Abstract
The degrees of effectiveness of reverse transcription (RT)-PCR, virus isolation, and antigen enzyme-linked immunosorbent assay (ELISA) for the detection of influenza A virus were evaluated with nasopharyngeal swabs from 150 patients (1 week to 86 years old) with influenza A virus infection. RT-PCR had a sensitivity for influenza A virus in stock virus preparations 103 times higher than virus isolation and 106 to 107 times higher than ELISA. The detection rate achieved by RT-PCR in clinical samples was clearly higher (93%) than that by virus isolation (80%) and ELISA (62%). Despite low overall detection rates achieved by antigen ELISA, samples from patients younger than 5 years old yielded higher-than-average rates in this rapid assay (88%). The likelihood of negative results in the ELISA increased significantly with increasing age of the patient (P < 0.01). The degrees of effectiveness of RT-PCR and virus isolation were not influenced by the age of the patient. Neither influenza immunizations nor the interval between onset of symptoms and laboratory investigation (mean, 4.7 days; standard deviation, 3.3 days) affected results obtained by the three test systems. Our results demonstrate that the ELISA is reliable for rapid laboratory diagnosis of influenza in infants and young children, but for older patients application of RT-PCR or virus isolation is necessary to avoid false negative results.
Distinguishing influenza illness from infections with other pathogens can be very difficult (11, 14). Therefore, rapid and sensitive laboratory confirmation of clinically suspected influenza is required in order to allow physicians to promptly initiate appropriate antiviral therapy and avoid costly testing and treatment for other pathogens. In addition, a sensitive and specific virus detection assay is a prerequisite for reliable epidemiological data, which are essential for the surveillance and the control of influenza outbreaks (3). Virus isolation, the standard diagnostic technique, is very sensitive, but results require several days to become available and rapid assays are of relatively low sensitivity (20). This compromises their usefulness for effective clinical management. Especially in the elderly, low influenza virus detection rates during influenza epidemics (13, 14), despite frequent hospitalizations (23) and consultations of physicians (15) for influenza-like illnesses, may not only indicate inadequate virological surveillance but may also suggest a limited usefulness of the currently available rapid diagnostic tools.
For these reasons, rapid and highly sensitive influenza-specific reverse transcription (RT)-PCR assays have been developed (1, 4) that may be used for typing and subtyping of influenza viruses (5, 25) or for detection in a multiplex PCR format (12). Despite their higher in vitro sensitivity, RT-PCR assays exhibited no clear advantage over virus isolation in terms of sensitivity, specificity, and virus detection rates when applied to clinical samples from pediatric patients (6, 20). Nevertheless, in one study including samples from patients of all age groups, an increased detection rate was found when RT-PCR was used (12). The results of these studies suggest that the effectiveness of influenza A virus detection assays may be variable with patients of different age groups.
The aim of the present study was therefore to analyze the results of two rapid virus detection assays—antigen enzyme-linked immunosorbent assay (ELISA) and RT-PCR—and those obtained by virus isolation, the standard test, with regard to the age of the patients. In order to develop a clinically relevant basis for choosing from among the available influenza virus tests, we also analyzed the influence of an influenza vaccination before the current season and the time between onset of symptoms and laboratory investigation on the results of the three assays.
Results of this analysis clearly demonstrate that the age of the patients should be considered when choosing the test method for rapid laboratory confirmation of clinically suspected influenza A virus infection.
MATERIALS AND METHODS
Patients and specimens.
The study was conducted during the period of epidemic activity of influenza in the winter season of 1999 to 2000, which lasted from week 52 of 1999 to week 6 of 2000. During this 7-week period 765 nasopharyngeal swabs (NPS) were collected from patients with acute respiratory tract infections by physicians of in- and outpatient units of hospitals, by general practitioners, and by pediatricians. NPS were taken with a swab from both the nose and the throat of the patients. The swab was squeezed out in 500 μl of minimal essential medium (MEM) (GIBCO, Life Technologies, Lofer, Austria) and was discarded thereafter. These specimens were collected from patients 1 week to 90 years old (mean, 7.9 years; standard deviation [SD], 15.8 years). Immediately after delivery, clinical samples were vortexed thoroughly, diluted 1:3 in MEM, and split into aliquots before being screened for influenza A viruses by ELISA, virus isolation, and RT-PCR.
