Abstract
An important role for immunoglobulin M (IgM) during early acute virulent Toxoplasma gondii infection was identified using IgM−/− mice that lack surface and secretory IgM but maintain normal B-cell functionality and isotype class switching. Following intraperitoneal inoculation with the virulent RH strain, IgM−/− mice displayed significantly fewer peritoneal parasites than wild-type (WT) mice, which correlated with increased tachyzoite dissemination to the liver, lung, and spleen in IgM−/− mice compared with WT mice. Early splenic T-cell activation, as measured by CD69 expression, was augmented in IgM−/− mice, and serum and peritoneal cavity gamma interferon levels were also elevated in IgM−/− mice compared with WT controls. Consequently, the difference in parasite dissemination was not attributable to an impaired proinflammatory immune response in the IgM−/− mice. Specific IgM was found to bind to tachyzoites in vivo in WT mice, and this correlated with an increased ability of antiserum collected from WT mice at day 6 postinfection to block tachyzoite cell invasion, compared with comparable serum collected from IgM−/− mice at the same time point. Tachyzoite invasion of host cells was similar if parasites were incubated with WT or IgM−/− nonimmune serum, suggesting that natural IgM does not function to limit parasite dissemination during early T. gondii infection. Our results highlight an important role for parasite-specific IgM in limiting systemic dissemination of tachyzoites during early acute T. gondii infection.
Toxoplasma gondii is an obligate intracellular protozoan parasite of significant public health importance, being a major cause of congenital infection and abortion as well as a significant and often fatal infection in the immune compromised. The early acute stage of infection is characterized by widespread tachyzoite dissemination and tissue damage. The rapid onset of cellular immunity controls parasite replication, causing encystment of the parasites in skeletal muscle and the central nervous system, forming the life-long chronic stage of infection (reviewed in reference 26). T. gondii tachyzoites are found only transiently in the extracellular milieu as they invade new host cells. As is typical for intracellular parasites, protection against both acute and chronic disease is mediated primarily by type 1 responses, with CD8+ T cells playing the most significant role (34, 40, 41). Thus, SCID mice can survive early acute infection via gamma interferon (IFN-γ) production by NK cells but ultimately succumb during late acute infection with uncontrolled parasite replication (17, 18, 38). Depletion of CD4+ and CD8+ T cells during chronic infection results in parasite reactivation and the development of toxoplasmic encephalitis (1, 5, 12). Protection during acute infection is correlated primarily with IFN-γ production by CD8+ T cells, rather than granule-dependent cytolytic pathways; however, perforin-dependent cytolytic mechanisms are protective during chronic infection (9, 40). Although cell-mediated immunity is important during T. gondii infection, a number of studies have also highlighted the importance of antibody during infection. Thus, antibodies are required for effective vaccination against virulent RH challenge (37) and in the prevention of toxoplasmic encephalitis during chronic infection (21, 23). Opsonization of tachyzoites with specific and nonspecific antibodies may also enhance neutrophil toxoplasmastatic activity, prevent the suppression of macrophage proinflammatory cytokine production, and increase phagolysosomal fusion and parasite killing of macrophages (4, 7, 24, 43). In vitro experiments have shown that antibodies to T. gondii apical membrane antigen 1 (AMA-1) and the major tachyzoite surface antigens, SAG-1 and SAG-2, can inhibit cellular invasion (14-16, 28-30, 42) and activate the complement cascade, which can kill tachyzoites (11, 39).
Studies on the importance of antibody during T. gondii infection have almost entirely focused on the role of immunoglobulin G (IgG) isotypes. Consequently, the importance of antibody during early acute infection is still unclear. Natural IgM is an important link between the innate and specific immune responses through its ability to trap and increase the immunogenicity of pathogens early during infection and by enhancing filtration in the spleen (reviewed in reference 32). Thus, the early neutralizing and agglutination capacity of IgM is important during lymphocytic choriomeningitis virus, vaccinia virus, vesicular stomatitis virus, influenza virus, and Listeria monocytogenes infection, where natural IgM prevents early pathogen dissemination and reduces microbial/viral titers in the blood and peripheral organs (2, 3, 13, 31). Injection of natural IgM also causes maturation of the IgG response in secretory IgM-negative mice, indicating that natural IgM is in part required for the development of the specific response (6, 10, 25). With specific relevance to T. gondii infection, IgM has been shown to increase tachyzoite killing by neutrophils (24) and is a potent activator of complement that may be toxoplasmocidal (22). However, the in vivo significance of IgM remains to be defined.
In the present study, therefore, we have investigated the importance of IgM during acute T. gondii infection by utilizing IgM−/− mice, which retain normal B-cell function and isotype switching but lack surface and secreted IgM (27). IgD replaces IgM as the primary antibody produced during the humoral response in IgM−/− mice, and all B-cell receptors are comprised of IgD in place of IgM. We demonstrate that while no significant role for natural IgM was found, T. gondii-specific IgM prevents cellular invasion and limits tachyzoite systemic dissemination during early acute T. gondii infection.
MATERIALS AND METHODS
Mice.
IgM−/− mice on the BALB/c background, engineered as described previously (27), were bred and maintained at the University of Strathclyde and the Trudeau Institute under barrier conditions. Age- and sex-matched BALB/c mice (hereafter referred to as wild-type [WT] mice) were used as controls.
Infections.
IgM−/− and WT mice were infected intraperitoneally (i.p.) with 100,000 tachyzoites of the virulent RH strain. In separate experiments, mice were infected with 200,000 green fluorescent protein-expressing RH tachyzoites (RH-GFP) parasites. Tachyzoites were obtained from continuous in vitro culture (37). Mice were killed on day 6 or earlier of infection, and extracellular peritoneal tachyzoite burdens were ascertained microscopically using a hemocytometer. Parasite dissemination into the liver, lung, and spleen as well as total peritoneal parasite numbers were determined by Taqman analysis or by flow cytometry (RH-GFP parasites) as stated.
