Cholesterol, which is required for viability and cell proliferation, is a major sterol of mammalian cells. More than 90% of cellular cholesterol is located at the plasma membrane. Several microorganisms and bacterial products target lipid rafts, membrane microdomains of eukaryotic cells enriched in cholesterol, sphingolipids, and certain proteins. Cholesterol confined in lipid rafts is a crucial component required by microorganisms, directly or indirectly, to enter or exit the intracellular compartment. It is also required for cytolytic activity of cholesterol-dependent cytolysins formerly known as thiol-activated toxins. The object of this review is to provide a brief synopsis of our understanding of the role of cholesterol in microbial invasion of mammalian cells. The interaction of microbial toxins and pathological proteins such as prions or β-amyloid with cholesterol is beyond the scope of this article; however, these topics are addressed in recently published comprehensive reviews and articles (13, 52, 58).
Cholesterol biosynthesis.
Cholesterol was discovered in 1815 by the French chemist M. E. Chevreul as a component of human gallstones (59). During the 20th century a great deal of work led to the elucidation of the structure of cholesterol and the complexity of its biosynthesis. Cholesterol consists of four hydrocarbon rings, which are strongly hydrophobic; however, the hydroxyl (OH) group attached to one end of cholesterol is weakly hydrophilic, meaning that cholesterol is also amphiphatic. A complicated pathway that involves multiple enzymes and a variety of cofactors are required to accomplish the biosynthesis of cholesterol from acetyl coenzyme A (acetyl-CoA) (5). Cells produce cholesterol or are able to draw it from extracellular sources with lipoproteins. Cellular cholesterol is also continuously lost to the outside circulation. The endoplasmic reticulum (ER) serves as the major site of cholesterol synthesis. A class of cellular proteins named caveolins bind cholesterol at a 1:1 ratio and are involved in transport of de novo-synthesized cholesterol from the ER to the plasma membrane. Cellular homeostasis of cholesterol involves the regulation of its total cellular level and its distribution between membranes and within a given membrane (6, 7). As an indispensable constituent of plasma membranes, cholesterol affects properties and functions of membrane proteins such as receptors, enzymes, or ion channels (14).
Cholesterol maintains the integrity of lipid rafts.
Once in the plasma membrane, cholesterol is not uniformly distributed but is preferentially confined to lipid rafts. The concept of lipid rafts was formulated more than 15 years ago and evolved from the observation that cholesterol and sphingolipids, along with specific classes of membrane proteins, form insoluble complexes following membrane solubilization with a cold mild detergent called Triton X-100 (39). A number of descriptive terms have been proposed for the insoluble membrane fraction, including glycosphingolipid-enriched membrane domains (GEMs), detergent-resistant membranes (DRMS), and Triton-insoluble floating fraction (TIFF) (40, 50). Cholesterol condenses the packing of sphingolipid molecules in the exoplasmic leaflet of the bilayer by occupying the spaces between the saturated hydrocarbon chains of the sphingolipids. The exoplasmic assemblage of sphingolipids and cholesterol is linked to the underlying cytoplasmic leaflet and forms a separate phase, a liquid ordered (Lo) phase. The Lo phase is dispersed in the liquid-disordered phase constituting the more loosely packed fluid matrix of the membrane. Studies using photonic force microscopy in live fibroblasts determined that the size of an individual raft is about 50 nm, which implies that only a limited set of proteins is incorporated into a raft (37, 60). Caveolae are flask-shaped invaginations of the plasma membrane found in many cell types, and they serve mainly to store and downregulate raft proteins. They usually remain attached to the cell surface but can pinch off from the plasma membrane and enter the endocytosis pathway upon encountering viral particles.
Cholesterol is required to maintain the functionality (signaling capacity) of lipid rafts.
More than 100 proteins have been suggested to be associated with lipid rafts (10). A class of proteins that has attracted much attention is that of glycosylphosphatidylinositol (GPI)-anchored proteins, heterotrimeric G proteins, and doubly acylated src family kinases. The GPI-anchored proteins are ubiquitously expressed in various tissues and encompass such functionally diversified proteins as enzymes, adhesion molecules, receptors, and surface antigens. These proteins are targeted to rafts by modification with saturated chain lipid groups, which pack well into an ordered lipid environment. Most of the membrane-spanning proteins and prenyl groups are excluded from lipid rafts. The cross-linking of receptors embedded in small raft domains results in the coalescence of rafts into larger assemblies that bring receptors into proximity with high concentrations of second-messenger molecules, thus initiating signaling cascades (51). Binding of an antibody or ligand to human GPI-anchored proteins (CD14, CD24, CD48, CD55, or CD59) or to murine molecules (Thy-1 or Ly-6) can cause the activation of T cells as measured by cell proliferation, the production of interleukin-2, or the influx of extracellular calcium (18, 53). The GPI anchor proved to be essential for T-cell activation, because transmembrane versions of the proteins could not transduce signals. The finding that proteins inserted only into the outer leaflet are able to initiate signaling events led to the concept of a signal transducer(s) associated with GPI-anchored proteins (23).