All 150 patients with samples that yielded an influenza A virus-positive result in at least one of the tests applied (ELISA, virus isolation, or RT-PCR) were included in this study for further evaluation of the effectiveness of the tests with respect to patient-related variables. For this purpose, the following information was obtained by questionnaires, which were sent to the attending physicians and had to be filled out for each individual patient: the age of the patients, the date of onset of illness, clinical signs and symptoms, duration of the symptomatic period, and a history of influenza immunizations before the season of 1999 to 2000. Date of testing and of final diagnosis was assessed through the electronic medical record system of the Institute of Virology, Vienna, Austria.
Detection of influenza A virus in nasopharyngeal secretions. (i) Virus isolation in tissue culture and typing of the isolates.
Virus isolation in tissue culture and typing of the isolates were carried out on Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas, Va.) according to standard procedures (19).
The influenza A virus strains isolated during the study period were typed and subtyped according to general recommendations (19) by immunofluorescence staining using monoclonal antibodies MAB8252 (H1N1) and MAB8254 (H3N2) (Chemicon International, Inc., Temecula, Calif.).
(ii) Detection of influenza virus antigen by ELISA.
The in-house ELISA used was carried out as previously described (22). Briefly, the aliquots tested by ELISA were sonicated, and 50-μl aliquots of these samples were added to the wells of U-shaped Removastrips (Dynatech, Plochingen, Germany), coated with influenza virus nucleoprotein-specific guinea pig antiserum and incubated overnight at 37°C. After washing, influenza virus nucleoprotein-specific monoclonal antibodies (50 μl per well; Chemicon International, Inc.) were added and incubated for 1 h at 37°C. Species-specific biotinylated sheep antibodies against mouse immunoglobulin (Amersham International Plc., Amersham, United Kingdom) and afterwards streptavidin-peroxidase (Boehringer GmbH, Mannheim, Germany) were added, and the mixture was again incubated for 1 h at 37°C. Finally, 50 μl of substrate (o-phenylenediamine, 1 mg/ml; 0.1% perhydrol) was added to each well. The reaction was stopped after 30 min by the addition of 100 μl of 2 N H2SO4 per well, and the absorbance at 492 nm was measured. The cutoff to consider specimens positive for influenza A virus was determined by calculating the mean absorbance plus two times the SD from specimens negative for influenza viruses by virus isolation.
Detection of influenza A virus RNA sequences. (i) Preparation of samples for RT-PCR.
Immediately after diluting and splitting the NPS for the different tests, 1 μl of RNase inhibitor (Boehringer GmbH) in a final concentration of 0.01 U/μl was added to the portion designated for testing by RT-PCR. The specimen was vortexed thoroughly, and subsequently, viral RNA was extracted from 140 μl of the sample by using QIAamp Viral RNA kits (QIAGEN, Hilden, Germany).
(ii) Primer selection and sequences.
For RT and the first step of the PCR, influenza A virus type-specific primers previously described by Claas et al. (5) were used. These primers bind to the highly conserved region of the influenza A virus genome coding for nonstructural proteins and amplify a fragment of 190 bp (nucleotides 467 to 656). The first-step primers for this were InfA-P (5′-AAG GGC TTT CAC CGA AGA GG-3′) and InfA-P2 (5′-CCC ATT CTC ATT ACT GCT TC-3′).
In order to increase the sensitivity of the RT-PCR assay, nested primers for a second step of amplification were used. Primer selection for the second step was based on the published genomic sequences of the nonstructural segment of influenza A viruses from GenBank (Bethesda, Md.) by alignment and consequent comparison of these sequences by the commercial software MegAlign (DNASTAR Inc., Madison, Wis.). Primer binding sites were chosen in order to achieve highest homology to the genomic sequences of human influenza A viruses A(H1N1), A(H3N2), and A(H2N2).
The primers used for nested PCR amplify a fragment of 146 bp (nucleotides 493 to 638). The secondary-step primers for this were InfA-P3 [5′-TTG TTG GCG AAA T(CT)T CAC C-3′] and InfA-P4 (5′-TCT CCA AGC GAA TCT CTG TAG-3′).