Cytokine ELISA.
Cytokines were measured as previously described (35). Briefly, rat anti-mouse interleukin-12 (IL-12) (P40/P70; clone C15.6; BD Pharmingen, San Diego, Calif.), rat anti-mouse IL-10 (clone JES5-2A5; BD Pharmingen), and rat anti-mouse IFN-γ (clone R4 6A2; BD Pharmingen) were used as capture antibodies at concentrations of 10 μg/ml, 1 μg/ml, and 2 μg/ml in 0.5 M Tris-HCl, pH 8.9, buffer, respectively. Fifty microliters of capture antibody was added to each well, and plates were incubated overnight at 4°C. Following blocking with 10% skimmed milk (IFN-γ enzyme-linked immunosorbent assay [ELISA]) or 2% bovine serum albumin (IL-10 and IL-12 ELISAs), 50-μl aliquots of samples were applied undiluted or at a 1/10 dilution. Murine recombinant IL-12 (P70; BD Pharmingen), murine recombinant IL-10 (BD Pharmingen), and murine recombinant IFN-γ (BD Pharmingen) were used as standards at concentrations of 0 to 8 ng/ml. Following 2 h of incubation at 25°C, 100 μl biotin-labeled secondary antibody, rat anti-mouse IL-12 (P40/P70) monoclonal antibody (MAb) (clone C17.8; BD Pharmingen) at 1 μg/ml, rat anti-mouse IL-10 MAb (clone SXC-1; BD Pharmingen) at 2 μg/ml, or rat anti-mouse IFN-γ MAb (clone XMG1-2; BD Pharmingen) at 0.5 μg/ml was added in 2% bovine serum albumin-PBS, pH 7. After 1 h of incubation, 100 μl streptavidin-alkaline phosphatase (BD Pharmingen) was added at a dilution of 1/2,000 to each well, after which 100 μl of p-nitrophenyl phosphate at 1 mg/ml (Sigma-Aldrich, St. Louis, Mo.) in alkaline substrate buffer (Sigma-Aldrich) was added to each well. Once a color change developed, the absorbance was read at 405 nm (SpectraMax 190; Molecular Devices).
Real-time PCR.
Tissue IFN-γ, IL-12, and T. gondii SAG-1 (P30) mRNA levels were determined by real-time PCR (Taqman) normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as previously described (19, 20). T. gondii parasite burdens were calculated using a standard curve previously produced by adding known quantities of tachyzoites to tissue and then preparing cDNA. Primer and probe sequences as previously described (33) were supplied by Daniel Douek (NIH Vaccine Research Center, Bethesda, MD) or designed using Primer Express software (Perkin-Elmer). All primer probes, except for SAG-1, amplify cDNA but not genomic DNA. The primers were as follows: p30-forward, TTTCCGAAGGCAGTGAGACG; p30-reverse, GGATCCGATGCCATAGCG; p30-probe, TTGCCGCGCCCACACTGATG; IFN-γ forward, CATTGAAAGCCTAGAAAGTCTGAATAAC; IFN-γ reverse, TGGCTCTGCAGGATTTTCATG; IFN-γ probe, TCACCATCCTTTTGCCAGTTCCTCCAG; IL-12 p40 forward, GGAAGCACGGCAGCAGAATA; IL-12 p40 reverse, AACTTGAGGGAGAAGTAGGAATGG; IL-12 p40 probe, CATCATCAAACCAGACCCGCCCAA; GAPDH forward, CTCGTCCCGTAGACAAAATGG; GAPDH reverse, AATCTCCACTTTGCCACTGCA; GAPDH probe, CGGATTTGGCCGTATTGGGCG; IL-10 forward, GAAGACCCTCAGGATGCGG; IL-10 reverse, ACCTGCTCCACTGCCTTGCT; IL-10 probe, TGAGGCGCTGTCATCGATTTCTCCC.
Evaluation of cellular immune responses during virulent T. gondii infection.
The effects of IgM absence on early CD4+ and CD8+ T-cell activation during virulent T. gondii infection were investigated by flow cytometry. On day 5 of infection, spleen cells were stained with anti-CD4 (GK1.5), anti-CD8 (Ly-2), anti-CD69 (H1-2F3), and anti-CD44 (1M7). Comparisons of the numbers and frequencies of peritoneal cells in WT and IgM−/− mice during RH infection were performed on days 3, 4, 5, and 6 of infection using anti-Ly-6G (Gr-1), anti-F4-80 (BM8), anti-NK 1.1 (PK136), anti-CD19 (6B5), anti-CD4 (GK1.5), anti-CD8 (Ly-2), and anti-CD44 (1M7). All antibodies were purchased from Pharmingen or E-biosciences (San Diego, Calif.).
Assessment of complement-mediated tachyzoite killing.
Complement-mediated killing was evaluated using the Sabin-Feldman dye test (36). Live tachyzoites stain blue after incorporation of the dye, whereas dead parasites remain unstained. RH strain tachyzoites were obtained from the peritoneal cavity of 2-day-infected mice. Fifty microliters of inactive IgM−/− or WT plasma (heated at 56°C for 30 min) was distributed in a 96-well plate, and dilutions of 1/10 to 1/10,000 were performed using PBS, pH 7.2. Fifty microliters of tachyzoite suspension (5 × 107/ml) was added to each sample well. Fifty microliters of complement suspension (guinea pig whole complement sera; Sigma) was then added to each sample well. The plate was incubated at 37°C for 1 h. Following incubation the plate was stored on ice until the assay was complete. Live and dead tachyzoites were differentiated using Sabin-Feldman dye (0.25% methylene blue in alkaline soda-borax buffer [9.73 ml of 0.53% Na2CO3 plus 0.27 ml of 1.91% Na2B4O7 · 10H2O]). Quantification was carried out using a hemocytometer.
