The incidence of vector-borne diseases such as malaria, dengue, African sleeping sickness, and tick-borne fevers is increasing. Given that current global control efforts have met with limited success, the need to develop novel interventions has become all the more vital. Successful discovery of such interventions is contingent on improving our understanding of how the pathogen interacts with its vertebrate host and also with its invertebrate vector. In this review we examine a premise developed more than 20 years ago (94) that emphasized the role of protein-carbohydrate interactions in microbial pathogenesis, and we present it in the context of vector-pathogen dynamics.
Glycans are “essential macromolecules for numerous cellular processes, such as signaling, structural-support, cell-cell interaction, cell-matrix adhesion, growth, protection and trafficking” (94). Their ubiquitous yet tissue-specific occurrence as cell surface glycoconjugates has, over evolutionary time, been exploited by several pathogenic microorganisms as receptors for attachment and invasion (95). While evidence for microbial adherence to mammalian glycolipids and glycoproteins continues to grow (for reviews see references 17, 18, 58, 95, and 115), only recently has evidence for a “protein-carbohydrate recognition strategy” for vector host-pathogen interactions emerged. This review focuses on the most recent advances that describe adherence mechanisms of three different classes of pathogens—bacteria, viruses, and protozoan parasites—to their obligate arthropod vectors. We also discuss how the protein-carbohydrate recognition strategies in both vector and mammalian life stages are apparently conserved and how this conservation could lead to the development of novel strategies for intervention.
THE MIDGUT: THE PRINCIPAL PATHOGEN GATEWAY
The majority of vector-borne pathogens are acquired when the arthropod vector ingests an infective blood meal (113). Consequently, the arthropod midgut serves as both barrier and gateway to pathogen invasion (97, 107). The luminal face of the midgut epithelium is covered by a dense array of glycoconjugates that may act as a “glycan receptor-buffet” for a myriad of pathogens (107). Arthropod midgut glycoconjugates are involved in general tissue structure and digestion (96, 107). Also populating the midgut surfaces of arthropods are glycoconjugates that are involved in innate immunity (26, 79). Included, but not necessarily exclusively belonging to this immune subset, are carbohydrate binding proteins (CBPs).
Protein-carbohydrate recognition can be mediated by cooperative hydrogen bonding, metal coordination, and van der Waals and hydrophobic interactions between the numerous hydroxyl groups that are present on glycans and amino acids within carbohydrate recognition domains (CRDs) (for reviews, see references 31 and 112). Accordingly, CBPs have generally been classified into two major groups according to topological features of their CRDs (31, 93). Group I proteins, exemplified by bacterial transport proteins, bear hidden or buried combining sites within pockets that completely enclose the carbohydrate ligand. On the other hand, group II proteins maintain shallow binding sites that allow for multiple binding events with oligosaccharide ligands. Members of group II include the classical lectin families—legume lectins, C-type lectins (CTL), galectins (formerly referred to as S-type lectins), and other plant lectins. Also included in group II are lectins and lectin-like proteins from human and animal pathogens, bacterial toxins (e.g., Bacillus thuringiensis toxin) (4), and anticarbohydrate antibodies (32, 114).
To date, most of the invertebrate CBPs characterized are members of group II lectins. Invertebrate lectins function as mediators of cell adhesion, opsonization, phagocytosis, and cytolysis and as defense molecules of the innate immune system for the recognition of nonself carbohydrate molecules on invading pathogens (51, 112). Several invertebrate protein families involved in innate immunity and other functions also exhibit carbohydrate binding activity. While many of these molecules, including scavenger receptors (86) and lipopolysaccharide-binding and glucan-binding pattern recognition receptors (PRRs) (59), fall into one of the classical CBP groups, e.g., C-type lectins (123), others have not been classified specifically as CBPs though they bear CRDs characteristic of certain classical lectins. Their putative function as PRRs is further complicated by their conflicting and unintended role in facilitating pathogen survival, dissemination, and subsequent transmission to vertebrate hosts by serving as homing receptors, attachment anchors, and differentiating factors (10, 123).