RT.
For RT an aliquot (10 μl) of the extracted RNA was added to the reaction mixture, yielding a total volume of 50 μl. The mixture consisted of 10 μl of EZ buffer (5× buffer [Gene Amp Kit]; Perkin-Elmer/Cetus Corp., Norwalk, Conn.), 4 μl of Mn(OAc)2 (25 mM solution), 8 μl of deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dUTP), 25 pmol of each primer, 2 μl of recombinant Tth DNA polymerase (2.5 U/μl [Gene Amp Kit]; Perkin-Elmer/Cetus), and 15 μl of double-distilled H2O. The reaction mixtures were incubated at 60°C for 30 min.
Amplification.
Immediately after RT at 60°C, the reaction mixture (cDNA) was incubated in a DNA Thermal Cycler (Perkin-Elmer/Cetus) through 40 cycles of programmed amplification (denaturation at 94°C, 20 s; annealing at 50°C, 30 s; extension at 72°C, 30 s; final incubation for 5 min at 72°C). Nested PCR was performed on 2 μl of the first-step RT-PCR with a mixture consisting of 5 μl of EZ buffer (10× buffer; Perkin-Elmer/Cetus), 4 μl of MgCl2 (25 mM solution), 8 μl of deoxynucleoside triphosphates (dATP, dCTP, dGTP, dUTP), 25 pmol of each primer, 0.2 μl of Taq-Gold DNA polymerase (5 U/μl; 250 U of AmpliTaq Gold DNA Polymerase; Perkin-Elmer/Cetus), and 29 μl of double-distilled H2O in a final volume of 50 μl. The thermocycling procedure included 30 cycles of amplification (denaturation at 94°C, 15 s; annealing at 50°C, 30 s; extension at 72°C, 20 s; final incubation for 5 min at 72°C).
Each PCR experiment included two to five positive controls, several negative controls, and two or three respiratory virus-positive specimens (respiratory syncytial virus, enteroviruses, rhinoviruses, coronavirus, parainfluenza viruses, or adenoviruses) interposed between the samples tested. If a negative control or one of the specimens positive for any of the other respiratory viruses had tested positive in the influenza A virus-specific RT-PCR, which may indicate a contamination, the whole run would have been discarded. Nevertheless, these contamination controls were consistently negative.
Visualization of PCR amplicons.
The PCR amplicons, in 10-μl volumes, were analyzed by gel electrophoresis and ethidium bromide staining on 3% NuSieve Agarose Gel (FMC, Rockland, Maine) with 0.5 μg of ethidium bromide per ml in the gel.
Statistical analysis.
Comparison of two groups was carried out using the Mann-Whitney U test. Multivariate logistic regression was used to analyze the relationship between test results obtained by ELISA, RT-PCR, and virus isolation and patient-related variables, especially for correction of confounding by age. Comparison of the three methods with regard to their rate of positivity in clinical samples was done by the Cochran Q test, followed by pairwise comparisons applying McNemar tests with Bonferroni correction for multiple testing. In all statistical tests a P of <0.05 was considered statistically significant. All statistical analyses were performed using the commercial software SPSS 10.0 (SPSS Inc., Chicago, Ill.).
RESULTS
Sensitivities of RT-PCR, virus isolation, and ELISA for influenza A virus in stock virus preparations.
In order to assess the sensitivities of the three test systems used, stock virus preparations of cell-adapted strains of influenza A/Texas(H1N1), influenza A/Nanchang(H3N2), and influenza A/Sydney(H3N2) were tested by RT-PCR, virus isolation, and ELISA. These preparations, containing virus at a concentration of 105 50% tissue culture infective doses (TCID50)/ml, were diluted in 10-fold steps in MEM, vortexed thoroughly, separated into three aliquots, and tested twice by RT-PCR, virus isolation, and ELISA.
In these experiments the detection limit of ELISA was a virus concentration of 103 TCID50/ml (Table 1). RT-PCR had an in vitro sensitivity for all influenza virus subtypes of 0.001 TCID50/ml—103 times higher than the sensitivity of virus isolation and 106 to 107 times higher than that of ELISA.
TABLE 1.
