Abstract
Nocardia paucivorans represents a new species of the genus Nocardia that has recently been isolated from bronchial secretions of a patient with chronic lung disease. Here, we report on the course of a disseminated infection caused by this species: i.e., cerebral and subsequent meningeal manifestations, isolation from the cerebrospinal fluid, and in vitro susceptibility to various antimicrobial agents.
CASE REPORT
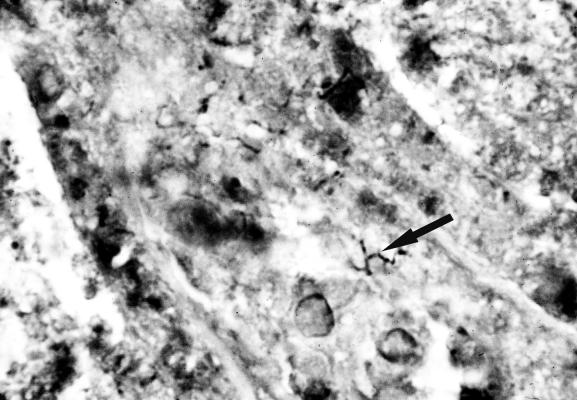
A 63-year-old man presented to the emergency department of this hospital in July 2000 with a 10-day history of headaches and mild ataxia on the right-hand side. Cranial computed tomography showed a cerebellar mass with surrounding edema, without signs of intracranial bleeding, and the result was confirmed by magnetic resonance imaging. The patient's condition improved with conservative treatment. A few days after admission, a blood pressure crisis occurred, resulting in an impaired neurological status that persisted despite prompt sufficient control of systemic blood pressure. Imaging demonstrated a minor perilesional bleeding in the cerebellum as well as an increase in the size of the original mass. The patient underwent craniotomy, during which the mass, now identified as an abscess, was removed. Histological investigation of the biopsies yielded granulomatous inflammation. Additionally, infiltration of the tissue by dark brown to black branching rods was seen in Grocott-stained sections, a typical feature of nocardiae (Fig. 1). Brain abscess material was inoculated in brain heart broth (BHB) and blood (BA) and chocolate (CA) agars and incubated at 36°C in an atmosphere enriched with 5% CO2. However, no growth was found after 10 days of incubation. Failure to isolate the pathogen despite its histological demonstration may have been due to sampling error. A decreased number of CD4+ T cells (172 per μl) was found, demonstrating an immunodeficiency of unknown origin, since human immunodeficiency virus types 1 and 2 antibody and p24 antigen tests were negative. The patient received a course of intravenous trimethoprim-sulfamethoxazole, which was replaced by amikacin plus a carbapenem because of systemic side effects. This therapy was administered for 6 weeks, with imipenem given during the first week and meropenem, which was better tolerated, given during the remaining 5 weeks. The patient made a slow recovery and was discharged with minor neurological deficits (tremor and dysdiadochokinesis). The CD4+ T-cell count remained low (maximum count, 414 per μl). Treatment of the nocardiosis was continued with oral minocycline.
FIG. 1.
Nocardiae (arrow) in a Grocott-stained histological section of the brain abscess material. Magnification, ×500.
Antibiotic therapy was discontinued after a duration of 6 months. The patient developed headaches and singultus within 2 weeks, resulting in readmission to the hospital. A lumbar puncture at readmission showed 1,306 granulocytes per mm3 in the cerebrospinal fluid (CSF) without detection of infectious agents upon microscopy. Treatment for suspected bacterial meningitis was initiated with ceftriaxone plus ampicillin. However, the patient's condition did not improve under this regimen, and no fast-growing bacteria could be isolated. Therefore, therapy was changed after 3 days to amikacin plus meropenem, assuming a meningeal manifestation of nocardiosis. The administration of amikacin plus ampicillin plus clavulanate during the 4th week worsened the side effects, leading to reinitiation of the former regimen.