Determination of antibody-parasite binding.
The presence of IgM in WT mice that could bind to tachyzoites in vivo by day 5 of infection was demonstrated in two ways. The first method counted antibody-coated parasites obtained directly ex vivo. RH tachyzoites were harvested from the peritoneum of IgM−/− and WT mice on day 5 of infection and were subsequently stained with anti-T. gondii-biotin (CR1241RB; Cortex Biochemicals), followed by streptavidin-phycoerythrin (PE), and with either fluorescein isothiocyanate (FITC)-labeled anti-mouse IgM, IgG1, or IgG2a. In vitro culture-derived RH tachyzoites were utilized as negative controls. The second method determined the presence of IgM in serum that could bind tachyzoites. RH tachyzoites, obtained from in vitro culture, were incubated with WT or IgM−/− nonimmune serum or day 5 antiserum at 1:10 or 1:50 dilution for 20 min, washed, and stained with FITC-labeled anti-mouse IgM, IgG2a, or IgG1 as well as anti-T. gondii-biotin (followed by streptavidin-PE). Parasites were treated with Fc block (24G.2) before incubation with specific antibody.
Antibody inhibition assay.
The ability of antibody to limit tachyzoite cellular invasion was investigated as previously described (37) Briefly, 5 × 104 RH-GFP were added to an HS-68 fibroblast monolayer in the presence or absence of 10% IgM−/− or WT nonimmune serum or day 5 to 6 antisera. Following 24, 48, and 72 h of incubation, HS-68 cells were trypsinized and the percentage of RH-GFP-invaded cells was determined via flow cytometry.
Statistics.
Statistical significance was determined using Student's t test unless otherwise stated, with a P value of <0.05 taken as significant.
RESULTS
Comparison of parasite burden in WT and IgM−/− mice after T. gondii infection.
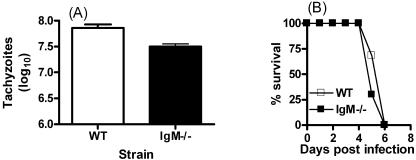
WT mice displayed significantly elevated numbers of peritoneal extracellular tachyzoites on day 6 of RH infection, compared with IgM−/− mice (P < 0.05) (Fig. 1A). Before day 6, the numbers of extracellular tachyzoites in WT and IgM−/− mice did not differ significantly (results not shown). The difference on day 6 was independent of sex, as male and female WT mice displayed significantly greater extracellular tachyzoite numbers than IgM−/− male or female mice (results not shown). However, IgM−/− mice did not succumb to virulent T. gondii infection more rapidly than WT mice (Fig. 1B).
FIG. 1.
The absence of IgM significantly alters numbers of peritoneal extracellular tachyzoites during infection with virulent T. gondii. WT and IgM−/− mice were infected i.p. with T. gondii RH strain parasites obtained from continuous in vitro culture. (A) Extracellular peritoneal parasite numbers were ascertained on day 6 of infection using a hemocytometer. (B) Survival of IgM−/− mice compared with WT mice following infection. Results are the mean + standard deviation of three to five mice/group and are representative of three separate experiments.
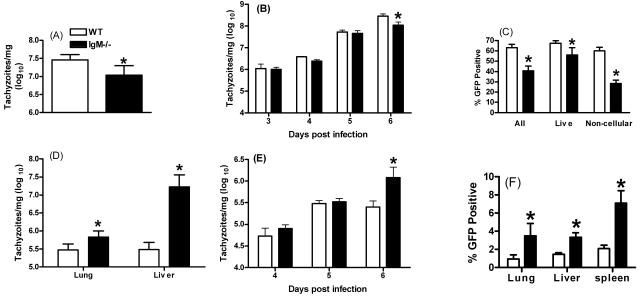
Tachyzoite numbers were also quantitated by Taqman analysis, which measured levels of transcripts of the tachyzoite surface antigen, SAG-1 (P30), and by flow cytometry, using RH-GFP. Taqman analysis of peritoneal cavity contents revealed that WT mice had consistently higher levels of parasite mRNA than IgM−/− mice on day 6 of infection (Fig. 2A), but not on days 3, 4, or 5 (Fig. 2B). In agreement, significantly increased frequencies of GFP-fluorescing events were observed in the peritoneum of WT mice, compared with IgM−/− mice, on day 6 of infection with RH-GFP parasites (Fig. 2C). Interestingly, the most dramatic difference was observed when comparing the numbers of RH-GFP parasites in gates that excluded host cells by forward and side scatter but which included extracellular tachyzoites. Thus, although the frequency of GFP-positive cells was significantly higher in WT mice on day 6 compared with IgM−/− mice (P = 0.019), the difference was particularly evident when gates were set on small noncellular events (P = 1.93 × 10−06). This suggests that tachyzoites were maintained extracellularly to a higher degree in WT mice than in IgM−/− mice.
FIG. 2.
IgM is required to limit systemic dissemination during virulent T. gondii infection. WT and IgM−/− mice were infected i.p. with T. gondii RH strain parasites or RH strain GFP-labeled parasites. (A and B) On day 6 of infection (A) and days 3 to 6 of infection (B), parasite numbers were measured by real-time PCR (Taqman) specific for SAG-1. (C) Parasites were also quantified by detecting the frequencies of RH-GFP-positive events by flow cytometry on day 6. (D and E) Parasite systemic dissemination was examined by measuring parasite numbers in the liver and lung on day 6 by Taqman (D) or in liver on days 4 to 6 (E). (F) Parasite dissemination on day 6 was also measured by detecting RH-GFP-positive events in the liver, lung, and spleen by flow cytometry. The * denotes a statistically significant difference (P < 0.05). Results are the mean + standard deviation of three to five mice/group and are representative of three separate experiments.