Sensitivities of various assays for different influenza A virus subtypes in stock virus preparations
| Influenza A virus subtype | Concn of stock virus (TCID50/ml) | Highest dilution yielding a positive result
|
||
|---|---|---|---|---|
| RT-PCR | Virus isolation | ELISA | ||
| A/Nanchang(H3N2) | 105.0 | 10−8 | 10−5 | 10−2 |
| A/Sydney(H3N2) | 105.0 | 10−8 | 10−5 | 10−1 |
| A/Texas(H1N1) | 105.0 | 10−8 | 10−5 | 10−2 |
Detection of influenza A virus in NPS by RT-PCR, virus isolation, and ELISA. (i) Study population.
Table 2 summarizes the characteristics of the 150 patients with laboratory- confirmed influenza A virus infection. The median age was 5.1 years (mean, 20.5 years; SD, 24.4 years), ranging from 1 week to 86 years. Children constituted the largest study population, since 49.3% (74 of 150) of all patients were younger than 5 years old and 12% (18 of 150) were 5 to 19 years old. The study population was 51% female (77 of 150 patients), and 60% (90 of 150) of all patients were hospitalized. The hospitalization rate was highest in children younger than 5 years old (89%) and lowest in those 20 to 40 years old (5%). Patients above the age of 40 years had a hospitalization rate of 32%, reflecting the increased risk of a severe course of influenza in this age group.
TABLE 2.
Characteristics of the investigated study population
| Characteristic | No. of patients for which information was available | Value for age (yr) group
|
|||
|---|---|---|---|---|---|
| 0-4 | 5-19 | 20-40 | >40 | ||
| No. of patients | 150 | 74 | 18 | 20 | 38 |
| No. (%) of patients hospitalized | 150 | 66 (89) | 11 (61) | 1 (5) | 12 (32) |
| No. of patients with symptoms | 71 | 41 | 5 | 6 | 19 |
| Duration (days) of symptoms [mean (SD)] | 7.2 (2.8) | 8.2 (3.6) | 6.8 (3.8) | 9.0 (5.3) | |
| Interval between onset and testing | |||||
| No. of patients | 118 | 62 | 10 | 15 | 31 |
| Mean (SD) interval (day) | 5.2 (3.4) | 5.7 (4.7) | 4.0 (1.9) | 3.7 (2.8) | |
| No. (%) of patients with reduced immunocompetencea | 150 | 2 (3) | 2 (11) | 2 (10) | 2 (5) |
| No. (%) of patients infected despite vaccinationb | 78 | 0 (0) | 0 (0) | 3 (38) | 3 (14) |
Immunosuppression because of solid-organ (n = 5) or bone marrow (n = 3) transplantation.
Vaccine composition of inactivated influenza virus strains corresponded to the circulating strain (A/H3N2).
In the patients for whom information was available, the duration of the symptomatic period ranged from 3 to 21 days (mean, 7.7 days; SD, 3.8 days), and samples were tested after a mean interval of 4.7 days (SD, 3.3 days; range, 1 to 20 days) after the onset of symptoms. Samples from patients younger than 20 years were tested clearly later after the onset of symptoms (median, 5.0 days) than those obtained from patients above this age (median, 3 days; P = 0.007 [Mann-Whitney U]). This difference may be explained by the different hospitalization rates in these two age groups (Table 2) and by the fact that for patients younger than 20 years samples were normally not taken and tested before the severity of the symptoms required hospitalization (median interval between onset of influenza and testing in hospitalized patients, 5.0; median interval in outpatients, 3.0 days; P = 0.013 [Mann-Whitney U]).
Eight (5.3%) of the 150 patients were immunosuppressed because of solid-organ (n = 5) or bone marrow (n = 3) transplantation. Despite being vaccinated against influenza during the preepidemic period of 1999 to 2000, 6 of 78 (7.7%) patients were infected by influenza A virus (A/H3N2). These six patients were not immunocompromised as a consequence of transplantation and were ≥20 years old (mean, 47.2 years; SD, 21.0 years).
(ii) Effectiveness of tests.