After 6 weeks of intravenous therapy, treatment was changed to oral levofloxacin and minocycline based upon susceptibility test results and accounting for multiple drug intolerances. The patient was discharged home with this therapy and since then has been seen regularly in our outpatient clinic. The leukocyte count in the CSF decreased, but it remained mildly elevated for more than 10 weeks before falling to 10/mm3. The pathological findings in magnetic resonance imaging of the brain remained unchanged, but the neurological symptoms have improved.
CSF specimens for bacterial culture obtained on readmission were inoculated in liquid media (BHB and the commercially available VITAL blood culture system [bioMérieux S.A., Marcy l'Etoile, France] for aerobic and anaerobic incubation), as well as on BA, CA, and brain heart agar (BHA). Agars and liquid media showed no signs of bacterial growth after 7 days of incubation. Subcultures of the aerobic blood culture medium (not detected as positive by the automatic fluorescence readout system) on BA, CA, and BHA yielded yellowish colonies after 5 days of incubation at 36°C in an atmosphere enriched with 5% CO2, whereas there was no bacterial growth in subcultures of the BHB. Growth was most abundant on CA; the colonies developed white aerial hyphae after 3 to 4 days of incubation. On microscopic examination of BHA, microcolonies showed irregular branching. Growth of nocardiae (confirmed by subculture and microscopy) was also found on Loewenstein-Jensen agar after 2 weeks of incubation.
The Gram- and Ziehl-Neelsen-stained preparations from single colonies showed mostly gram-positive branched rods and coccoid fragments, as well as partially-acid-fast rods and coccoid fragments, respectively.
The morphology of the colonies and the typical Gram and acid-fast stains led to the diagnosis of Nocardia species. Tests for basic biochemical reactions, such as oxidative and fermentative glucose use, gelatin liquefaction, nitrate reduction, and urea hydrolysis, were negative. In addition, the isolate showed remarkable susceptibility to a wide range of antimicrobial agents (disk diffusion test on Mueller-Hinton agar [data not shown]). Subsequent sequence determination of a 600-bp fragment amplified from the 3′ end of the 16S rRNA gene identified the isolate as Nocardia paucivorans. The diagnosis was confirmed by the German national consultant laboratory for actinomycetes.
Comparative in vitro susceptibility testing including the N. paucivorans type strain (DSM 44386T) was performed on Mueller-Hinton agar at 36°C in an atmosphere enriched with 5% CO2 by using the Etest system, and results were read after 24 and 48 h. Strains of Nocardia nova (ATCC 33726) and Nocardia farcinica (ATCC 3308) along with previously published results of broth dilution testing for these organisms (16, 18) were used as controls. The results from our isolate confirmed those obtained by disk diffusion testing (Table 1). Slightly higher MICs were found for the N. paucivorans type strain, for which no susceptibility data have been available. The results from the control strains were comparable to those published previously (16, 18).
TABLE 1.
MICs of the N. paucivorans CSF isolate, the N. paucivorans type strain N. nova, and N. farcinica as read by Etest on Mueller-Hinton agar
| Agent | MIC (μg/ml) for:
|
|||
|---|---|---|---|---|
|
N. paucivorans
|
N. nova ATCC 33726 | N. farcinica ATCC 3308 | ||
| CSF isolate | DSM 44386T | |||
| Amikacin | 0.064 | 0.047 | 0.25 | 1.5 |
| Ampicillin | 0.094 | 0.75 | 0.19 | 2 |
| Amoxicillin-clavulanate | 0.094 | 2 | ≥256 | 2 |
| Ampicillin-sulbactam | 0.064 | 0.19 | 0.125 | 1.5 |
| Cefotaxime | 0.032 | 0.38 | 1 | 16 |
| Ceftriaxone | 0.094 | 0.75 | 3 | ≥32 |
| Imipenem | 0.047 | 0.094 | 0.064 | 0.5 |
| Meropenem | 0.047 | 0.125 | 0.064 | 0.75 |
| Tetracycline | ≤0.016 | 0.023 | 8 | 3 |
| Ciprofloxacin | 0.023 | 0.023 | 4 | 1 |
| Levofloxacin | 0.047 | 0.047 | 12 | 0.5 |
| Trimethoprim-sulfamethoxazole | ≤0.002 | ≤0.002 | 1.5 | 0.016 |
| Gentamicin | 3 | 6 | 16 | 128 |
| Chloramphenicol | 12 | 12 | 2 | 4 |
| Clarithromycin | 0.064 | 2 | ≤0.016 | 4 |
Discussion.