To investigate whether the reduced numbers of peritoneal parasites observed in IgM−/− mice during virulent infection were the result of increased systemic dissemination to other tissues, we determined the relative numbers of tachyzoites in the liver and lungs of infected mice via Taqman and flow cytometry. Taqman analysis revealed elevated dissemination to the lung and liver in IgM−/− mice compared with WT mice, with significantly increased frequencies of infected cells in the liver and lungs found on day 6 of infection (P < 0.01) (Fig. 2D), but not before in the liver (Fig. 2E) or lungs (results not shown). Similarly elevated levels of disseminated parasites were seen by day 6 of infection by flow cytometry (Fig. 2F). Significantly elevated frequencies of parasitized cells were also observed in the spleen of IgM−/− mice on day 6 of infection (P < 0.003), but no difference was observed in blood (results not shown).
Evaluation of immunity in IgM−/− and WT mice during infection.
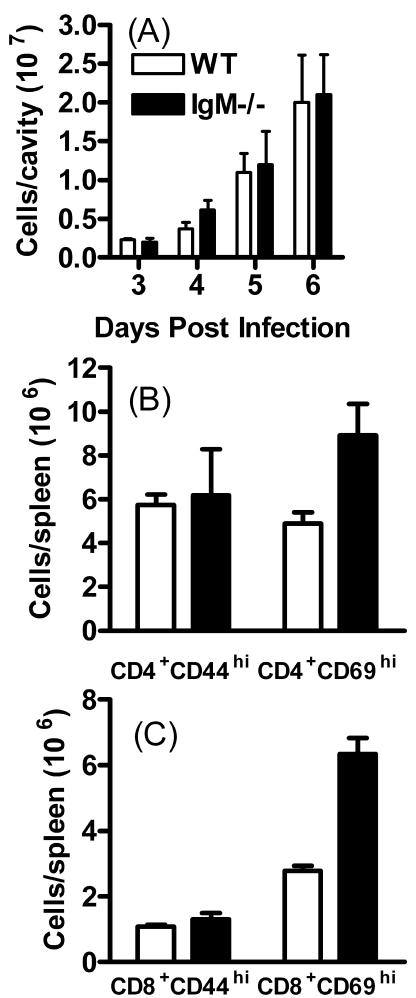
The difference in parasite dissemination observed in IgM−/− mice following virulent T. gondii infection was consistent with a direct functional role for IgM in preventing cellular invasion at the site of infection, thereby lessening dissemination of parasites within host cells to distant sites. However, the number, frequency, and activation state of peritoneal cells in IgM−/− mice could also influence tachyzoite numbers. To address this possibility, the numbers of peritoneal cells on days 3, 4, 5, and 6 of virulent infection were determined, and markers of early activation of CD4+ and CD8+ T cells in the peritoneal cavity and spleen were evaluated. There was no significant alteration in the absolute number of peritoneal cells in IgM−/− mice compared with WT mice on days 3, 4, 5, or 6 of infection (Fig. 3A), and numbers of Gr-1+, CD19+, F4-80+, and NK1.1+ cells were similar in both groups (results not shown). The numbers of CD69hi spleen-derived CD4+ and CD8+ T cells were significantly higher in IgM−/− mice on day 5 of infection compared with WT controls (P < 0.05) (Fig. 3B and C). This was attributable both to greater splenomegaly and increased frequencies of activated cells in the IgM−/− mice. Similarly, on day 9 of infection, more activated CD4+ and CD8+ T cells were found in the peritoneal cavity and spleen of IgM−/− mice (P < 0.05). This was attributable to elevated total cell numbers in IgM−/− mice, compared with WT mice, rather than a significant change in the frequency of activated cells (results not shown).
FIG. 3.
Cellular immunity is not impaired in IgM−/− mice during virulent T. gondii infection. WT and IgM−/− mice were infected i.p. with T. gondii RH strain parasites. (A) On days 3, 4, 5, and 6 of infection, total peritoneal cell numbers in WT and IgM−/− mice were ascertained using a hemocytometer. On day 5 of infection, early splenic CD4+ (B) and CD8+ (C) T-cell activation in IgM−/− and WT mice was investigated by determining the level of CD69 expression by flow cytometry Results are the mean + standard deviation of three to five mice/group and are representative of two experiments.
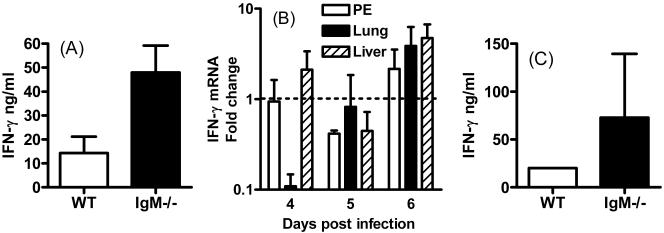
To further assess whether cellular immunity was comparable in WT and IgM−/− mice during virulent T. gondii infection, serum and peritoneal exudate IFN-γ, IL-12, and IL-10 cytokine levels were determined, and IFN-γ and IL-12 mRNA levels in liver, lungs, and peritoneum were measured. Levels of IL-12 and IL-10 protein in peritoneum and serum were comparable in WT and IgM−/− mice on days 5 and 6 postinfection (results not shown), and this correlated with equivalent levels of peritoneal IL-12 mRNA in IgM−/− and WT mice on days 5 and 6 postinfection (results not shown). Peritoneal IFN-γ protein levels and peritoneal, liver, and lung IFN mRNA levels were, however, significantly elevated in IgM−/− mice on day 6 postinfection (P < 0.05) (Fig. 4A and B). Serum IFN-γ levels were also elevated in IgM−/− mice, but the difference was not significant (Fig. 4C). IL-12 and IFN-γ liver, lung, and peritoneal mRNA levels and IL-12 and IFN-γ peritoneal and serum protein levels were also comparable in WT and IgM−/− mice on day 9 of infection (results not shown), indicating that cytokine responses were not significantly impaired in the absence of IgM during T. gondii infection.
