Abstract
A nationwide study on candidemia was conducted in Iceland from 1980 to 1999. The annual incidence increased from 1.4 cases/100,000 inhabitants/year between 1980 and 1984 to 4.9 cases/100,000 inhabitants/year between 1995 and 1999 (P < 0.0001). Candidemia episodes at university hospitals increased from 0.15/1,000 admissions to 0.55/1,000 admissions (P < 0.0001). Candida albicans was the predominant species responsible (64.4%). The national import of fluconazole increased approximately fourfold during the second half of the study, but increased resistance to this agent was not observed.
The incidence of invasive fungal infections is increasing in many hospitals. The National Nosocomial Infections Surveillance (NNIS) program for U.S. hospitals documented a doubling in the rate of nosocomial fungal infections between 1980 and 1990 (4). From 1995 to 1996, Candida species was the fourth most common cause of nosocomial bloodstream infections (BSIs) in the United States (13). In recent years, some studies have reported an increase of candidemia due to Candida non-albicans species, with the threat of increased mortality and antifungal drug resistance (1, 10, 16, 21). The epidemiology of candidemia has been studied primarily in selected hospitals (2, 7, 22); few studies have focused on this problem on a nationwide basis (15, 17). The purpose of this study was to examine candidemia in Iceland during a 20-year period with respect to epidemiology and clinical mycology. Due to the high quality and accessibility of demographic and medical data, Iceland, a 103,000-km2 island in the north Atlantic ocean with 285,000 inhabitants, is well suited for epidemiological studies.
All patients in Iceland with yeast isolated from blood from 1 January 1980 to 31 December 1999 were identified retrospectively by a nationwide search in microbiology databases. During the first decade of the study, a Bactec (Becton Dickinson Microbiology Systems) radiometric system was most widely used. During the second decade of the study, the nonradiometric systems Bactec, Difco ESP (Becton Dickinson), and bioMérieux Vital (bioMérieux) were used. There are 2 university or university-affiliated hospitals and 14 county hospitals in the country. Three clinical microbiology laboratories process blood cultures from all of the hospitals. An episode of yeast BSI was defined as at least one blood culture positive for yeast. Episodes were considered separate if they occurred more than 2 weeks apart.
Viable yeast BSIs were subcultured on Sabouraud agar (Oxoid). Species identification was based on germ tube production, distinctive color, and morphology on CHROMagar (Hardy Diagnostics) and sugar assimilation profiles by using the API id32C system (bioMérieux). The MICs of amphotericin B, fluconazole, and itraconazole were determined by using an Etest (AB Biodisk) according to instructions from the manufacturer (Antifungal susceptibility testing of yeasts, Etest technical guide 4, AB Biodisk, Solna, Sweden, 1997). The plates were incubated at 35°C for 48 h before reading the MICs. The National Committee for Clinical Laboratory Standards (NCCLS) breakpoint criteria for antifungal susceptibility were used (9).
National import figures on antifungal agents for the period from 1980 to 1999 were obtained from the Icelandic Association of Importers of Pharmaceuticals. The number of defined daily doses (DDD) per packing was calculated, and the import was expressed as DDD per 1,000 inhabitants per year. Information about national demographics, including age distribution, for the period from 1980 to 1999 was obtained from the Bureau of Statistics in Iceland. Information on admissions to pediatric, medical, and surgical wards at the university hospitals for each year of the study was obtained from annual hospital reports. The chi-square test for linear trend was used to compare incidence rates between study periods, and the chi-square test was used to compare fungal species distribution and proportions of blood cultures positive for yeasts between the first and second halves of the study period.
In the 20-year period from 1980 to 1999, 172 episodes of BSI with yeasts, predominantly candidemia, were diagnosed in 165 patients in Iceland. Children (≤16 years) comprised 11 (7%) of the patients, and adults comprised 154 (93%) of the patients. Six patients had two or more separate episodes, occurring at least 2 weeks apart. The nationwide annual incidence of candidemia and number of candidemic episodes per 1,000 admissions to the university hospitals is shown in Fig. 1. A vast majority of the patients (87%) came from the university hospitals. Figure 2 shows the age-specific incidence for the first and second halves of the observation period, respectively. During the second decade of the study, the use of blood cultures at the two university hospitals increased from 71,002 vials between 1990 and 1994 to 93,032 between 1995 and 1999. The proportion of blood cultures positive for yeasts increased slightly, from 0.187% between 1990 and 1994 to 0.204% between 1995 and 1999, but this increase was not significant (P = 0.464).