Further studies convincingly showed that cross-linking of GPI-anchored decay-accelerating factor (DAF; also called CD55) with antibodies results in protein tyrosine phosphorylation and association of two protein tyrosine kinases, p56lck and p59fyn (48). At present, it is accepted that signaling molecules are targeted to cytoplasmic leaflets of lipid rafts by myristoylation or palmitoylation (47).
Treatment of cells in culture with the compound methyl-β-cyclodextrin (M-β-CD) results in depletion of cholesterol from the plasma membrane followed by dissociation of proteins from rafts (51). M-β-CD and other cyclic oligosaccharides named β-cyclodextrins are able to dissolve lipids in their hydrophobic cores and are very efficient at stimulating the removal of cholesterol from a variety of cells in culture (11). The mechanism that allows cyclodextrins to remove cholesterol from cell membranes is related to their ability to reduce the activation energy for cholesterol efflux. Treatment of cells with cholesterol-sequestering agents such as nystatin and filipin or inhibition of cholesterol biosynthesis with the inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (statins) is another approach aimed at manipulating raft constituents that results in dissociating protein from rafts. Treatment with lovastatin followed by cholesterol depletion with M-β-CD was also used to disrupt raft integrity (21).
Microorganisms target components of cholesterol-enriched microlipid domains (lipid rafts) as their cellular receptors.
GPI-anchored proteins, cholesterol, or unidentified components of lipid rafts are utilized by several microorganisms as their cellular receptors. Uropathogenic and diarrheagenic Escherichia coli bearing Dr family adhesins (Afa/Dr adhesins) were among the first discovered to bind to a GPI-anchored DAF (CD55), a member of the complement regulatory proteins (32, 33). Interaction between Afa/Dr adhesins and DAF leads to the formation of characteristic clusters of DAF receptor molecules around the sites of adherent bacterial cells (15, 16). The raft marker ganglioside GM1, VIP/21 caveolin, and α5β1 integrin were also mobilized around adhering Dr-positive E. coli (20). Besides CD55, E. coli bearing the Afa/Dr family of adhesins recognizes CD66e (carcinoembryonic antigen [CEA]) and is able to recruit CEA-related cell adhesion molecules (CEACAM) 1 and 6 to sites of bacterial adherence (4). DAF is also targeted by certain viruses of the family Picornaviridae and has been recognized as the cellular receptor for at least six echovirus serotypes as well as for coxsackieviruses A21, B1, B3, and B5 (3, 29, 56). Cardiovirulent coxsackievirus strain B3 binds to the SCR-2 and SCR-3 domains of DAF, similarly to Afa/Dr adhesins. Coxsackievirus A21 binds to the SCR-1 domain of DAF but also requires intercellular adhesion molecule 1 (ICAM-1) for productive infection (46). E. coli expressing FimH adhesin binds to CD48, a GPI-linked receptor present in the lipid rafts of mast cells (1).
Mycobacteria, which bind to a variety of cell surface receptors on macrophages, including complement, mannose, Fc, or scavenger receptors, are also capable of interacting directly with the plasma membrane cholesterol. Rich in glycolipid, the mycobacterial cell wall contains a putative high-affinity cholesterol-binding site. It has been postulated that cholesterol may function as a direct “docking site” for mycobacteria and stabilize their interaction with membranes (36).
Cholesterol appears to be involved in the adhesion process of Helicobacter pylori. This notion came from the observation that H. pylori is consistently found in the mucus close to the intercellular junctions of epithelial cells—the same sites characterized by high concentrations of cholesterol (17). In addition, H. pylori and other Helicobacter species are capable of accumulating pure cholesterol in the membrane fraction from the culture medium. Proteinase K pretreatment of H. pylori prevented interactions between bacteria and cholesterol. This might suggest that proteinaceous components of the bacterial surface are involved in this process. It remains to be elucidated whether uptake of cholesterol by H. pylori protects the bacteria in the gastric environment.