FIG. 4.
IFN-γ production is enhanced in IgM−/− mice during virulent T. gondii infection. WT and IgM−/− mice were infected i.p. with T. gondii RH strain parasites. IFN-γ levels were measured by either ELISA or real-time PCR. (A) On day 6 of infection, peritoneal exudate IFN-γ levels were compared in IgM−/− and WT mice. (B) On days 4, 5, and 6 of infection, peritoneal cavity, lung, and liver IFN-γ mRNA levels in IgM−/− and WT mice were examined. The change in expression in IgM−/− mice was calculated compared with WT controls, and a value of 1 indicates equivalent expression in IgM−/− and WT groups. Note the log scale. (C) On day 6 postinfection, serum IFN-γ levels were also quantified in IgM−/− and WT mice. Results are the mean + standard deviation of three to five mice/group and are representative of two experiments.
Comparison of the ability of IgM−/− and WT antiserum to activate complement and kill tachyzoites.
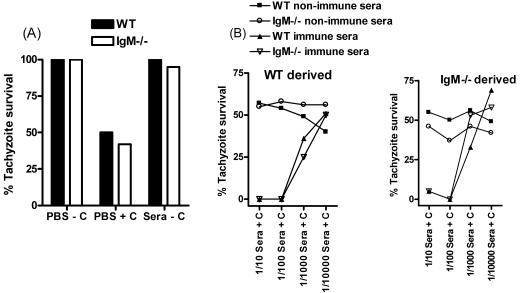
IgM is a potent activator of the classical complement cascade (8). Therefore, the differential abilities of WT and IgM−/− sera to activate complement and kill tachyzoites as a factor contributing to the elevated tissue parasite burdens in IgM−/− mice were also investigated. The results showed that tachyzoites derived directly from 2-day-infected mice had the capacity to activate the complement cascade (Fig. 5A), possibly a result of nonspecific antibody binding to the parasite surface (43). Nevertheless, parasites obtained from WT and IgM−/− mice were similarly able to activate the classical complement cascade, and incubation of parasites with WT and IgM−/− nonimmune serum did not enhance parasite killing above the direct ex vivo level witnessed when parasites were incubated with PBS (Fig. 5B). Thus, natural IgM does not appear to significantly induce parasite killing through the complement cascade. However, incubation of tachyzoites with WT and IgM−/− T. gondii antisera and complement resulted in 0% parasite survival at plasma dilutions of 1/10 and 1/100, indicating increased complement fixing ability of antisera compared to nonimmune plasma (Fig. 5B). No difference in complement fixing ability between WT and IgM−/− antisera was observed, highlighting the absence of intrinsic differences between plasma from these mice at activating the classical complement cascade.
FIG. 5.
WT and IgM−/− antisera activate the complement cascade and induce tachyzoite killing with similar efficacy, and tachyzoites derived from WT and IgM−/− mice are equally susceptible to complement-mediated killing. Tachyzoites were obtained from either WT or IgM−/− mice and were incubated with guinea pig whole complement sera and either PBS, WT, or IgM−/− heat-inactivated nonimmune serum or day 5 antisera. Complement-mediated killing was quantified by hemocytometer using the Sabin-Feldman dye test. (A) The ability of parasites derived from 2-day-infected mice to activate the classical complement cascade directly ex vivo was examined. (B) Tachyzoites were obtained from WT and IgM−/− mice, and the complement-fixing abilities of WT and IgM−/− nonimmune serum and day 5 antiserum were investigated. Results represent two separate experiments.
Parasite-specific IgM in WT mice.
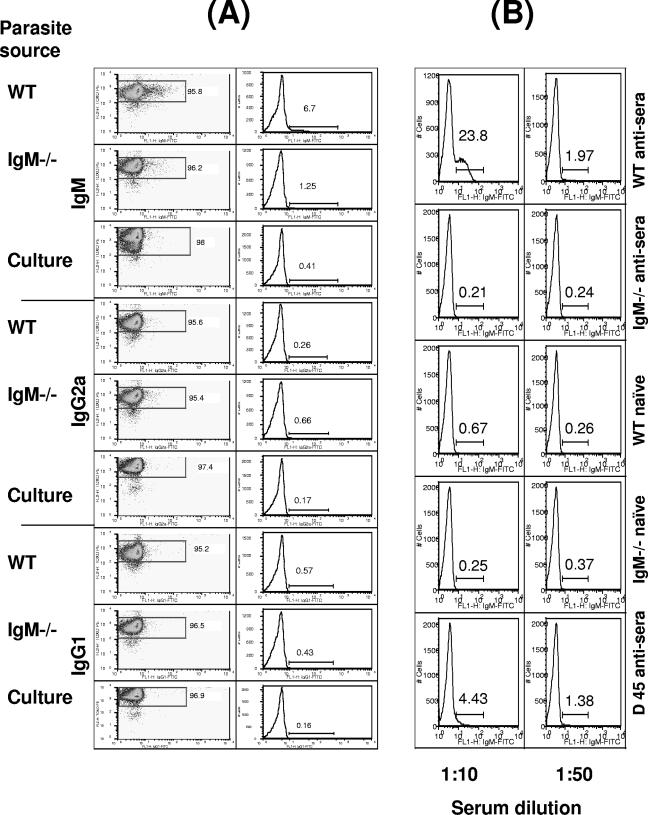
To elucidate the mechanism whereby IgM limits parasite dissemination, the ability of IgM to bind directly to the surface of tachyzoites was first investigated. In preliminary experiments, we determined by ELISA that serum levels of T. gondii-specific IgM were undetectable on days 2 or 3 of infection with RH tachyzoites, rose moderately on day 4, and attained high titers by days 5 and 6 (results not shown). Our results show that a proportion of parasites obtained on day 5 of infection in WT mice have IgM bound to their surface ex vivo (Fig. 6A). In contrast, parasites obtained from IgM−/− mice or from in vitro culture were not positive for surface IgM. No difference in IgG2a or IgG1 binding was observed between tachyzoites derived from WT and IgM−/− mice, suggesting no intrinsic difference in the IgG response during early T. gondii infection (Fig. 6A). The presence of IgM that can recognize tachyzoites in WT mice during early acute T. gondii infection was further shown by incubating in vitro-derived tachyzoites with WT or IgM−/− naïve serum or day 5 antiserum. A high percentage of tachyzoites incubated with WT antisera were positive for membrane-bound IgM when analyzed by flow cytometry (Fig. 6B). In contrast, no IgM was detected when tachyzoites were incubated with IgM−/− sera. In addition, no difference in IgG2a or IgG1 bound to parasites was observed following incubation with IgM−/− or WT sera (results not shown).