Of the 150 influenza A virus-positive specimens, 86 were tested by all three assays and 64 were tested by virus isolation and ELISA. Overall detection rates achieved by ELISA, virus isolation, and RT-PCR were 62% (93 of 150), 80% (121 of 150), and 93% (80 of 86), respectively. All of the 121 influenza A virus-positive isolates were subtyped as A/H3N2. Since the RT-PCR negative results may have been caused by nonspecific inhibitors of RT and amplification in the six samples influenza A virus-positive by virus isolation, 8 μl of these samples was spiked with 2 μl of influenza A stock virus (final concentration, 101 TCID50/ml). All the spiked samples were found to be influenza A virus positive by RT-PCR, and therefore, a false-negative test result due to nonspecific inhibition of the RT-PCR in these six samples could be ruled out.
A highly significant result was obtained when the rate of positivity was compared in the 86 samples tested by all three assays (P < 0.01 [Cochran Q test]). ELISA had a significantly lower detection rate (30 of 86 specimens) than the other two assays, virus isolation (63 of 86 specimens; P < 0.01 [McNemar test, Bonferroni correction]) and RT-PCR (80 of 86 specimens; P < 0.01 [McNemar test, Bonferroni correction]), and virus isolation had significantly lower detection rates than RT-PCR (P < 0.01 [McNemar test, Bonferroni correction]).
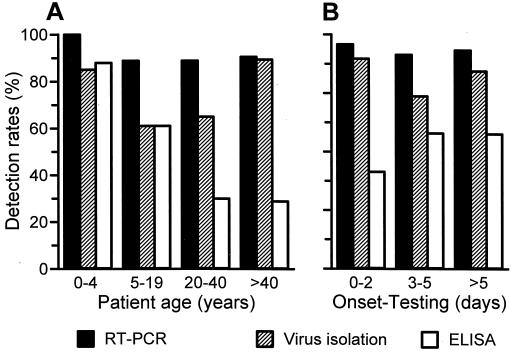
Results obtained by the three different test systems in relation to patient-related variables are summarized in Table 3. In children younger than 5 years, nearly similar detection rates were obtained by RT-PCR, virus isolation, and ELISA (Fig. 1A). Virus detection rates achieved by ELISA decreased with increasing age, and older patients were significantly more likely to test ELISA negative than younger ones. Neither the effectiveness of virus isolation nor that of RT-PCR was significantly reduced in any of the age groups, although virus isolation exhibited a somewhat lower detection rate in patients 5 to 19 years old.
TABLE 3.
Effectiveness of various assays for the diagnosis of influenza A virus infection
| Patient-related variable | Value for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ELISA
|
Virus isolation
|
RT-PCR
|
|||||||
| Negative | Positive | P | Negative | Positive | P | Negative | Positive | P | |
| Age | |||||||||
| No. of patients | 57 | 93 | 29 | 121 | 6 | 80 | |||
| Median (yr) | 38.0 | 1.8 | <0.01b | 14.5 | 3.9 | 0.88b | 39.9 | 26.8 | 0.26b |
| Interval between onset and testing | |||||||||
| No. of patients | 55 | 63 | 23 | 95 | 5 | 69 | |||
| Median (days) | 3.0 | 4.0 | 0.61c | 4.0 | 4.0 | 0.75c | 5.0 | 3.0 | 0.86c |
| Duration of symptoms | |||||||||
| No. of patients | 29 | 42 | 11 | 60 | 2 | 42 | |||
| Median (days) | 7.0 | 7.0 | 0.58b | 4.0 | 7.0 | 0.18b | 9.5 | 7.0 | 0.46b |
| No. (total) of patients with influenza and reduced immunocompetencea | 2 (57) | 6 (93) | 0.44b | 1 (29) | 7 (121) | 0.62b | 0 (6) | 4 (80) | 0.85b |
| No. (total) of patients with influenza despite vaccination | 3 (32) | 3 (45) | 0.66b | 2 (14) | 4 (63) | 0.33b | 0 (2) | 6 (46) | 0.91b |
Immunosuppression because of solid-organ (n = 5) or bone marrow (n = 3) transplantation.
Logistic regression.
Logistic regression, corrected for age.
FIG. 1.
