49-52. [DOI] [PubMed] [Google Scholar]
- 6.Brenner, D. J., G. R. Fanning, A. V. Rake, and K. E. Johnson. 1972. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J. Bacteriol. 109:953-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouqui, P., B. La Scola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184-189. [DOI] [PubMed] [Google Scholar]
- 8.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolan, M. J., M. T. Wong, R. L. Regnery, J. H. Jorgensen, M. Garcia, J. Peters, and D. Drehner. 1993. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann. Intern. Med. 118:331-336. [DOI] [PubMed] [Google Scholar]
- 10.Drancourt, M., R. J. Birtles, G. Chaumentin, F. Vandenesh, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat scratch disease. Lancet 347:441-443. (Erratum, Lancet 347:842.) [DOI] [PubMed] [Google Scholar]
- 11.Drancourt, M., V. Moal, P. Brunet, B. Dussol, Y. Berland, and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infection in a seronegative hemodialyzed patient. J. Clin. Microbiol. 34:1158-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Droz, S., B. Chi, E. Horn, A. G. Steigerwalt, A. M. Whitney, and D. J. Brenner. 1999. Bartonella koehlerae sp. nov., isolated from cats. J. Clin. Microbiol. 37:1117-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Bruneel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis. A study of 48 patients. Medicine 80:245-251. [DOI] [PubMed] [Google Scholar]
- 14.Gasquet, S., M. Maurin, P. Brouqui, H. Lepidi, and D. Raoult. 1998. Bacillary angiomatosis in immunocompromised patients: a clinicopathological and microbiological study of seven cases and review of literature. AIDS 12:1793-1803. [DOI] [PubMed] [Google Scholar]
- 15.Gradon, J. D., and D. S. Stein. 1993. Association between Rochalimaea infection and cat-scratch disease. Clin. Infect. Dis. 17:287-288. [DOI] [PubMed] [Google Scholar]
- 16.Grimont, P. A. D., M. Y. Popoff, F. Grimont, C. Coynault, and M. Lemelin. 1980. Reproducibility and correlation study of three deoxyribonucleic acid hybridization procedures. Curr. Microbiol. 4:325-330. [Google Scholar]
- 17.Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. Heller, R. W. Kasten, K. Yamamoto, and Y. Piemont. 1997. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J. Clin. Microbiol. 35:2120-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlow, E., and D. Lane. 1988. Monoclonal antibodies, growing hybridomas, p. 139-282. In E. Harlow and D. Lane (ed.), Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Heller, R., M. Artois, V. Xemar, D. De Briel, H. Gehin, B. Jaulhac, H. Monteil, and Y. Piemont. 1997. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J. Clin. Microbiol. 35:1327-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khammas, K. M., E. Ageron, P. A. D. Grimont, and P. Kaiser. 1989. Azospirillum irakense sp. nov. a nitrogen-fixing bacterium associated with rice roots and rhizosphere soil. Res. Microbiol. 140:679-693. [DOI] [PubMed] [Google Scholar]
- 21.Koehler, J. E., C. A. Glaser, and W. Tappero. 1994. Rochalimaea henselae infection: a new zoonosis with the domestic cat as a reservoir. JAMA 271:531-535. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.La Scola, B., B. Davoust, M. Boni, and D. Raoult. Lack of correlation between serology, Bartonella DNA detection within fleas and results of blood culture in a Bartonella infected stray cat population. Clin. Microbiol. Infect., in press. [DOI] [PubMed]
- 24.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang, Z., and D. Raoult. 2000. Differentiation of Bartonella species by a microimmunofluorescence assay, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblotting. Clin. Diagn. Lab. Immunol. 7:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matar, G. M., B. Swaminathan, S. B. Hunter, L. N. Slater, and D. F. Welch. 1993. Polymerase chain reaction-based restriction fragment length polymorphism analysis of a fragment of the ribosomal operon from Rochalimaea species for subtyping. J. Clin. Microbiol. 31:1730-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurin, M., R. J. Birtles, and D. Raoult. 1997. Current knowledge of Bartonella species. Eur. J. Clin. Microbiol. Infect. Dis. 16:487-506. [DOI] [PubMed] [Google Scholar]