Cholesterol-rich domains are also targeted by chlamydiae, which are strictly intracellular bacterial pathogens. After binding to HeLa cells, chlamydial elementary bodies remain associated with lipid rafts. It has been postulated that a putative host cell receptor for chlamydiae may reside in lipid rafts or colocalize to lipid rafts following binding of chlamydial ligand (19). A role for GPI-anchored proteins in chlamydial binding has not been demonstrated.
Although cholesterol depletion usually does not affect microbial binding, there are a few exceptions. Plasma membrane cholesterol plays a key role in Leishmania donovani infection by affecting the function of the receptor(s) involved in the nonopsonic attachment of the parasite (38). Depletion of cholesterol from macrophages with M-β-CD resulted in a significant (∼45%) reduction in macrophage-parasite interaction relative to that with untreated control cells. The reduction in binding of the parasite to cholesterol-depleted macrophages can be reversed by replenishment of cholesterol, reinforcing the specific requirement for cholesterol in the infection process. These results show that cholesterol depletion may lead to alterations in the interaction of cholesterol with one or more of the many receptors that have been proposed to have a role in the attachment of the parasite. The early contact of Shigella flexneri with epithelial cells is initiated within lipid rafts by interaction of IpaB, a component of the bacterial type III secretion apparatus, with the mammalian surface protein CD44. Cholesterol depletion and resultant disruption of lipid rafts inhibited this interaction and decreased bacterial binding to the epithelial cells (24). While there is no evidence that cholesterol interacts directly with Leishmania or Shigella, these findings may indicate that membrane cholesterol affects the function of surface receptors targeted by these pathogens. The modulatory role of cholesterol in the function of membrane receptors, such as the oxytocin receptor and the cholecystokinin receptor, has been demonstrated previously (14, 59).
Cholesterol depletion hampers microbial entry into intracellular compartments.
The interaction of microorganisms with the components of lipid rafts mediates microbial uptake by phagocytic or epithelial cells. The process of entry appears to be cholesterol dependent regardless of the specific molecules in lipid rafts targeted by microorganisms. Pharmacological depletion or sequestration of plasma membrane cholesterol significantly decreases internalization. Treatment of the HeLa epithelial cell line or Chinese hamster ovary cells expressing human CD55 with M-β-CD resulted in the inhibition of entry of uropathogenic E. coli producing Dr fimbriae (44). Treatment of mouse mast cells with the cholesterol-binding agent filipin inhibited the internalization of FimH-expressing E. coli mediated via binding to the GPI-anchored, mannose-containing molecule CD48 (49). Cholesterol and GPI-anchored proteins were found to be essential for uptake of Mycobacterium kansasii by macrophages. Although internalization of M. kansasii is mediated by CR3, antibodies against several GPI-anchored proteins were found to inhibit mycobacterial phagocytosis (35). It has been concluded that internalization of M. kansasii requires the association of CR3 with a GPI-anchored protein and relocation to a cholesterol-rich lipid raft. CR3 not associated with GPI proteins remains outside the microlipid domains and does not mediate phagocytosis of M.kansasii. Sequestration of plasma membrane cholesterol with filipin or nystatin, or cholesterol depletion with M-β-CD, significantly reduces phagocytosis of mycobacteria. Similar findings on cholesterol requirements in murine macrophages have been reported for Mycobacterium bovis BCG (35).
Cholesterol is also required for efficient entry of Chlamydia trachomatis. Binding of chlamydial elementary bodies to lipid rafts may lead to the coalescence of rafts into larger entities and subsequent internalization. Extraction of plasma membrane cholesterol resulted in a slight decrease in the total number of cell-associated chlamydiae and in significant (90%) inhibition of internalized chlamydiae (19).
Studies on phagocytosis of the intracellular pathogen Brucella suis demonstrated that while cholesterol depletion or sequestration significantly decreased nonopsonic phagocytosis, this treatment did not prevent opsonic phagocytosis mediated by the Fc receptor (30). Listeria monocytogenes is a rare example of a gram-positive pathogen that requires cholesterol-dependent lipid domain integrity for entry into nonphagocytic epithelial cells (45). Invasion by Listeria monocytogenes is promoted by interaction of the bacterial invasion proteins internalin and InIB with the surface receptors E-cadherin and hepatocyte growth factor receptor. Cholesterol depletion with M-β-CD prevents invasion by Listeria.