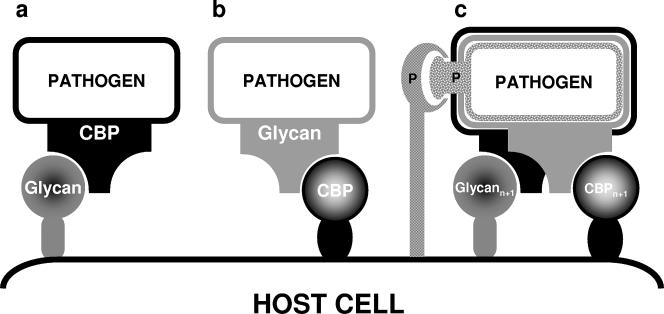
The putative dual role of invertebrate CBPs as PRRs and as pathogen receptor targets suggests at least four mechanisms by which protein-carbohydrate recognition events may facilitate pathogen survival and development in the arthropod host. The first mechanism involves pathogen carbohydrate binding adhesins that utilize arthropod host midgut glycans as initial attachment receptors preceding invasion of the midgut epithelium. The second mechanism is the promotion of attachment by arthropod CBPs themselves via recognition of glycans that are present either on glycosylated capsules or on membrane-bound glycoproteins of these pathogens. The third mechanism involves soluble CBP of vector origin that may act as a linker recognizing glycans on both the midgut and pathogen surfaces. CBP affinities for glycans are relatively weak. Hence, CBP binding requires a multiplicity of interactions between the CRDs and carbohydrate moieties. Therefore, one can speculate that the fourth and final possibility is that following the initial glycan recognition event, other coreceptors are recruited to strengthen the interaction. These molecules may be other CBPs on the pathogen, on the host cell, or a combination of both. Alternatively, coreceptors can be molecules that utilize protein-protein interactions between surface proteins at or proximal to the attachment site in a “zipper” or “Velcro” attachment effect (Fig. 1). As will become evident, this is a strategy that is common to most vector-borne pathogens.
FIG. 1.
Model for protein-carbohydrate adhesion strategies for vector-borne pathogens. (a) Pathogen CBP-host cell glycan interaction. (b) Pathogen glycan present on membrane or envelope glycoconjugates is bound by host cell CBP. (c) Reciprocal recognition, i.e., the “zippered” or “Velcro” effect, wherein multiple (n + 1) CBP-glycan interactions, including putative protein-protein (P) coreceptor interactions, mediate adhesion.
ADHERENCE TO THE RULE: CONSERVED THEMES IN MICROBIAL ATTACHMENT TO INVERTEBRATE HOST TISSUES
The bulk of the evidence on the critical role of protein-carbohydrate interactions has been gained from the study of the interaction of vector-borne pathogens with their mammalian hosts. Several examples of putative adhesins and/or glycan receptors of vector-borne pathogens are presented in Table 1. For several of the examples that will be discussed, the mechanism of that interaction still remains poorly understood. In such cases an analysis of the attachment/invasion process in mammals may provide some insight into the nature of the mechanism in the invertebrate.
TABLE 1.