Effect of patient-related variables on detection rates of the influenza A virus-specific RT-PCR, of virus isolation, and of ELISA. (A) Age of patient at time of the influenza A virus infection. (B) Interval between onset of influenza-related symptoms and time of testing of the nasopharyngeal secretion.
In order to exclude confounding of results by selecting patients according to their age for testing in the three different assays, we reanalyzed results obtained by the three different test systems in relation to patient-related variables for those 86 patients whose samples were tested by all three assays. Twenty-seven (31.4%) of these 86 patients were younger than 5 years (mean, 28.8 years; SD, 24.5 years). Results of this group of patients were similar to those of the total study population of 150 patients; increased patient age was the only factor significantly associated with an increased likelihood for ELISA-negative results (P < 0.01 [logistic regression]).
Information on the date of the onset of symptoms was available from 118 of the 150 patients, and 95% of all samples were tested within 10 days after the onset of symptoms. When corrected for age, the detection rates of the three test systems were not significantly influenced by the interval between onset of symptoms and NPS testing (Table 3 and Fig. 1B).
A history of vaccination against influenza viruses or a reduced immunocompetence did not influence the effectiveness of any of the three assays to detect influenza A virus in NPS (Table 3).
DISCUSSION
The results of this study clearly demonstrate the high sensitivity of our newly developed RT-PCR for the detection of influenza A virus in stock virus preparations and in clinical specimens. In a considerable proportion of the clinical specimens, influenza A virus could be detected only by RT-PCR. Although these positive results could not be confirmed by virus isolation in tissue culture, the consistently negative cross-contamination and respiratory virus-positive controls rendered nonspecific positive results very unlikely.
On the other hand, despite its high sensitivity, the RT-PCR assay failed to detect influenza A virus in six specimens positive by virus isolation. One possible explanation for this failure may be the nature of the samples tested. RNases are present in various quantities in specimens collected from the respiratory tract and may gradually digest viral RNA not protected by the viral envelope (16). Thereby, the sensitivity of the RT-PCR may be reduced in clinical specimens that contain large amounts of RNase and low concentrations of viral RNA. As a consequence, virus isolation in tissue culture remains indispensable for laboratory confirmation of influenza virus infections although different influenza virus strains might also vary in their capability to grow in tissue culture.
As far as detection of antigen is concerned, we observed a high effectiveness of the ELISA for the rapid detection of influenza A virus in NPS from patients younger than 5 years and its limitations when applied to patients above this age. Detection rates of influenza A virus in NPS achieved by RT-PCR and virus isolation were not significantly influenced by the age of the patients studied. Therefore, the RT-PCR assay in a nested format clearly represents the most-sensitive test for the early confirmation of influenza A virus infections in adolescents and adults.
Basically, two explanations seem to be conceivable for the relationship between patient age and detection rates achieved by ELISA: patterns of virus shedding and differences in the quality of specimens between age groups. An association between patient age, patterns of virus shedding, and the ability of laboratory tests to detect influenza A viruses in NPS has been suspected but not yet established. The only available studies addressing this issue are those investigating the immunogenicity and the virus shedding patterns following vaccination with live attenuated influenza vaccines (2, 8, 18, 21). In these studies, children shed virus in nasal washes for up to 2 weeks after vaccination (2, 18) compared to a shedding of not more than 1 week in adults (8, 21).
Attributable to the high antigenic variability of the virus, influenza virus can reinfect any individual (10), and this results in long-lived but subtype-specific immunity. Heterosubtypic immunity, which follows an infection with an influenza virus of a different subtype or strain, protects only weakly against reinfection (24) but may alter the pattern of virus shedding.
Therefore, the reduced effectiveness of the ELISA for the rapid diagnosis of influenza in adolescents and adults may be explained by the decreasing virus concentrations in their NPS with accumulating infections with increasing age. The children in our study were most likely experiencing their first infection with influenza A virus, and therefore, most probably were shedding the virus in higher quantities and for a longer period than older patients who were experiencing reinfections. Since the same results were obtained in the total study population of 150 patients and the 86 patients tested by all three assays, confounding by conditions related to a patient's age is a very unlikely explanation for the observed association between the age of the patient and ELISA results.