Lipid rafts are present in the plasma membranes of erythrocytes, terminally differentiated cells, and nonendocytic cells, which are targeted by vacuolar pathogens such as the malaria parasite, Plasmodium falciparum (42). Depletion of cholesterol blocks raft assembly and inhibits the formation of new vacuoles, strongly suggesting that rafts play a critical role in establishing a parasitic vacuole. Cholesterol depletion led to a significant reduction in the number of intracellular Leishmania amastigotes due to decreased binding of parasite promastigotes to cholesterol-depleted macrophages.
Cellular infection with poliovirus (PV) is initiated by binding of the virus capsid to its cellular receptor, CD155. The binding is associated with a conformational transition in the PV capsid, which changes the sedimentation coefficient of the virion from 160S to 135S.
Removal of cholesterol with M-β-CD resulted in inhibition of PV infection, which was partially compensated for by restoring cholesterol levels in cells. In contrast to nonenveloped viruses such as echovirus 1 and 11 or simian virus 40, poliovirus and poliovirus receptors do not colocalize to lipid rafts. Consequently, the loss of lipid raft integrity does not explain how M-β-CD inhibits PV entry. However, treatment with M-β-CD may affect other cholesterol-dependent cellular functions such as membrane fluidity, the electrical properties of ion channels, or cellular signaling pathways. It has been proposed that local recruitment of the PV receptor is required to catalyze the conformational transition from 160S to 135S. This process may be affected by impaired membrane fluidity due to cholesterol depletion. Entry of echovirus 11, which is mediated by interaction with DAF, is also inhibited by the lipid raft disruptors M-β-CD and nystatin (56).
Overall, microbial pathogens exploit a variety of mechanisms to gain access to intracellular compartments. Plasma membrane cholesterol might play a dual role in microbial internalization as a binding site for microbial pathogens and as an integrating constituent of lipid rafts providing a platform for efficient initiation of signaling cascades. The role and repertoire of signaling molecules recruited to lipid rafts in the process of microbial invasion have been characterized only for a limited number of microorganisms. The Rho family GTPase member Rac1 has recently been shown to play an essential role in the invasion of bladder epithelial cells by FimH-expressing E. coli (28). The invasion of epithelial cells by E. coli expressing Afa/Dr adhesins triggers a signaling pathway(s) involving protein tyrosine kinases, phospholipase Cγ, phosphatidylinositol 3-kinase, protein kinase C, and Rho GTPase Cdc42, leading to rearrangements of the cytoskeleton and pseudopod elongation (4, 34). The inhibition of invasion of numerous microbial pathogens by cholesterol sequestration or depletion supports the argument that cholesterol is a key molecule involved in the invasion process.
Lipid raft cholesterol is required for viral assembly and budding and for bacterial escape.
The finding that virions of influenza viruses contain large amounts of detergent-insoluble complexes confirmed the hypotheses formulated in the 1970s that viral budding could occur, not randomly, but from specialized domains in a biological membrane (43). The influenza virus hemagglutinin glycoprotein, which mediates virus-cell attachment and membrane fusion, is targeted to lipid rafts. Mutations in the transmembrane domain of hemagglutinin reduced association with rafts, reduced budding, and significantly decreased infectivity (57). Human immunodeficiency virus type 1 (HIV-1) buds selectively from lipid rafts and incorporates GPI-anchored proteins, such as the inhibitors of complement pathway CD55 and CD59, cholesterol, and sphingolipids into the viral envelope (31, 41). The presence of CD55 and CD59 in the viral envelope may help the virus to avoid complement-mediated lysis. Inhibition of cholesterol synthesis decreased the production of HIV-1 from infected cells. The filoviruses Ebola virus and Marburg virus, released from infected cells, incorporated the raft-associated molecule GM1, suggesting that viral assembly and budding take place in lipid rafts (2). Lipid rafts also serve as a cellular location for measles virus assembly. In infected cells, measles virus proteins attach to low-density detergent-insoluble complexes that are disrupted by cyclodextrin (27). Plasma membrane cholesterol is required to expel internalized bacteria to the extracellular environment. This interesting phenomenon has been described for E. coli expressing FimH, which mediates entry to mast cells in an attenuated phagocytic pathway. The expulsion of bacterial cells appears to depend on the level of plasma membrane cholesterol, since treatment of mast cells with M-β-CD significantly reduces the number of bacteria discharged (49).
Cellular cholesterol affects the intracellular lifestyle of microbial pathogens.