Vector-borne pathogen protein-carbohydrate interactionsa
| Pathogen | Vector | Pathogen ligand/receptor | Vector host ligand/receptor | Vertebrate host ligand/receptor | Reference(s) |
|---|---|---|---|---|---|
| Borrelia burgdorferi | Ixodid ticks | OspA, OspB, OspC | TROSPA | GalCer, LacCer, gangliosides | 11, 46, 80, 81, 84, 85, 101, 121, 126 |
| DbpA, DbpB, Bgp | Decorin, collagen I, dermatan sulfate, heparin | 42, 47, 84, 85, 126 | |||
| Anaplasma phagocytophilum | Ixodid ticks | P-selectin mimic, MSP2 | Fucosylated PSGL-1 | 23, 40, 63 | |
| Anaplasma marginale | MSP1a, MSP1b, MSP2 | Midgut lectin or galectin? | 10, 22, 23, 24, 26, 37, 55, 68 | ||
| Dengue fever virus | Aedes spp. (mosquitoes) | E glycoprotein, domain III | Heparan sulfate, chondroitin sulfate | Heparan sulfate, chondroitin sulfate | 3, 16, 43, 62 |
| 40- and 45-kDa midgut glycoproteins, 67- and 80-kDa glycoproteins | 49, 66 | ||||
| Midgut C-type lectin? | 29, 33, 100 | ||||
| Mannose | DC-SIGN/L-SIGN | 48, 59, 66, 74, 117 | |||
| Sindbis virus | Culex spp. (mosquitoes) | E1 glycoprotein | Midgut C-type lectin? | DC-SIGN/L-SIGN | 48, 59, 66, 74 |
| E2 glycoprotein | 74 | ||||
| Trypanosoma brucei spp. | Glossina spp. (tsetse) | EPEET | ConA-like lectin | 71, 118 | |
| GPEET | Gal- or Glc-specific lectin | 50, 60 | |||
| TsetseEP | 14, 44, 45 | ||||
| Leishmania spp. | Phlebotomus spp. (sandflies) | LPG | Galectin | 54, 75, 87, 91, 98 | |
| Plasmodium spp. | Anopheles spp. (mosquitoes) | CTRP | Midgut GAG? | 2, 13, 34, 65 | |
| WARP | Midgut GAG? | 2, 34, 65, 125 | |||
| CS, TRAP | Salivary gland GAG? | Hepatocyte HSPG | 2, 13, 35, 36, 83, 103, 110 | ||
| LCCL | Galactose? | 110, 111 | |||
| O-linked glycans? | 6, 27, 28, 128 |
Summary of presently known and/or putative pathogen receptors or ligands involved in adhesion of vector-borne pathogens to mammalian and vector host cells.
Bacterial pathogens.
The tick-borne rickettsial pathogens Anaplasma phagocytophilum and Anaplasma marginale utilize host cell lectin-like molecules for attachment. The A. marginale major surface protein 1 (MSP1) complex is a heterodimer composed of MSP1a and MSP1b, and these subunits have been shown to be the primary rickettsial adhesins involved in attachment to tick and bovine cells, respectively (23, 24). Antibodies against MSP1a or tick midgut antigens block A. marginale transmission to ticks (7). The N terminus of MSP1a bears O-linked glycans on several motifs typical of mucins (37). Removal of these glycans from the MSP1a functional domain resulted in a significant decrease in binding efficiency. This suggests that the glycans on MSP1a contribute extensively to attachment to tick cells and that MSP1a behaves primarily as a receptor for a tick CBP. Although several other tick-borne rickettsial pathogens express heavily glycosylated outer membrane proteins, only MSP1a and the Ehrlichia ruminatum mucin-like outer membrane protein have been shown to have a role in pathogen transmission through ticks (21, 22, 68). Several studies have already reported lectin or lectin-like activity in the hemolymph, midgut, and salivary glands of soft and hard ticks (40, 55, 56, 61, 63). However, only recently has a tick galectin-like protein (AY208827) been isolated and molecularly characterized from the midguts of Rhipicephalus appendiculatus ticks (A. Mulenga, personal communication).
Conversely to its putative mechanism of attachment in ticks, A. phagocytophilum uses bacterial lectin-like recognition for attachment to mammalian cells. A. phagocytophilum efficiently invades mammalian neutrophils through P-selectin mimicry (46). Fucosylation of the leukocyte P-selectin glycoprotein ligand (PSGL-1) was found to be important, since PSGL-1-expressing cells that were deficient in fucosyltransferase (Fuc-TVII) were inefficiently bound by neutrophils (11). An A. marginale glycosylated adhesin, MSP2, may likewise mimic lectins, since it has been shown to adhere to bovine erythrocytes (J. de la Fuente, personal communication).