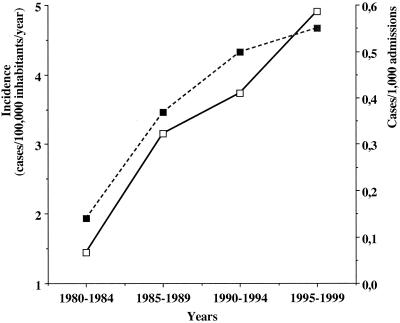
FIG. 1.
Increasing incidence (cases/100,000 inhabitants/year) of candidemia in Iceland during a 20-year period, from 1980 to 1999. The open squares with solid lines represent the population-based incidence, whereas the filled squares with dashed lines depict the incidence as a proportion of 1,000 admissions to the university hospitals in the country. As shown, the nationwide incidence increased 3.5-fold during the 20 years of the study, from 1.4 between 1980 and 1984 to 4.9 between 1995 and 1999 (P < 0.0001). The number of candidemic episodes per 1,000 admissions at the two university hospitals also increased significantly, from 0.15/1,000 admissions between 1980 and 1984 to 0.55/1,000 admissions between 1995 and 1999 (P < 0.0001).
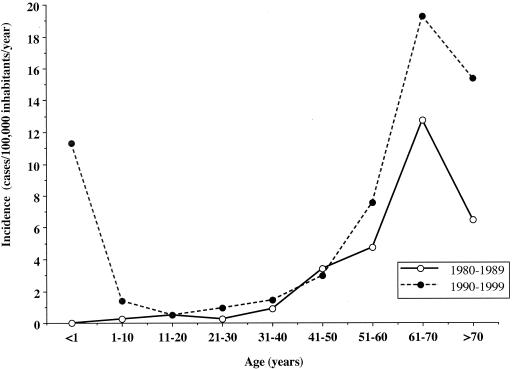
FIG. 2.
Age-specific incidence of fungal BSIs in Iceland from 1980 to 1999 calculated for the first and second decades of the study. As shown, the age-specific incidence was similarly distributed in the first (open circles, solid lines) and the second (filled circles, dashed lines) decades, with the exception of the youngest age group (<1 year old). The incidence was low in people aged 5 to 40 years but started to rise among people aged 41 to 50 years. Incidence subsequently increased with advancing age, peaking among patients aged 61 to 70 years (1980 to 1989, 12.7 cases/100,000 inhabitants/year; 1990 to 1999, 19.3 cases/100,000 inhabitants/year). From 1980 to 1989, no children of <1 year of age were diagnosed with candidemia. After 1989, however, the incidence followed a biphasic pattern, with a high incidence occurring in the youngest age group (<1 year, 11.3 cases/100,000 inhabitants/year). Most of the children younger than 1 year old were preterm infants in the neonatal ICU at the time of diagnosis.
Table 1 shows the yeast species that were cultured from the blood of Icelandic patients from 1980 to 1999. Species distribution remained relatively stable in the country, with C. albicans causing around two-thirds of infections (range, 63.4 to 65.3%) and Candida non-albicans species causing one-third. There was no significant change in yeast species distribution between the first and second halves of the study (P = 0.35). Species identification of all available isolates revealed Candida dubliniensis in four cases.
TABLE 1.
Yeast strains isolated from blood cultures in Iceland from 1980 to 1999
| Yeast species | No. of strains isolated from:
|
Total no. (% of total no.) | |||
|---|---|---|---|---|---|
| 1980-1984 | 1985-1989 | 1990-1994 | 1995-1999 | ||
| C. albicans | 11 | 26 | 32 | 45 | 114 (64.4) |
| C. glabrata | 0 | 6 | 7 | 9 | 22 (12.4) |
| Candida parapsilosis | 0 | 5 | 4 | 8 | 17 (9.6) |
| Candida tropicalis | 0 | 2 | 4 | 4 | 10 (5.6) |
| C. dubliniensis | 0 | 0 | 0 | 4 | 4a (2.3) |
| Candida lusitaniae | 0 | 0 | 1 | 0 | 1 (<1) |
| Candida famata | 0 | 0 | 1 | 0 | 1 <1 |
| Hansenula anomala | 0 | 1 | 0 | 0 | 1 <1 |
| C. krusei | 1 | 0 | 0 | 0 | 1 (<1) |
| Yeast not identified | 5 | 1 | 0 | 0 | 6 (3.4) |
| Total | 17 | 41 | 49 | 70 | 177b |
C. dubliniensis was identified for the first time in Iceland in four cases. Originally, these isolates had been identified as other Candida species: two were identified as C. albicans and one was identified as C. tropicalis, and yeast had not been identified in one case.