The ubiquitous nocardiae are aerobic actinomycetes found worldwide in soil, sand, and house dust (10, 12). The organisms are characterized as gram-positive, partially-acid-fast branched rods that form irregular branching colonies on agar; aerial hyphae may be present in some species. The respiratory tract and skin are the sites of entry in most cases of nocardiosis, and nosocomial outbreaks of wound infections have been reported (19). The course of the disease may be subclinical and self-limiting, but chronic disease can develop. The nocardiae can spread from the site of entry to the brain, kidneys, eyes, bones, joints, and other organ systems. Immunocompromised hosts (e.g., patients with AIDS, malignancies, diabetes, or under immunosuppressive therapy after transplantation or for rheumatological disorders) are more frequently affected, but cases among immunocompetent patients have also been reported (6-8, 11, 13, 15). The antibiotics most frequently used are trimethoprim-sulfamethoxazole or minocycline in localized or mild cases and amikacin, imipenem, or cefotaxime in severe cases, such as disseminated infection. Recommendations for the duration of treatment in cases of cerebral nocardiosis vary from 6 to 12 months in immunocompetent hosts, and some authors recommend even longer treatment regimens in immunocompromised individuals (2, 10). There is no standard method for in vitro susceptibility testing of Nocardia species, but disk diffusion, Etest, and broth and agar dilution methods have been described (3, 4, 10, 16-18). While high rates of resistance to multiple classes of antibiotics have been reported for various clinical and environmental Nocardia isolates (4, 9, 14, 18), data on N. paucivorans are not available.
Here, we report a case of cerebral infection with the recently defined species N. paucivorans, which is closely related to the Nocardia asteroides complex (20). Histologically it was diagnosed as a cerebellar nocardial abscess (Fig. 1), and microbiologically after its spread to the meninges, representing a rare manifestation of nocardiosis (5, 10).
Different factors may have contributed to the failure to cure the disease and the consequent relapse in this patient despite in vitro susceptibility of the isolate to all drugs administered. Because the specific immune response to nocardiae is mainly T-cell mediated (1), the patient's immunosuppression is likely to have played a role in addition to drug- and pathogen-related factors. These include the duration of treatment, as well as poor penetration of minocycline into the CSF and its bacteriostatic mode of action. Additionally, L-forms of Nocardiae, which can persist within host tissues, have been described (1).
Ours is the first reported case of N. paucivorans isolated from the CSF of a patient with intracranial or intrathecal manifestation of nocardiosis. It demonstrates that this species can cause severe systemic infections similar to those caused by other medically important Nocardia species, such as N. asteroides, N. farcinica, and N. otitidiscaviarum. The remarkably low level of antimicrobial resistance associated with the few unique biochemical characteristics of both the isolate reported here and the type strain of N. paucivorans necessitates its identification by molecular methods.