The strategy of attachment to tick cells through the use of endogenous arthropod lectins followed by the use of bacterial lectin-like and non-CBP molecules in mammalian cell invasion is not completely mirrored in other vector-borne bacterium models. Borrelia burgdorferi, the causative agent of Lyme disease, expresses two major outer surface (lipo)proteins (OspA and OspB) that are crucial for spirochete adherence to, and survival in, the tick midgut (34, 80, 101, 121). For instance, anti-OspA and anti-OspB antibodies could confer transmission-blocking immunity in the tick midgut (80). Additionally, through the use of recombinant protein constructs, it was shown that the two binding domains, found at the center and at the carboxy terminus of OspA, that are responsible for attachment to the tick were also capable of self-binding, thereby promoting coattachment of other spirochetes to tick midguts (80). However, based on the use of deglycosylating enzymes, it was suggested that OspA adhesion is not mediated by glycans on tick midgut surface glycoconjugates (80). These initial conclusions alone can be problematic, since mammalian deglycosidases may not work efficiently on either bacterial or arthropod glycoproteins due to unique carbohydrate branching modifications. Recently, the tick receptor for OspA (TROSPA) was identified (81). Bacterially expressed recombinant TROSPA, which is essentially a deglycosylated form of the native 55-kDa tick midgut-specific glycoprotein, was capable of binding to OspA. The data support the idea that OspA-TROSPA affinity is indeed glycan independent. OspC, which is not expressed in resident spirochetes until their egress from the midgut into the hemolymph, has also been shown to be important for adherence and subsequent invasion of the tick salivary glands (82). ospC knockouts adhered to and survived in the midgut but were incapable of invading the tick salivary glands. To date, the glycoconjugate receptors for both OspB and OspC are unknown, and therefore the roles of CBPs and glycans in this context remain unclear.
While the action of the Osp family of proteins is certainly critical for spirochete adherence to tick tissues, they are not the only surface proteins that could be involved in glycan-mediated adherence. In fact, the Osp family of proteins may not act as CBPs for tick host cell glycoconjugates at all. That function may rely on other outer membrane proteins, such as those in the B. burgdorferi genome that have been identified as bearing the von Willebrand factor (vWF) A domain (GenBank/EMBL/DDBJ accession no. AAC65016) (105). This motif is well represented in other arthropod-borne pathogens (65) and may therefore play an accessory adhesion role early in the process of spirochete attachment to the tick gut. It has been shown that B. burgdorferi can bind glycosaminoglycans (GAGs) (52). Therefore, the presence of vWF A domains, which have affinity for polyanionic oligosaccharides (65), provides one possible mechanism. In addition, an alternative mechanism, whereby Borrelia-tick interaction follows a reciprocal molecular system of recognition, has been proposed (41). The authors argue that B. burgdorferi spirochetes express several glycosylated surface molecules that are components of the “slime layer,” including OspA, OspB, and a major extracellular 83-kDa glycoprotein (30, 47). These molecules could be targets of tick midgut tissue CBPs, as was described previously for tick-borne rickettsiae.
Research exploring the mechanisms by which Borrelia spirochetes invade mammalian tissues has found that borreliae employ several surface proteins with carbohydrate binding properties, allowing them to bind to several mammalian cell types. This cosmopolitan specificity may be indicative of the affinity of these spirochetes for common extracellular matrix (ECM) components, including glycolipids. Two B. burgdorferi decorin binding adhesins or lipoproteins (Dbp's), Dbp A and DbpB, have been characterized recently (42). Both recognize decorin (a collagen- and ECM-associated proteoglycan) (42), collagen type I lattices (126), and dermatan sulfate and heparin in mammalian tissues (84). Another Borrelia GAG-binding protein (Bgp), which was identified by its ability to agglutinate erythrocytes, also has binding specificity for heparin (85). It appears that Dbp's and Bgp's are complex molecules bearing recognition domains with affinity for both core protein and oligosaccharide. At present, it is not clear how these additional adhesins work in concert with the Osp proteins to promote attachment to mammalian tissues.