- 28.Norman, A. F., R. L. Regnery, P. Jameson, C. Greene, and D. C. Krause. 1995. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 33:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins, B. A., B. Swaminathan, L. A. Jackson, D. J. Brenner, J. D. Wenger, R. L. Regnery, and D. J. Wear. 1992. Case 22-1992. Pathogenesis of cat scratch disease. N. Engl. J. Med. 327:1599-1601. [DOI] [PubMed] [Google Scholar]
- 30.Philip, R. N., E. A. Casper, W. Burgdorfer, R. K. Gerloff, L. E. Hugues, and E. J. Bell. 1978. Serologic typing of rickettsiae of the spotted fever group by micro immunofluorescence. J. Immunol. 121:1961-1968. [PubMed] [Google Scholar]
- 31.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 32.Regnery, R. L., B. E. Anderson, B. E., J. E. Clarridge, M. C. Rodriguez-Barradas, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Relman, D. A., J. S. Loutit, T. M. Schmidt, S. Falkow, and L. S. Tompkins. 1990. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N. Engl. J. Med. 323:1573-1580. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Barradas, M. C., R. J. Hamill, E. D. Houston, P. R. Georgiou, J. E. Clarridge, R. L. Regnery, and J. E. Koehler. 1995. Genomic fingerprinting of Bartonella species by repetitive element PCR for distinguishing species and isolates. J. Clin. Microbiol. 33:1089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux, V., and D. Raoult. 1995. Inter- and intraspecies identification of Bartonella (Rochalimea) species. J. Clin. Microbiol. 33:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roux, V., and D. Raoult. 1995. The 16S-23S rRNA intergenic spacer region of Bartonella (Rochalimaea) species is longer than usually described in other bacteria. Gene 156:107-111. [DOI] [PubMed] [Google Scholar]
- 37.Sander, A., C. Bühler, K. Pelz, E. Von Cramm, and W. Bredt. 1997. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J. Clin. Microbiol. 35:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sander, A., M. Ruess, K. Deichmann, N. Böhm, and W. Bredt. 1998. Two different genotypes of Bartonella henselae in children with cat-scratch disease and their pet cats. Scand. J. Infect. Dis. 30:387-391. [DOI] [PubMed] [Google Scholar]
- 39.Slater, L. N., D. F. Welch, D. Hensel, and D. W. Coody. 1990. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N. Engl. J. Med. 323:1587-1593. [DOI] [PubMed] [Google Scholar]
- 40.Slater, L. N., D. F. Welch, and K. W. Min. 1992. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch. Intern. Med. 152:602-606. [PubMed] [Google Scholar]
- 41.Stoler, M. H., T. A. Bonfiglio, R. T. Steigbigel, and M. Pereira. 1983. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am. J. Clin. Pathol. 80:714-718. [DOI] [PubMed] [Google Scholar]
- 42.Varela, G., J. W. Vinson, and C. Molina-Pasquel. 1969. Trench fever. II. Propagation of Rickettsia quintana on cell-free medium from the blood of two patients. Am. J. Trop. Med. Hyg. 18:708-712. [PubMed] [Google Scholar]
- 43.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss, E., D. A. Dasch, D. R. Woodman, and J. C. Williams. 1978. Vole agent identified as a strain of the trench fever rickettsia, Rochalimaea quintana. Infect. Immun. 19:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch, D. F., D. A. Pickett, L. N. Slater, A. G. Steigerwalt, and D. J. Brenner. 1992. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J. Clin. Microbiol. 30:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto, K., B. B. Chomel, R. W. Kasten, C. C. Chang, T. Tseggai, P. R. Decker, M. Mackowiak, K. A. Floyd-Hawkins, and N. C. Pedersen. 1998. Homologous protection but lack of heterologous-protection by various species and types of Bartonella in specific pathogen-free cats. Vet. Immunol. Immunopathol. 65:191-204. [DOI] [PubMed] [Google Scholar]
- 47.Zeaiter, Z., P.-E. Fournier, and D. Raoult. 2002. Genomic variation of Bartonella henselae strains detected in lymph nodes from patients with cat scratch disease. J. Clin. Microbiol. 40:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]