In the period from 1980 to 1999, there were 172 cases of candidemia diagnosed in Iceland, but in five of these cases, two different yeast species were cultured from the same individual.
The MICs of amphotericin B, fluconazole, and itraconazole were determined for 99 strains dating from 1991 to 1999 (Table 2). All were susceptible to amphotericin B (MIC, ≤1 μg/ml), 97% were susceptible to fluconazole (MIC, ≤8 μg/ml), and 87% were susceptible to itraconazole (MIC, ≤0.125 μg/ml). During the study period, the number of isolates classified as susceptible-dose dependent for itraconazole increased from 6.7% (3 of 45) between 1991 and 1995 to 16.7% (9 of 54) between 1996 and 1999, but this increase was not statistically significant.
TABLE 2.
In vitro susceptibility of 99 fungal BSIs to amphotericin B, itraconazole, and fluconazole
| Antifungal agent | Species | No. of isolates | MIC (μg/ml)
|
MIC50c | MIC90d | ||
|---|---|---|---|---|---|---|---|
| Mean | Median | Range | |||||
| Amphotericin B | C. albicans | 67 | 0.08 | 0.064 | 0.016-0.25 | 0.064 | 0.125 |
| C. glabrata | 12 | 0.23 | 0.22 | 0.125-0.38 | 0.19 | 0.25 | |
| C. parapsilosis | 11 | 0.15 | 0.125 | 0.125-0.25 | 0.125 | 0.190 | |
| C. tropicalis | 5 | 0.21 | 0.25 | 0.125-0.25 | 0.25 | 0.25 | |
| C. dubliniensis | 4 | 0.03 | 0.275 | 0.023-0.047 | 0.023 | 0.047 | |
| Itraconazole | C. albicans | 67 | 0.04 | 0.023 | 0.008-0.75 | 0.023 | 0.094 |
| C. glabrataa | 12 | 0.46 | 0.315 | 0.125-2 | 0.25 | 0.50 | |
| C. parapsilosis | 11 | 0.08 | 0.064 | 0.008-0.19 | 0.064 | 0.125 | |
| C. tropicalis | 5 | 0.03 | 0.016 | 0.006-0.064 | 0.016 | 0.064 | |
| C. dubliniensis | 4 | 0.08 | 0.0945 | 0.008-0.125 | 0.064 | 0.125 | |
| Fluconazole | C. albicans | 67 | 0.36 | 0.19 | 0.094-8 | 0.190 | 0.380 |
| C. glabratab | 12 | 12.67 | 8 | 4-48 | 8.0 | 24 | |
| C. parapsilosis | 11 | 1.60 | 1 | 0.19-8 | 1.0 | 2.0 | |
| C. tropicalis | 5 | 1.13 | 0.38 | 0.125-4 | 0.380 | 4.0 | |
| C. dubliniensis | 4 | 2.14 | 0.22 | 0.125-8 | 0.190 | 8.0 | |
Ten strains of C. glabrata were classified as susceptible-dose dependent to itraconazole (MIC, 0.25 to 0.5 μg/ml), and one strain was resistant (MIC, ≥1 μg/ml).
Three strains of C. glabrata were classified as susceptible-dose dependent to fluconazole (MIC, 16 to 32 μg/ml).
MIC at which 50% of the isolates tested are inhibited.
MIC at which 90% of the isolates tested are inhibited.
The national import of antifungal agents from 1990 to 1999 is shown in Table 3. Fluconazole was approved for oral and parenteral use in 1990. During the period from 1991 to 1999, the import of oral formulations increased from 9.4 to 47.6 DDD/1,000 inhabitants/year (406%) and the import of fluconazole for parenteral use increased from 0.5 to 1.8 DDD/1,000 inhabitants/year (260%).
TABLE 3.