FIG. 6.
IgM is present in WT day 5 antisera and binds directly to tachyzoites in vivo. WT and IgM−/− mice were infected i.p. with T. gondii RH strain parasites, and on day 5 postinfection peritoneal tachyzoites were harvested. Parasites were also obtained from in vitro culture. Tachyzoites were incubated with anti-T. gondii-biotin (streptavidin-PE) and FITC-labeled anti-mouse IgM, IgG2a, or IgG. Separately, tachyzoites were obtained from continuous in vitro culture and incubated with WT or IgM−/− nonimmune serum or day 5 antiserum before being incubated with FITC-labeled anti-mouse IgM and anti-T. gondii-biotin (streptavidin-PE). The percentage of parasites with surface-bound antibody was then evaluated by flow cytometry. (A) The presence of IgM, IgG2a, and IgM on tachyzoites obtained from WT and IgM−/− mice was determined. (B) Parasites obtained from in vitro culture were incubated with WT or IgM−/− sera at 1:10 and 1:50 dilutions, and the presence of anti-T. gondii IgM was investigated. The results are representative of two separate experiments.
IgM directly limits cellular entry by tachyzoites.
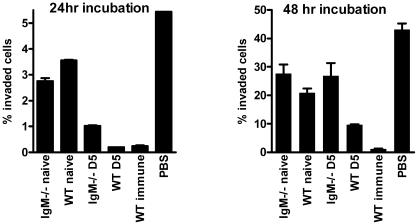
Following the demonstration that IgM directly binds to tachyzoites, the ability of IgM to block cellular entry by parasites was examined. Our results clearly indicate that WT day 5 antiserum is significantly more effective at blocking tachyzoite cellular invasion than IgM−/− day 5 antiserum following 24 h and 48 h of incubation (P < 0.05) (Fig. 7). No significant difference in the ability of nonimmune IgM−/− or WT serum to limit tachyzoite entry was observed, again demonstrating that specific, rather than natural, IgM is important during virulent T. gondii infection.
FIG. 7.
WT IgM-containing day 5 antisera is significantly more effective than IgM−/− day 5 antisera at blocking tachyzoite cellular invasion. RH-GFP-labeled tachyzoites were incubated on a fibroblast monolayer with WT or IgM−/− nonimmune serum or day 5 antiserum. After 24 h or 48 h, the fibroblasts were obtained and the frequencies of infected cells were ascertained by flow cytometry. The results are representative of three separate experiments and show the mean + the standard deviation.
DISCUSSION
In this study we have demonstrated an important inhibitory role for IgM in limiting tachyzoite host cell invasion and systemic dissemination during virulent T. gondii RH infection. Early T-cell activation and proinflammatory cytokine responses were, if anything, enhanced in IgM−/− mice compared with WT controls, suggesting that IgM is protective during T. gondii infection through direct antiparasitic mechanisms, rather than through the initiation of cellular responses. Indeed, IgM was observed to bind directly to tachyzoites in vivo, and WT, but not IgM−/−, antiserum significantly reduced the ability of tachyzoites to invade cells. The protective capacity of IgM appears limited to specific IgM, rather than natural IgM, as no difference in parasite invasion was observed using WT or IgM−/− nonimmune serum.
The increased extracellular tachyzoite burdens observed in the peritoneum of WT mice on day 6 of virulent T. gondii infection, although initially surprising due to our hypothesized protective role for IgM during T. gondii infection, are in fact in agreement with previous reports that have highlighted the ability of antibody to prevent tachyzoite host cell invasion (14-16, 28-30). We determined that the increased extracellular tachyzoite counts in WT mice compared with IgM−/− mice were a direct consequence of increased cellular invasion and dissemination in IgM−/− mice. Real-time PCR as well as flow cytometry analysis highlighted the important role of IgM in limiting tachyzoite systemic dissemination during virulent T. gondii infection. IgM−/− mice exhibited significantly elevated frequencies of RH-GFP-positive cells in the liver, lung, and spleen compared with WT mice. However, total cell numbers in tissues were comparable in WT and IgM−/− mice; thus, there were more cells harboring tachyzoites in IgM−/− mice. Furthermore, Taqman analysis using non-GFP-expressing RH parasites also consistently revealed increased systemic dissemination in IgM−/− mice. Peritoneal parasite levels and systemic dissemination were similar in WT and IgM−/− mice prior to day 6 of infection, which was probably associated with the failure of WT mice to produce significant quantities of T. gondii-specific IgM earlier than day 5 of infection.