REFERENCES
- 1.Beaman, B. L., and L. Beaman. 1994. Nocardia species: host-parasite relationships. Clin. Microbiol. Rev. 7:213-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaman, B. L., P. Boiron, L. Beaman, G. H. Brownwell, K. Schaal, and M. E. Gombert. 1992. Nocardia and nocardiosis. J. Med. Vet. Mycol. 30(Suppl. 1):317-331. [PubMed] [Google Scholar]
- 3.Biehle, J. R., J. R. Cavallieri, M. A. Saboulle, and L. J. Getsinger. 1994. Comparative evaluation of the Etest for susceptibility testing of Nocardia species. Diagn. Microbiol. Infect. Dis. 19:101-110. [DOI] [PubMed] [Google Scholar]
- 4.Boiron, P., and F. Provost. 1988. In-vitro susceptibility testing of Nocardia species and its taxonomic implications. J. Antimicrob. Ther. 22:623-629. [DOI] [PubMed] [Google Scholar]
- 5.Bross, J. E., and G. Gordon. 1991. Nocardial meningitis: case reports and review. Rev. Infect. Dis. 13:160-165. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez, D. C., and S. J. Antony. 1999. Actinomyces and Nocardia infections in immunocompromised and nonimmunocompromised patients. J. Natl. Med. Assoc. 91:35-39. [PMC free article] [PubMed] [Google Scholar]
- 7.Fleetwood, I. G., J. M. Embil, and I. B. Ross. 2000. Nocardia asteroides cerebral abscess in immunocompetent hosts: report of three cases and review of surgical recommendations. Surg. Neurol. 53:605-610. [DOI] [PubMed] [Google Scholar]
- 8.Jones, N., M. Khoosal, M. Louw, and A. Karstaedt. 2000. Nocardial infection as a complication of HIV in South Africa. J. Infect. 41:232-239. [DOI] [PubMed] [Google Scholar]
- 9.Khan, Z., H. Al-Sayer, T. Das Chugh, R. Chandy, F. Provost, and P. Boiron. 2000. Antimicrobial susceptibility profile of soil isolates of Nocardia asteroides from Kuwait. Clin. Microbiol. Infect. 6:94-98. [DOI] [PubMed] [Google Scholar]
- 10.Lerner, P. I. 1996. Nocardiosis. Clin. Infect. Dis. 22:891-905. [DOI] [PubMed] [Google Scholar]
- 11.Lucas, R. E., and P. K. Armstrong. 2000. Two cases of mycetoma due to Nocardia brasiliensis in central Australia. Med. J. Aust. 172:167-169. [DOI] [PubMed] [Google Scholar]
- 12.McNeil, M. M., and J. M. Brown. 1994. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin. Microbiol. Rev. 7:357-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng, C. S., and W. C. Hellinger. 1998. Superficial cutaneous abscess and multiple brain abscesses from Nocardia asteroides in an immunocompetent patient. J. Am. Acad. Dermatol. 39:793-794. [DOI] [PubMed] [Google Scholar]
- 14.Shishido, H., K. Deguchi, S. Miyake, S. Akagawa, and Y. Yoshizawa. 1998. Multiple drug-resistant Nocardia asteroides isolated from a patient with pulmonary nocardiosis. Respir. Med. 92:873-875. [DOI] [PubMed] [Google Scholar]
- 15.Torres, O. H., P. Domingo, R. Pericas, P. Boiron, J. A. Montiel, and G. Vazquez. 2000. Infection caused by Nocardia farcinica: case report and review. Eur. J. Clin. Microbiol. Infect. Dis. 19:205-212. [DOI] [PubMed] [Google Scholar]
- 16.Wallace, R. J., B. A. Brown, M. Tsukamura, J. M. Brown, and G. O. Onyi. 1991. Clinical and laboratory features of Nocardia nova. J. Clin. Microbiol. 29:2407-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace, R. J., E. J. Septimus, D. M. Musher, and R. R. Martin. 1977. Disk diffusion susceptibility testing of Nocardia species. J. Infect. Dis. 135:568-576. [DOI] [PubMed] [Google Scholar]
- 18.Wallace, R. J., Jr., M. Tsukamura, B. A. Brown, J. Brown, V. A. Steingrube, Y. Zhang, and D. R. Nash. 1990. Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J. Clin. Microbiol. 28:2726-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger, P. N., J. M. Brown, M. M. McNeil, and W. R. Jarvis. 1998. Nocardia farcinica sternotomy site infections in patients following open heart surgery. J. Infect. Dis. 178:1539-1543. [DOI] [PubMed] [Google Scholar]
- 20.Yassin, A. F., F. A. Rainey, J. Burghardt, H. Brzezinka, M. Mauch, and K. P. Schaal. 2000. Nocardia paucivorans sp. nov. Int. J. Syst. E vol. Microbiol. 50:803-809. [DOI] [PubMed] [Google Scholar]