Borrelia spirochetes also have affinity for three neutral mammalian glycosphingolipids, galactocerebroside (GalCer), lactosylceramide (LacCer), and ceramide trihexoside, as well as two gangliosides (3). GalCer is one of many types of glycosphingolipids which have already been shown to be receptors for pathogenic microorganisms (57). The spirochete ligand for GalCer is most likely not OspA or OspB, since neither the recombinant OspA protein nor the monoclonal antibody to OspB blocked attachment to GalCer (3). On the other hand, these authors suggest that OspB may bind LacCer. Additionally, monosaccharide competitors were unable to block attachment. Taken together, the data indicate a role for multiple sugars (in a defined array) or even ceramide itself as a host receptor for bacterial attachment (57), an interaction that is most likely mediated by a poorly understood class of adhesins.
The current data suggest that many of the molecules that are involved in vector and mammalian host adhesion may have promiscuous binding specificities, which have complicated their study. Yet this promiscuity does not seem to affect host species specificity or even tissue specificity. “Promiscuity,” as opposed to “nonspecificity,” suggests a consistent recognition of a defined molecular pattern, e.g., a carbohydrate ligand that may be present on a wide range of glycoconjugates. Alternatively, a peptide domain that shares structural features with an oligosaccharide, i.e., a peptide carbohydrate mimic, may also be recognized (17). It is increasingly apparent that the pathogen's choice of strategy at various stages of development is the direct result of coevolution with corresponding hosts. As will be discussed, the same strategies are used by vector-borne viral and parasite pathogens.
Viral pathogens.
The genus Flavivirus (family Flaviviridae) includes several arthropod-borne human pathogens. Four of these viruses, yellow fever virus, Japanese encephalitis virus, West Nile virus, and dengue fever virus (DEN), are transmitted by mosquitoes. Tick-borne encephalitis virus, as its name indicates, is transmitted by soft Argasid ticks. Flaviviruses are small enveloped particles that are believed to enter vertebrate and invertebrate host cells through attachment via the large enveloped glycoprotein E (also known as the viral attachment protein) followed by receptor-mediated endocytosis (117).
The mammalian host receptors for the E glycoproteins of several flaviviruses and other viral families have been found to be heparan sulfate (HS) and chondroitin sulfate, which are highly sulfated, heterogeneous polyanionic GAGs that occur on a wide variety of ECM and cell membrane glycoproteins and proteoglycans (95). HS-containing proteoglycans mediate endocytosis through either clathrin-coated pits or membrane rafts (5, 43, 62). HS-dependent viral recognition, however, is believed to be restricted to the initial attachment event preceding invasion (62). Potential HS-binding sites have been identified for the DEN type 2 (DEN-2) glycoprotein, which has been shown to have strong binding affinity for a decasaccharide form of highly sulfated HS (16).