A second explanation for this association may be the difference in the quality and quantity of NPS obtained from patients of different age groups. Samples used for this analysis were collected by well-trained and experienced physicians and hospital staff in the course of routine investigation of acute respiratory tract infections, and they were delivered without delay to the laboratory by professional and specialized companies. Consequently, these specimens were of the highest quality attainable under the circumstances of routine investigation of acute respiratory tract infection. Still, sample collection may be more difficult in adults than in children since adults may resist more strongly the taking of swabs or blow their noses more often and thereby reduce the quantity of virus in NPS. Due to the higher quantity of virus necessary for an ELISA-positive result, as demonstrated by testing stock virus preparations, the likelihood of an ELISA-positive result will be lower in adults, even if virus shedding were equal in children and adults. As a consequence, there is a considerable potential for significantly increasing detection rates of all test systems by improving and standardizing the quantity and quality of clinical samples, for example by instructing the patients not to blow their noses some time before NPS collection. Nevertheless, despite their undeniable limitations samples collected from the respiratory tract are the only ones that can be used for the early and reliable diagnosis of influenza A virus infection.
As far as the interval between onset of symptoms and testing is concerned, no influence on the detection rates achieved by the three assays was observed. It can be assumed that the relatively long interval in the children and adolescents investigated is due to the fact that their NPS were not collected before the severity of symptoms required hospitalization.
Six of 78 patients investigated were infected by influenza A/H3N2 viruses despite immunoprophylaxis with a homologous inactivated influenza vaccine. Nevertheless, these immunizations had no effect on the detection rates of the three assays. Although the results should be interpreted with caution due to the low number of patients investigated, they are consistent with the findings of other studies. A significant reduction in the duration and magnitude of virus shedding was only observable in patients previously immunized with live attenuated vaccine, but not in those immunized with inactivated vaccines (7, 9, 17).
In conclusion, given the high sensitivity of virus isolation in cell culture and its additional importance for identification of the predominant circulating types, subtypes, and strains of influenza virus and for formulation of vaccine for the coming year, this assay remains essential in the diagnosis of influenza despite the relatively long time required for results to become available. In addition, our findings demonstrate that the effectiveness of ELISA differs significantly between age groups of patients with influenza virus infection. A reliable rapid laboratory diagnosis can be achieved in infants and young children by ELISA, but application of an RT-PCR assay is necessary for the sensitive and rapid detection of influenza A virus in NPS obtained from older patients.
Acknowledgments
We thank Edith Mlynar, Ursula Sinzinger, Heide Dippe, and Thomas Urbanek for their excellent technical assistance and Steven L. Allison and Franz X. Heinz for critical reading of the manuscript.
REFERENCES
- 1.Atmar, R. L., B. D. Baxter, E. A. Dominguez, and L. H. Taber. 1996. Comparison of reverse transcription-PCR with tissue culture and other rapid diagnostic assays for detection of type A influenza virus. J. Clin. Microbiol. 34:2604-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belshe, R. B., and L. P. Van Voris. 1984. Cold-recombinant influenza A/California/10/78 (H1N1) virus vaccine (CR-37) in seronegative children: infectivity and efficacy against investigational challenge. J. Infect. Dis. 149:735-740. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49(RR-3):1-32. [Google Scholar]