Bacterial entry into intracellular compartments might interfere with the host sterol biosynthesis pathway. Intracellular infection of macrophages or epithelial cells with Salmonella enterica serovar Typhimurium leads to cholesterol accumulation in the Salmonella phagosome (9). At the terminal stages of infection, as much as 30% of the total cellular cholesterol resides in the Salmonella phagosome. The accumulation of cholesterol in the Salmonella phagosome was found to be linked to intracellular replication of bacteria and is presumably dependent on a Salmonella pathogenicity island (SPI-2). Interestingly, the GPI-anchored protein CD55 was recruited to the Salmonella vacuoles. The significance of this finding remains obscure. The mechanism of the striking redistribution of cellular cholesterol to the Salmonella phagosome is unknown. One can speculate that cholesterol may play a role in nutrient acquisition by bacteria entrapped within vacuoles or that the accumulation of cholesterol may prevent phagolysosomal fusion.
Mycobacteria resist lysosomal delivery, in contrast to normal phagocytosis, in which phagosomal contents are delivered to lysosomes. This inhibition of lysosomal delivery is dependent on processes affected by the mycobacteria. Living mycobacteria, once phagocytosed by macrophages, reside within organelles that contain markers of early, but not of late, endosomal compartments. Recently, a protein termed TACO (tryptophan-aspartate-containing coat protein) was identified in phagosomes containing viable mycobacteria (12). This molecule was not present in phagosomes harboring killed bacilli or in any of the endosomal/lysosomal organelles purified from uninfected cells. TACO prevents maturation into or fusion with lysosomes, allowing the mycobacteria to survive within the phagosome. In uninfected macrophages, TACO molecules are associated with a cortical microtubule network, whereas in macrophages containing viable mycobacteria, TACO relocalizes quickly to phagosomal membranes. The process of TACO incorporation into the phagosomal membrane is cholesterol dependent. Mycobacteria entering macrophages via cholesterol-enriched membrane domains are sequestered in TACO-coated phagosomes, which prevent lysosomal delivery and ensure intracellular survival.
Interference with cholesterol homeostasis modulates the course of the infectious process.
The practicality of topical application of β-cyclodextrin (β-CD) has recently been tested in a severe combined immunodeficient (SCID) mouse model of HIV infection in which the animals carry human peripheral blood leukocytes (HuPBLs). In these mice, vaginal transmission of HIV-1 is mediated by transepithelial migration of HIV-infected human leukocytes. Depletion of epithelial cholesterol by topical application of 2-hydroxypropyl β-cyclodextrin (2OHp-β-CD) administered intravaginally prior to challenge with HIV-1-infected cells effectively blocked viral transmission without damage to vaginal mucosae (22). It is likely that migration through the epithelium involves, as an initial step, interaction between lymphocytes and/or macrophages and epithelial cells. Clustering of lipid rafts on the cell membrane results in enhancement of cell binding and migration, while disruption of rafts with β-CDs diminishes cell-cell interactions and hampers cell migratory properties (55). Moreover, cholesterol depletion results in decreased production of significantly less infectious HIV-1 virions (25, 55). In addition, β-CDs might disrupt the cell signaling pathway required for transepithelial migration of HIV-infected cells (22). Cyclodextrins have been used clinically as food additives or as molecular complexing agents that can increase the solubility and stability of some poorly soluble drugs, which have then been administered by the intravenous route (54). Current studies indicate that 2OHp-β-CD can be safely applied to the vaginal mucosae and is substantially less toxic than a subclinical concentration of the widely used spermicide nonoxynol-9. The HuPBL-SCID model of vaginal HIV-1 transmission might be useful for investigating cell-associated HIV-1 transmucosal transmission, as well as for screening reagents for their potential efficacy in preventing sexual HIV-1 transmission.
The drugs collectively named statins, which inhibit HMG-CoA reductase in the pathway of cholesterol biosynthesis, were found to decrease intracellular bacterial proliferation (8). Lovastatin at nanomolar concentrations reduced growth of Salmonella serovar Typhimurium 6- to 10-fold in mouse macrophages. Statins were also effective at reducing the proliferation of S. enterica in animals. Mice treated with atorvastatin 7 days prior to intraperitoneal infection contained 65% fewer bacteria in their spleens than untreated controls. This effect does not depend on lowering plasma cholesterol levels, due to very low levels of low-density lipoproteins in rodents. Experiments with the inhibitor 4,4,10β-trimethyl-trans-decal-3β-ol, which blocks the conversion of squalene oxide to lanosterol and subsequently inhibits the synthesis of all cellular sterols, demonstrated that biosynthetic sterols are not critical for intracellular growth of S. enterica but rather indicated that bacteria need nonsterol intermediates for intracellular growth. The underlying mechanism of the antibacterial activity of statins remains unclear. It has been postulated that statins may interfere with the modulation of small GTPases, which are frequently exploited by pathogens. The antibacterial effect may also be related to induction of apoptosis in infected cells. Clinical retrospective analysis of bacteremic infections due to gram-negative organisms and Staphylococcus aureus showed significant reductions in overall and attributable mortality in patients taking statins compared with patients not taking statins (26). Although the mechanism of lower mortality remains unclear, it has been postulated that the beneficial effect may be related tothe antiinflammatory activity of HMG-CoA reductase inhibitors.