The results of this study suggest that IgM plays a direct functional role during virulent T. gondii infection by interfering with tachyzoite invasion. However, IgM has been reported to activate the complement cascade and enhance early specific cellular responses (8, 10). Therefore, we also investigated whether immune responses were altered during T. gondii infection in the absence of IgM. IgM−/− mice developed comparable, or slightly elevated, IFN-γ responses during infection, and increased early CD4+ and CD8+ T-cell activation, as measured by CD69 expression, was evident in IgM−/− mice on day 6 of infection. Thus, cellular responses were not adversely affected in IgM−/− mice during T. gondii infection. The augmented proinflammatory response observed in IgM−/− mice may be explained by elevated parasite dissemination to the spleen and peripheral organs compared with WT mice. However, as IgD replaces IgM in the humoral response in IgM−/− mice, we cannot discount that a previously unreported proinflammatory role for IgD may exist. Although IgM was present in WT day 5 and 6 antiserum and bound directly to tachyzoites, we found no difference in the complement fixing and parasite killing abilities of WT or IgM−/− antiserum, and parasites derived from WT and IgM−/− mice were equally susceptible to complement-mediated killing. Parasites derived ex vivo from IgM−/− and WT mice were able to directly activate the complement cascade to a similar extent as parasites incubated with nonimmune WT and IgM−/− serum. These results indicate that nonspecific and natural antibody binds to tachyzoites in vivo and may initiate the classical complement cascade. As no difference was observed in complement activation if the tachyzoites were obtained from WT or IgM−/− mice, it is likely that IgG rather than IgM preferentially binds to tachyzoites in vivo.
Cellular immunity was unimpaired in IgM−/− mice during virulent T. gondii infection, indicating that IgM limited parasite systemic dissemination through direct antiparasitic mechanisms. Consistent with this, in vitro experiments clearly demonstrated that IgM-containing antiserum was significantly more effective at blocking parasite cellular invasion than IgM−/− antiserum. Although the results in this study indicate an important role for IgM during virulent T. gondii infection, as IgD replaces IgM during the early humoral response in IgM−/− mice, it is also possible that T. gondii-specific IgD may have counterprotective properties during infection by increasing parasite invasion and dissemination. However, we have found no previous reports to support this possibility. WT and IgM−/− nonimmune sera were equally inefficient at blocking parasite cellular entry, showing that despite the importance of natural IgM during a number of infectious diseases (2, 13, 31), it does not significantly contribute to protection during virulent T. gondii infection. B cells and antibody have previously been shown to be required for effective vaccination against virulent T. gondii infection, as vaccinated μMT mice exhibit significantly higher tachyzoite burdens than similarly vaccinated WT control mice during RH challenge (37). These results highlight an important role for antibody in controlling RH tachyzoite entry of host cells and systemic dissemination during a challenge infection, where IgG isotypes are the predominant serum antibody. Therefore, although a protective role for IgG isotypes during T. gondii infection has previously been shown, our results are the first to clearly demonstrate that in a primary infection, IgM plays an important protective role before the IgG responses develop.
Studies have previously shown that antibodies to Toxoplasma gondii AMA-1, SAG-1, and SAG-2 are able to limit tachyzoite cellular invasion by blocking the initial attachment of the parasite to the host cell (14-16, 28-30). Although we have not identified the antigen that specific IgM recognizes on the tachyzoite surface, given the results from previous studies, it is possible that the target of T. gondii-specific IgM may be one of those antigens. Accordingly, antibodies against SAG1 have been shown to be directly protective in vivo. SAG-1-specific IgA antibodies are produced in the intestine following peroral infection and serve to inhibit infection of host cells and protect the mucosal surface (28). Surprisingly, IgM−/− mice, in spite of displaying significantly elevated parasite systemic dissemination during virulent T. gondii infection, did not succumb to infection more rapidly than WT mice. However, elevated IFN-γ levels and augmented early CD4+ and CD8+ T-cell activation were evident in IgM−/− mice compared with WT mice. Thus, the increased proinflammatory responses may have been sufficient to prolong the survival of IgM−/− mice despite elevated parasite burdens.
In summary, we have shown a significant modulatory role for specific IgM during early infection with virulent T. gondii. Less-extensive parallel studies using mice infected with mildly virulent strain ME49 or Beverly parasites revealed similarly augmented systemic dissemination of parasites in IgM−/− mice (results not shown). Thus, IgM is required to limit parasite entry of host cells and to reduce systemic dissemination. Our results underline the importance of antibody during virulent T. gondii infection and highlight a previously unreported early protective role for IgM during this infection.
Acknowledgments
This work was supported in part by USPHS grant AI46571 and by funds provided by the Trudeau Institute.