While HS appears to be a receptor for DEN invasion of mammalian cells/tissues, other host molecules (as coreceptors) may play a greater role in DEN infection of mosquito cells (39, 48, 49, 66). From vertebrate studies, it has been shown that DEN-4 adhesion to Vero cells requires the involvement of a 74-kDa glycoprotein and that recognition of the receptor is sensitive to periodate but not heparinase treatment (63). This suggests that DEN attachment in mosquito cells may follow a similar strategy, recognizing non-HS oligosaccharides on other surface glycoconjugates. DEN-2 has been shown to bind to two prominent glycoproteins of 6 and 80 kDa on Aedes albopictus C6/36 mosquito cells (74). On the other hand, DEN-4 recognized another two glycoproteins of approximately 40 and 45 kDa on both C6/36 cells and mosquito midguts. Unlike the GAG-dependent adhesion of envelope proteins to vertebrate cells (48), soluble GAG had no effect on DEN-2 adhesion to mosquito cells (48), suggesting the involvement of non-HS-bearing receptors. Unfortunately, since only neuraminidase was used to assess the carbohydrate components of the 67- and 80-kDa glycoproteins on C6/36 cells, which do not characteristically contain sialic acids, the exact nature of the carbohydrate moieties of the DEN-2 receptors remains unclear (100). Removal of glycans from the two DEN-4 mosquito glycoproteins did not affect viral adhesion, suggesting that viral CBPs are not involved (66). Antibodies against either the viral envelope or the mosquito glycoproteins blocked DEN-2 and DEN-4 attachment to mosquito cells (48). The likelihood that antibody-mediated steric hindrance is the basis for these results complicates their interpretation, since virus receptors may associate as a receptor complex with other molecules. Altogether, the data suggest that different DEN serotypes may use diverse glycoprotein receptors (many of which remain unknown) depending on the specific host and host cell type. Interestingly, the DEN-4 ∼45-kDa glycoprotein receptor is absent from the midguts of Anopheles albimanus (122). Since Anopheles albimanus is a poor vector for DEN-4, this glycoprotein may play a critical role in defining mosquito vector competence for DEN-4. The presence or absence of such accessory or coreceptor molecules apparently defines DEN tropism in mosquitoes (67). It remains to be seen if coreceptor complexes help define the limits of viral host range for different DEN serotypes or, for that matter, even for other arboviruses. The mosquito-borne Sindbis virus (SINV) (Alphavirus: Togaviridae) utilizes the E2 envelope glycoprotein for host cell tropism, receptor recognition, and general virulence (76). A deletion in the cell receptor and binding domain of the E2 glycoprotein results in a decrease in the infectivity of the virus for C6/36 cells and Aedes aegypti midguts. The E2 receptor on mosquito midgut microvilli has not yet been identified, but there is some evidence suggesting that, as with DEN-4, recognition of insect tissues is GAG independent (116).
Both the current DEN and SINV data preclude the involvement of viral CBP recognition of insect cell glycans. However, they do not rule out the potential role of neutral oligosaccharides on the viral membrane itself in attachment. The lectin concanavalin A (ConA), which preferentially binds mannose (Man), competitively inhibits DEN-2 attachment to insect cells by binding to Man residues on viral glycoproteins (61). Moreover, this recognition is specific, since soluble Man reversed the effects of ConA inhibition (49). Wheat germ agglutinin (WGA), with specificity for N-acetylglucosamine (GlcNAc), likewise inhibited DEN-2 attachment to vertebrate cells (61); however, its inhibitory activity was not determined for C6/36 cells. While CBPs from insect cell lines have not yet been identified, the evidence for the involvement of host cell-specific lectins in viral adhesion to vertebrate tissues is well supported.
C-type lectins are a prime example of host cell molecules that are used by pathogenic microorganisms, and their potential role in vector-pathogen interactions deserves further study. C-type lectins (a class of Ca2+-dependent lectins) or proteins bearing CRDs that are characteristic of mammalian C-type lectins have been described for Drosophila melanogaster (29) and Anopheles gambiae. In mammals, C-type lectins provide sentinel immune cells with a means of molecular pattern recognition. C-type lectins on the surfaces of dendritic cells, such as DC-SIGN (dendritic cell-specific ICAM3-grabbing nonintegrin) or L-SIGN (liver/lymph node-specific ICAM3-grabbing nonintegrin), have been shown to bind mannose on complex hybrid glycan structures present on the glycosylated viral envelope proteins (33). The binding of mannose increases viral binding affinity for the cell after initial HS recognition (33). Among the many arboviruses, DEN and SINV have been shown to utilize this strategy for viral tropism during vertebrate infection and dissemination (10, 60, 72). Additionally, there are early indications that N-linked glycosylation patterns on the envelope proteins of both these arboviruses are conserved between early-mosquito-stage virus (i.e., virions taken up in a blood meal from an infected vertebrate host) and virions in the salivary gland (53, 77, 92). Thus, it is possible that conserved hybrid glycan structures on virus envelope glycoproteins are recognized by C-type lectin-like molecules within the mosquito midgut and other tissues, in a manner similar to the role of DC-SIGN and L-SIGN during viral attachment to vertebrate cells.