In recent years, the concept of a “cholesterol connection” in infectious diseases has emerged from studies in the fields of microbiology and cell biology. The level of plasma membrane cholesterol appears to be critical in the regulation of microbial entry, intracellular trafficking, and exit (Table 1; Fig. 1). The feasibility of modulating the transmission or viability of pathogens by local cholesterol depletion or by treatment with HMG-CoA reductase inhibitors offers new perspectives for unconventional therapeutic intervention. Much more effort is needed to understand how interference with cholesterol homeostasis at the local or systemic level affects the host-pathogen interaction.
TABLE 1.
Modulatory effects of cholesterol on interactions of microbial pathogens with mammalian cells
| Effect of cholesterol depletion | Microbial pathogen | Reference(s) |
|---|---|---|
| Decrease of microbial | Leishmania donovani | 38 |
| binding to surface | Shigella flexneri | 24 |
| receptors | ||
| Reduction of microbial | E. coli Afa/Dr, E. coli FimH | 1, 44 |
| internalization | Brucella suis | 30 |
| Mycobacterium kansasii | 35 | |
| Chlamydia trachomatis | 19 | |
| Listeria monocytogenes | 45 | |
| Prevention of viral assembly | Influenza virus | 43 |
| and budding or expelling | HIV-1 | 22, 25 |
| of bacterial cells | Ebola virus | 2 |
| Marburg virus | 2 | |
| Measles virus | 27 | |
| E. coli FimH | 49 | |
| Decrease of intracellular survival | Mycobacterium spp. | 12 |
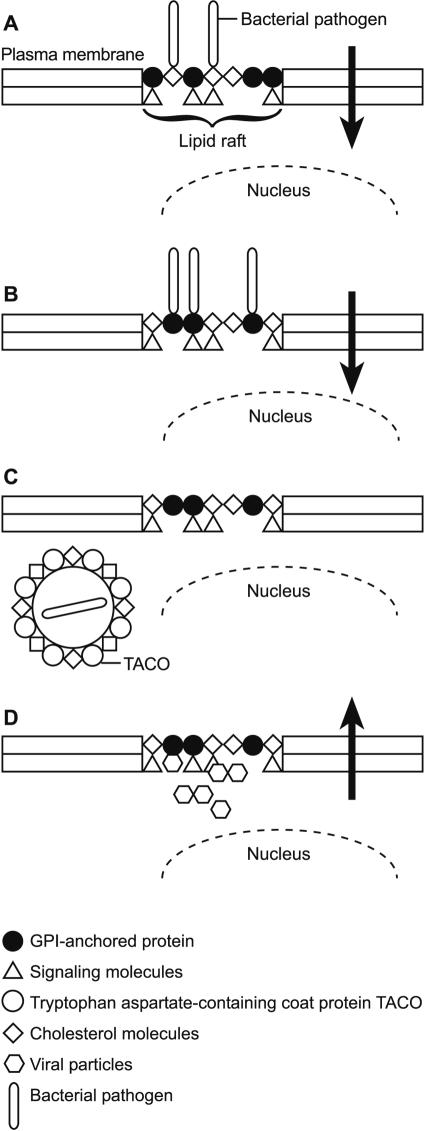
FIG. 1.
Conceptual model of cholesterol effects on the traffic of microbial pathogens into or out of the eukaryotic cell. (A) The bacterial pathogen interacts directly with membrane cholesterol, which serves as a “docking site” and stabilizes microbial interaction with membranes. (B) The bacterial pathogen interacts directly with a GPI-anchored protein receptor embedded in lipid rafts. Cholesterol is required to maintain lipid raft integrity. (C) The internalized microorganism resides within a phagosome with a cholesterol-enriched membrane. Cholesterol is required to prevent phagolysosomal fusion. (D) Viral particles (HIV-1) are targeted to lipid rafts. Cholesterol is required to maintain virion integrity and the release of infectious virions.