We thank Paula Lanthier for valuable technical assistance.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Araujo, F. G. 1991. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect. Immun. 59:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgarth, N., O. C. Herman, G. C. Jager, L. E. Brown, L. A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan, C., and M. Rollinghoff. 1999. How do protozoan parasites survive inside macrophages? Parasitol. Today 15:22-28. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. R., and R. McLeod. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438-3441. [PubMed] [Google Scholar]
- 6.Bruggemann, M., and K. Rajewsky. 1982. Regulation of the antibody response against hapten-coupled erythrocytes by monoclonal antihapten antibodies of various isotypes. Cell. Immunol. 71:365-373. [DOI] [PubMed] [Google Scholar]
- 7.Butcher, B. A., and E. Y. Denkers. 2002. Mechanism of entry determines the ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infect. Immun. 70:5216-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, N. R. 1985. The classical complement pathway: activation and regulation of the first complement component. Adv. Immunol. 37:151-216. [DOI] [PubMed] [Google Scholar]
- 9.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. A. Butcher, P. Caspar, S. Heiny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159:1903-1908. [PubMed] [Google Scholar]
- 10.Ehrenstein, M. R., T. L. O'Keefe, S. L. Davies, and M. S. Neuberger. 1998. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA 95:10089-10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhrman, S. A., and K. A. Joiner. 1989. Toxoplasma gondii: mechanism of resistance to complement-mediated killing. J. Immunol. 142:940-947. [PubMed] [Google Scholar]
- 12.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175-180. [PubMed] [Google Scholar]
- 13.Gobet, R., A. Cerny, E. Ruedi, H. Hengartner, and R. M. Zinkernagel. 1988. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 56:175-180. [DOI] [PubMed] [Google Scholar]
- 14.Godard, I., J. Estaquier, L. Zenner, M. Bossus, C. Auriault, F. Darcy, H. Gras-Masse, and A. Capron. 1994. Antigenicity and immunogenicity of P30-derived peptides in experimental models of toxoplasmosis. Mol. Immunol. 31:1353-1363. [DOI] [PubMed] [Google Scholar]
- 15.Grimwood, J., and J. E. Smith. 1996. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int. J. Parasitol. 26:169-173. [DOI] [PubMed] [Google Scholar]
- 16.Hehl, A. B., C. Lekutis, M. E. Grigg, P. J. Bradley, J. F. Dubremetz, E. Ortega-Barria, and J. C. Boothroyd. 2000. Toxoplasma gondii homologue of plasmodium apical membrane antigen 1 is involved in invasion of host cells. Infect. Immun. 68:7078-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, L. L. 1992. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect. Immun. 60:3719-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, L. L., K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley. 2003. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 197:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, L. L., P. Lanthier, J. Hoffman, and W. Chen. 2004. Vaccination protects B cell-deficient mice against an oral challenge with mildly virulent Toxoplasma gondii. Vaccine 22:4054-4061. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, L. L., and P. C. Sayles. 2002. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect. Immun. 70:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko, Y., Y. Takashima, X. Xuaun, I. Igarashi, H. Nagasawa, T. Mikami, and H. Otsuka. 2004. Natural IgM antibodies in sera from various animals but not the cat kill Toxoplasma gondii by activating the classical complement pathway. Parasitology 128:123-129. [DOI] [PubMed] [Google Scholar]
- 23.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 24.Konishi, E., and M. Nakao. 1992. Naturally occurring immunoglobulin M antibodies: enhancement of phagocytic and microbicidal activities of human neutrophils against Toxoplasma gondii. Parasitology 104:427-432. [DOI] [PubMed] [Google Scholar]
- 25.Lehner, P., P. Hutchings, P. M. Lydyard, and A. Cooke. 1983. IgM-mediated enhancement: dependency on antigen dose, T-cell requirement and lack of evidence for an idiotype-related mechanism. Immunology 50:503-509. [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman, L. A., and C. A. Hunter. 2002. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int. Rev. Immunol. 21:373-403. [DOI] [PubMed] [Google Scholar]
- 27.Lutz, C., B. Ledermann, M. H. Kosco-Vilbois, A. F. Ochsenbein, R. M. Zinkernagel, G. Kohler, and F. Brombacher. 1998. IgD can largely substitute for loss of IgM function in B cells. Nature 393:797-801. [DOI] [PubMed] [Google Scholar]
- 28.Mineo, J. R., R. McLeod, D. Mack, J. Smith, I. A. Khan, K. H. Ely, and L. H. Kasper. 1993. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 150:3951-3964. [PubMed] [Google Scholar]
- 29.Mineo, J. R., and L. H. Kasper. 1994. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp. Parasitol. 79:11-20. [DOI] [PubMed] [Google Scholar]
- 30.Mineo, J. R., I. A. Khan, and L. H. Kasper. 1994. Toxoplasma gondii: a monoclonal antibody that inhibits intracellular replication. Exp. Parasitol. 79:351-361. [DOI] [PubMed] [Google Scholar]
- 31.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 32.Ochsenbein, A. F., and R. M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624-630. [DOI] [PubMed] [Google Scholar]
- 33.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305-312. [DOI] [PubMed] [Google Scholar]
- 34.Parker, S. J., C. W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts, C. W., D. J. Ferguson, H. Jebbari, A. Satoskar, H. Bluethmann, and J. Alexander. 1996. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect. Immun. 64:897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabin, A. B., and H. A. Feldman. 1948. Dyes as microbiochemical indicators of a new immunity phenomenon affecting a protozoan parasite (Toxoplasma). Science 108:660-663. [DOI] [PubMed] [Google Scholar]
- 37.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 39.Schreiber, R. D., and H. A. Feldman. 1980. Identification of the activator system for antibody to Toxoplasma as the classical complement pathway. J. Infect. Dis. 141:366-369. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943-3946. [PubMed] [Google Scholar]
- 42.Velge-Roussel, F., I. Dimier-Poisson, D. Buzoni-Gatel, and D. Bout. 2001. Anti-SAG-1 peptide antibodies inhibit the penetration of Toxoplasma gondii tachyzoites into enterocyte cell lines. Parasitology 123:225-233. [DOI] [PubMed] [Google Scholar]
- 43.Vercammen, M., T. Scorza, A. el Bouhdidi, K. Van Beeck, Y. Carlier, J. F. Dubremetz, and H. verschueren. 1999. Opsonisation of Toxoplasma gondii tachyzoites with nonspecific immunoglobulins promotes their phagocytosis by macrophages and inhibits their proliferation in nonphagocytic cells in tissue culture. Parasite Immunol. 21:555-563. [DOI] [PubMed] [Google Scholar]