National importa of antifungal agents in Iceland from 1990 to 1999 expressed as DDD per 1,000 inhabitants per year
| Year | DDD/1,000 inhabitantsb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| AmB | ITZ | FLU | Keto | 5-FC | Terb | Nystatin | Griseo | |
| 1990 | 1.2 | 0.0 | 6.8 | 147.0 | 0.1 | 0.0 | 228.1 | 477.4 |
| 1991 | 1.8 | 0.0 | 9.9 | 130.4 | 0.1 | 0.0 | 239.7 | 399.6 |
| 1992 | 1.4 | 0.0 | 6.3 | 193.4 | 0.1 | 0.0 | 287.5 | 343.7 |
| 1993 | 0.7 | 0.0 | 15.6 | 112.8 | 0.0 | 227.9 | 244.2 | 166.7 |
| 1994 | 1.8 | 0.0 | 18.3 | 84.9 | 0.0 | 553.5 | 183.7 | 44.1 |
| 1995 | 1.4 | 38.4 | 19.2 | 86.8 | 0.0 | 604.9 | 168.4 | 34.9 |
| 1996 | 1.5 | 49.5 | 24.9 | 65.1 | 0.0 | 632.1 | 166.8 | 15.0 |
| 1997 | 1.1 | 84.1 | 32.1 | 91.8 | 0.0 | 694.9 | 223.4 | 7.4 |
| 1998 | 0.4 | 61.5 | 48.0 | 69.0 | 0.0 | 709.2 | 174.1 | 0.0 |
| 1999 | 2.0 | 54.3 | 49.3 | 55.0 | 0.0 | 858.5 | 140.4 | 0.0 |
The first year of registered import was as follows: amphotericin B, 1982; fluconazole, 1990; ketoconazole, 1985; itraconazole, 1995; 5-flucytosine, 1982;terbinafine, 1993; nystatin, before 1980; griseofulvin, before 1980.
DDD were as follows: amphotericin B (AmB), 35 mg; fluconazole (FLU), itraconazole (ITZ), and ketoconazole (Keto), 200 mg each; 5-flucytosine (5-FC), 10 g; terbinafine (Terb), 250 mg; nystatin, 1,500,000 IUs; griseofulvin (Griseo), 500 mg.
Several studies have shown a substantial increase in the incidence of candidemia in the past 2 decades. Data from the NNIS system on nosocomial BSIs showed an up to fivefold increase in incidence between 1980 and 1989 in the United States (3). According to another surveillance study from the NNIS program, between 1995 and 1996 Candida species was the fourth most common cause of nosocomial BSI (13). Our data show that the incidence of candidemia in Iceland has increased steadily and significantly over the past 2 decades. The incidence is somewhat higher than that documented in a nationwide study in Norway from 1991 to 1996 (an average of 0.17 per 1,000 discharges) (17) but similar to that observed in Israel in 1994 (0.50 per 1,000 admissions) (15). Although the use of blood cultures increased at the university hospitals, the proportion of cultures that turned out positive for yeasts remained stable. The observed increase may be due in part to improved detection, but other factors are likely to have played a role. Among them are the greater use of invasive devices and broad-spectrum antibacterial agents, more extensive surgical procedures, and advanced life support (5, 18). The length of stay at the university hospitals in Iceland, where 87% of the patients were diagnosed, did not increase in the past 2 decades and therefore does not explain our findings.
Many studies on the epidemiology of fungal BSIs have focused on selected hospitals or hospital wards, with different patient populations, and have reported a substantially higher incidence than is reported in this study. According to two studies on candidemia, one in Australia and one in the United States, the incidence was 1.5 and 3.3 episodes per 1,000 discharges, respectively (7, 19). Another study at the M.D. Anderson Cancer Center reported an incidence of 6 cases of fungemia per 1,000 admissions between 1988 and 1992 (2). A prospective multicenter study of candidemia at six sites in the United States concluded that the incidence was 9.8 cases/1,000 admissions in surgical intensive care units (ICUs) and 12.3 cases/1,000 admissions in neonatal ICUs (14).
Studies on candidemia and pathogen species distribution from different parts of the world generally agree that C. albicans is still the most commonly isolated fungal pathogen from blood, causing between 50 and 70% of infections (12, 14, 22). According to our data, approximately two-thirds (64.4%) of fungal BSIs in Iceland were caused by C. albicans and one-third were caused by Candida non-albicans species. These results are comparable to those reported from Norway (17) but somewhat higher than those reported from the United States, Canada, Latin America, and Europe (11). Among the different fungal species, we identified C. dubliniensis as the pathogen in four cases. This species, which was originally described by Sullivan and coworkers in 1995 (20), is germ tube positive and can therefore easily be mistaken for C. albicans (6). The proportion of candidemias caused by Candida non-albicans has not increased in the past 20 years according to our results. In contrast, other studies have reported a shift towards Candida non-albicans species in the past 5 to 10 years (2, 16). Antifungal prophylaxis with fluconazole may have played a role in this observed shift. In two studies, fluconazole prophylaxis was the single most important determinant for the relative increase in Candida krusei and Candida glabrata infections (2, 8). Fluconazole prophylaxis is used infrequently in Iceland, which may explain this difference.