Acknowledgments
We apologize to those colleagues whose work we could not mention here. For editorial and graphic assistance, we thank the Ob/Gyn Publication, Grant, and Media Support Director and staff: Robert G. McConnell, Kristi Barrett, John Helms, and Mindy Loya.
Editor: J. B. Kaper
REFERENCES
- 1.Baorto, D. M., Z. Gao, R. Malaviya, M. L. Dustin, A. van der Merwe, D. M. Lublin, and S. N. Abraham. 1997. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636-639. [DOI] [PubMed] [Google Scholar]
- 2.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger, C. N., O. Billker, T. F. Meyer, A. L. Servin, and I. Kansau. 2004. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol. Microbiol. 52:963-983. [DOI] [PubMed] [Google Scholar]
- 5.Bloch, K. 1965. The biological synthesis of cholesterol. Science 150:19-28. [DOI] [PubMed] [Google Scholar]
- 6.Brown, M. S., and J. L. Goldstein. 1976. Receptor-mediated control of cholesterol metabolism. Science 191:150-154. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. S., and J. L. Goldstein. 1999. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA 96:11041-11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catron, D. M., Y. Lange, J. Borensztajn, M. D. Sylvester, B. D. Jones, and K. Haldar. 2004. Salmonella enterica serovar Typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect. Immun. 72:1036-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catron, D. M., M. D. Sylvester, Y. Lange, M. Kadekoppala, B. D. Jones, D. M. Monack, S. Falkow, and K. Haldar. 2002. The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cell. Microbiol. 4:315-328. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee, S., and S. Mayor. 2001. The GPI-anchor and protein sorting. Cell. Mol. Life Sci. 58:1969-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian, A. E., M. P. Haynes, M. C. Phillips, and G. H. Rothblat. 1997. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38:2264-2272. [PubMed] [Google Scholar]
- 12.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert, R. J. 2002. Pore-forming toxins. Cell. Mol. Life Sci. 59:832-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimpl, G., K. Burger, and F. Fahrenholz. 1997. Cholesterol as modulator of receptor function. Biochemistry 36:10959-10974. [DOI] [PubMed] [Google Scholar]
- 15.Goluszko, P., R. Selvarangan, V. Popov, T. Pham, J. W. Wen, and J. Singhal. 1999. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect. Immun. 67:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessey, S. J., J. Spencer, J. I. Wyatt, G. Sobala, B. J. Rathbone, A. T. Axon, and M. F. Dixon. 1990. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut 31:134-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilangumaran, S., H. T. He, and D. C. Hoessli. 2000. Microdomains in lymphocyte signalling: beyond GPI-anchored proteins. Immunol. Today 21:2-7. [DOI] [PubMed] [Google Scholar]
- 19.Jutras, I., L. Abrami, and A. Dautry-Varsat. 2003. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect. Immun. 71:260-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kansau, I., C. Berger, M. Hospital, R. Amsellem, V. Nicolas, A. L. Servin, and M. F. Bernet-Camard. 2004. Zipper-like internalization of Dr-positive Escherichia coli by epithelial cells is preceded by an adhesin-induced mobilization of raft-associated molecules in the initial step of adhesion. Infect. Immun. 72:3733-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna, K. V., K. J. Whaley, L. Zeitlin, T. R. Moench, K. Mehrazar, R. A. Cone, Z. Liao, J. E. Hildreth, T. E. Hoen, L. Shultz, and R. B. Markham. 2002. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Investig. 109:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuraya, M., and T. Fujita. 1998. Signal transduction via a protein associated with a glycosylphosphatidylinositol-anchored protein, decay-accelerating factor (DAF/CD55). Int. Immunol. 10:473-480. [DOI] [PubMed] [Google Scholar]
- 24.Lafont, F., V. N. Tran, K. Hanada, P. Sansonetti, and F. G. van der Goot. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 26.Liappis, A. P., V. L. Kan, C. G. Rochester, and G. L. Simon. 2001. The effect of statins on mortality in patients with bacteremia. Clin. Infect. Dis. 33:1352-1357. [DOI] [PubMed] [Google Scholar]
- 27.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez, J. J., and S. J. Hultgren. 2002. Requirement of Rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 4:19-28. [DOI] [PubMed] [Google Scholar]
- 29.Martino, T. A., M. Petric, M. Brown, K. Aitken, C. J. Gauntt, C. D. Richardson, L. H. Chow, and P. P. Liu. 1998. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology 244:302-314. [DOI] [PubMed] [Google Scholar]