- 4.Cherian, T., L. Bobo, M. C. Steinhoff, R. A. Karron, and R. H. Yolken. 1994. Use of PCR-enzyme immunoassay for identification of influenza A virus matrix RNA in clinical samples negative for cultivable virus. J. Clin. Microbiol. 32:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claas, E. C., M. J. Sprenger, G. E. Kleter, R. van Beek, W. G. Quint, and N. Masurel. 1992. Type-specific identification of influenza viruses A, B and C by the polymerase chain reaction. J. Virol. Methods 39:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claas, E. C., A. J. van Milaan, M. J. Sprenger, M. Ruiten-Stuiver, G. I. Arron, P. H. Rothbarth, and N. Masurel. 1993. Prospective application of reverse transcriptase polymerase chain reaction for diagnosing influenza infections in respiratory samples from a children's hospital. J. Clin. Microbiol. 31:2218-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements, M. L., R. F. Betts, E. L. Tierney, and B. R. Murphy. 1986. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J. Clin. Microbiol. 23:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements, M. L., M. H. Snyder, S. D. Sears, H. F. Maassab, and B. R. Murphy. 1990. Evaluation of the infectivity, immunogenicity, and efficacy of live cold-adapted influenza B/Ann Arbor/1/86 reassortant virus vaccine in adult volunteers. J. Infect. Dis. 161:869-877. [DOI] [PubMed] [Google Scholar]
- 9.Couch, R. B., R. G. J. Douglas, D. S. Fedson, and J. A. Kasel. 1971. Correlated studies of a recombinant influenza-virus vaccine. 3. Protection against experimental influenza in man. J. Infect. Dis. 124:473-480. [DOI] [PubMed] [Google Scholar]
- 10.Cox, N. J., and K. Subbarao. 1999. Influenza. Lancet 354:1277-1282. [DOI] [PubMed] [Google Scholar]
- 11.Demicheli, V., T. Jefferson, D. Rivetti, and J. Deeks. 2000. Prevention and early treatment of influenza in healthy adults. Vaccine 18:957-1030. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, J. S., D. M. Fleming, and M. C. Zambon. 1997. Multiplex reverse transcription-PCR for surveillance of influenza A and B viruses in England and Wales in 1995 and 1996. J. Clin. Microbiol. 35:2076-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming, D. M., P. Chakraverty, C. Sadler, and P. Litton. 1995. Combined clinical and virological surveillance of influenza in winters of 1992 and 1993-4. BMJ 311:290-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming, D. M., and K. W. Cross. 1993. Respiratory syncytial virus or influenza? Lancet 342:1507-1510. [DOI] [PubMed] [Google Scholar]
- 15.Fleming, D. M., M. Zambon, and A. I. Bartelds. 2000. Population estimates of persons presenting to general practitioners with influenza-like illness, 1987-96: a study of the demography of influenza-like illness in sentinel practice networks in England and Wales, and in The Netherlands. Epidemiol. Infect. 124:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, D. P., J. D. Hayden, and P. Quirke. 1991. Extraction of nucleic acid from fresh and archival material, p. 29-50. In M. J. McPherson, P. Quirke, and G. R. Taylor (ed.), PCR: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 17.Johnson, P. R., S. Feldman, J. M. Thompson, J. D. Mahoney, and P. F. Wright. 1986. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J. Infect. Dis. 154:121-127. [DOI] [PubMed] [Google Scholar]
- 18.Lazar, A., N. Okabe, and P. F. Wright. 1980. Humoral and cellular immune responses of seronegative children vaccinated with a cold-adapted influenza A/HK/123/77 (H1N1) recombinant virus. Infect. Immun. 27:862-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lennette, E. H., and N. J. Schmidt. 1979. Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association, Inc., Washington, D.C.
- 20.Magnard, C., M. Valette, M. Aymard, and B. Lina. 1999. Comparison of two nested PCR, cell culture, and antigen detection for the diagnosis of upper respiratory tract infections due to influenza viruses. J. Med. Virol. 59:215-220. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, B. R., E. G. Chalhub, S. R. Nusinoff, J. Kasel, and R. M. Chanock. 1973. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J. Infect. Dis. 128:479-487. [DOI] [PubMed] [Google Scholar]
- 22.Sarkkinen, H. K., P. E. Halonen, and A. A. Salmi. 1981. Detection of influenza A virus by radioimmunoassay and enzyme-immunoassay from nasopharyngeal specimens. J. Med. Virol. 7:213-220. [DOI] [PubMed] [Google Scholar]
- 23.Simonsen, L., K. Fukuda, L. B. Schonberger, and N. J. Cox. 2000. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 181:831-837. [DOI] [PubMed] [Google Scholar]
- 24.Steinhoff, M. C., L. F. Fries, R. A. Karron, M. L. Clements, and B. R. Murphy. 1993. Effect of heterosubtypic immunity on infection with attenuated influenza A virus vaccines in young children. J. Clin. Microbiol. 31:836-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada, A., J. Imanishi, E. Nakajima, K. Nakajima, and S. Nakajima. 1991. Detection of influenza viruses in throat swab by using polymerase chain reaction. Microbiol. Immunol. 35:259-265. [DOI] [PubMed] [Google Scholar]