The antifungal susceptibility patterns revealed that 97% of the Icelandic strains were susceptible to fluconazole, despite an approximately fourfold increase in fluconazole import in the past decade. Little has been published about national consumption of antifungal agents. Our results can be compared to data from the study conducted in Norway, where the use of fluconazole increased from 8.0 to 16.1 DDD/1,000 inhabitants/year between 1991 and 1996 (17). During the same period, a greater increase in fluconazole import was seen in Iceland: from 9.9 to 24.9 DDD/1,000 inhabitants/year. The use of amphotericin B remained relatively stable.
In conclusion, this study has shown that, on a national level, the incidence of candidemia in Iceland has increased 3.5-fold over the past 2 decades, with the highest incidences of infection occurring in the youngest and older age groups. The proportion of infections caused by Candida non-albicans species has remained stable. Fluconazole use has increased approximately fourfold in the past decade, but the majority of strains are still susceptible to this agent.
Acknowledgments
This study was supported in part by a Landspitali University Hospital research fund and the Kristín Björnsdóttir memorial fund.
We thank Örn Ólafsson for assistance with statistical analysis of the data.
REFERENCES
- 1.Abbas, J., G. P. Bodey, H. A. Hanna, M. Mardani, E. Girgawy, D. Abi-Said, E. Whimbey, R. Hachem, and I. Raad. 2000. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch. Intern. Med. 160:2659-2664. [DOI] [PubMed] [Google Scholar]
- 2.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, and W. J. Martone. 1991. Secular trends in nosocomial primary bloodstream infections in the United States. Am. J. Med. 91:86-89. [DOI] [PubMed] [Google Scholar]
- 4.Beck-Sague, C., W. R. Jarvis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, et al. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin. Infect. Dis. 33:177-186. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, M. E., L. H. Harrison, M. Pass, A. N. Sofair, S. Huie, R. K. Li, C. J. Morrison, D. W. Warnock, and R. A. Hajjeh. 2000. Candida dubliniensis fungemia: the first four cases in North America. Emerg. Infect. Dis. 6:46-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser, V. J., M. Jones, J. Dunkel, S. Storfer, G. Medoff, and W. C. Dunagan. 1992. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 8.Marr, K. A., K. Seidel, T. C. White, and R. Bowden. 2000. Candidemia in allogenic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M-27A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, R. J. Hollis, and S. A. Messer for the SENTRY Participant Group. 1998. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada and South America for the SENTRY Program. J. Clin. Microbiol. 36:1886-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 14.Rangel-Frausto, M. S., T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, J. E. Edwards, Jr., W. Jarvis, J. Dawson, and R. P. Wenzel. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in 7 surgical intensive care units and 6 neonatal intensive care units. Clin. Infect. Dis. 29:253-258. [DOI] [PubMed] [Google Scholar]
- 15.Rennert, G., H. S. Rennert, S. Pitlik, R. Finkelstein, and R. Kitzes-Cohen. 2000. Epidemiology of candidemia—a nationwide survey in Israel. Infection 28:26-29. [DOI] [PubMed] [Google Scholar]
- 16.Rocco, T. R., S. E. Reinert, and H. H. Simms. 2000. Effects of fluconazole administration in critically ill patients: analysis of bacterial and fungal resistance. Arch. Surg. 135:160-165. [DOI] [PubMed] [Google Scholar]
- 17.Sandven, P., L. Bevanger, A. Digranes, P. Gaustad, H. H. Haukland, M. Steinbakk, and the Norwegian Yeast Study Group. 1998. Constant low rate of fungemia in Norway, 1991 to 1996. J. Clin. Microbiol. 36:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh, N. 2001. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin. Infect. Dis. 33:1692-1696. [DOI] [PubMed] [Google Scholar]
- 19.Stratov, I., T. Gottlieb, R. Bradbury, and G. M. O'Kane. 1998. Candidaemia in an Australian teaching hospital: relationship to central line and TPN use. J. Infect. 36:203-207. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 21.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercan, C. Doyer, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective multicenter surveillance study by the Invasive Fungal Infection Group. Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 22.Yamamura, D. L., C. Rotstein, L. E. Nicolle, and S. Ioannou. 1999. Candidemia at selected Canadian sites: results from the Fungal Disease Registry, 1992-1994. Can. Med. Assoc. J. 160:493-499. [PMC free article] [PubMed] [Google Scholar]