- 30.Naroeni, A., and F. Porte. 2002. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 70:1640-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki, B., J. Moulds, R. Hull, and S. Hull. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 56:1057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peiffer, I., A. L. Servin, and M. F. Bernet-Camard. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyron, P., C. Bordier, E. N. N′Diaye, and I. Maridonneau-Parini. 2000. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J. Immunol. 165:5186-5191. [DOI] [PubMed] [Google Scholar]
- 36.Pieters, J. 2001. Entry and survival of pathogenic mycobacteria in macrophages. Microbes Infect. 3:249-255. [DOI] [PubMed] [Google Scholar]
- 37.Pralle, A., P. Keller, E. L. Florin, K. Simons, and J. K. Horber. 2000. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148:997-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pucadyil, T. J., P. Tewary, R. Madhubala, and A. Chattopadhyay. 2004. Cholesterol is required for Leishmania donovani infection: implications in leishmaniasis. Mol. Biochem. Parasitol. 133:145-152. [DOI] [PubMed] [Google Scholar]
- 39.Rietveld, A., and K. Simons. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta 1376:467-479. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers, W., and J. Zavzavadjian. 2001. Glycolipid-enriched membrane domains are assembled into membrane patches by associating with the actin cytoskeleton. Exp. Cell Res. 267:173-183. [DOI] [PubMed] [Google Scholar]
- 41.Saifuddin, M., T. Hedayati, J. P. Atkinson, M. H. Holguin, C. J. Parker, and G. T. Spear. 1997. Human immunodeficiency virus type 1 incorporates both glycosylphosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J. Gen. Virol. 78:1907-1911. [DOI] [PubMed] [Google Scholar]
- 42.Samuel, B. U., N. Mohandas, T. Harrison, H. McManus, W. Rosse, M. Reid, and K. Haldar. 2001. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J. Biol. Chem. 276:29319-29329. [DOI] [PubMed] [Google Scholar]
- 43.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 44.Selvarangan, R., P. Goluszko, V. Popov, J. Singhal, T. Pham, D. M. Lublin, S. Nowicki, and B. Nowicki. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 68:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seveau, S., H. Bierne, S. Giroux, M. C. Prevost, and P. Cossart. 2004. Role of lipid rafts in E-cadherin- and HGF-R/Met-mediated entry of Listeria monocytogenes into host cells. J. Cell Biol. 166:743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenoy-Scaria, A. M., L. K. Gauen, J. Kwong, A. S. Shaw, and D. M. Lublin. 1993. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol. Cell. Biol. 13:6385-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenoy-Scaria, A. M., J. Kwong, T. Fujita, M. W. Olszowy, A. S. Shaw, and D. M. Lublin. 1992. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J. Immunol. 149:3535-3541. [PubMed] [Google Scholar]
- 49.Shin, J. S., Z. Gao, and S. N. Abraham. 1999. Bacteria-host cell interaction mediated by cellular cholesterol/glycolipid-enriched microdomains. Biosci. Rep. 19:421-432. [DOI] [PubMed] [Google Scholar]
- 50.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 51.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 52.Simons, M., P. Keller, J. Dichgans, and J. B. Schulz. 2001. Cholesterol and Alzheimer's disease: is there a link? Neurology 57:1089-1093. [DOI] [PubMed] [Google Scholar]
- 53.Stefanova, I., V. Horejsi, I. J. Ansotegui, W. Knapp, and H. Stockinger. 1991. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254:1016-1019. [DOI] [PubMed] [Google Scholar]
- 54.Stella, V. J., and R. A. Rajewski. 1997. Cyclodextrins: their future in drug formulation and delivery. Pharm. Res. 14:556-567. [DOI] [PubMed] [Google Scholar]
- 55.Stokes, C. L., P. B. Weisz, S. K. Williams, and D. A. Lauffenburger. 1990. Inhibition of microvascular endothelial cell migration by beta-cyclodextrin tetradecasulfate and hydrocortisone. Microvasc. Res. 40:279-284. [DOI] [PubMed] [Google Scholar]
- 56.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda, M., G. P. Leser, C. J. Russell, and R. A. Lamb. 2003. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA 100:14610-14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taraboulos, A., M. Scott, A. Semenov, D. Avrahami, L. Laszlo, and S. B. Prusiner. 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vance, D. E., and H. Van den Bosch. 2000. Cholesterol in the year 2000. Biochim. Biophys. Acta 1529:1-8. [DOI] [PubMed] [Google Scholar]
- 60.Varma, R., and S. Mayor. 1998. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394:798-801. [DOI] [PubMed] [Google Scholar]