It can be envisaged that specific domains of the viral envelope glycoprotein are responsible for recognizing different glycans, e.g., anionic and neutral oligosaccharides, on mosquito midgut microvillar glycoproteins during initial attachment. However, it is the involvement of the coreceptor, e.g., a host cell glycoprotein with or without lectin-like activity, that is crucial for complete viral adsorption. In line with this argument, reciprocal coreceptor recognition may impart two layers of host selectivity, i.e., viral tropism for specific mammalian and invertebrate tissues. This is consistent with the fact that not all mosquitoes are competent vectors for flaviviruses. Therefore, vector competence is dependent on the contribution of critical glycan, CBP, and other protein coreceptors. This recognition mechanism may explain how some arthropod vectors (e.g., ticks and mosquitoes) can be infected with so many viruses (other than flaviviruses), how some viruses (e.g., West Nile virus) can infect so many arthropods, or how some arthropods (e.g., anophelines) are resistant to viral infection. Clearly, the relative contributions of protein or glycan receptors to arbovirus infection of mosquitoes require further investigation.
Parasitic protozoans.
African trypanosomes, the causative agents of African sleeping sickness in humans and nagana in cattle, are transmitted by flies of the genus Glossina (tsetse flies). Trypanosomes display their major surface glycoproteins using a glycosylphosphatidylinositol (GPI) anchor, a glycosylation modification that is common to parasitic protozoans. Vertebrate bloodstream forms of Trypanosoma brucei express GPI-anchored variant surface glycoproteins that have been found to bear both high-mannose-type (Man9GlcNAc2) and complex hybrid N-linked glycans (50). Procyclic T. brucei, the parasitic stage in the tsetse fly midgut, expresses procyclins, which are GPI-anchored major surface acidic glycoproteins. Procyclins appear to be generally restricted to Man5GlcNAc2 structures, despite the predisposition of trypanosomes to produce more-complex structures (50). The occurrence of a single, dominant N-linked glycan structure on procyclins suggests that the oligosaccharide most likely serves a specific biological function in the fly midgut. Two forms of procyclins are expressed at different points during the life cycle of the trypanosome in the midgut. The GPEET form, which consists of repeating units of Gln-Pro-Glu-Glu-Thr (hence the single-letter acronym), persists for the first 7 days. This is followed by the expression of the EPEET form, which has a Glu-for-Gln substitution. The former is believed to have an aglycosylated N-terminal polypeptide chain, while the latter, which bears an N-linked Man5GlcNAc2 structure (50), is crucial for trypanosome survival in the midgut and subsequent travel to the salivary glands (71, 118).
It has been postulated that the attrition in numbers of procyclics that is observed during the first 4 days of infection in the fly midgut is a result of specific carbohydrate-lectin interactions, which in turn modulate the transmission of trypanosomes in the tsetse fly (118). Earlier work suggested the presence of a Gal- or Glc-specific lectin-like protein in the fly midgut, because the addition of either monosaccharide to a trypanosome-infectious blood meal resulted in higher infection rates in tsetse flies (118). The bacterial endosymbiont Sodalis glossinidius resides in the tsetse fly midgut, where it catalyzes the production of GlcNAc from chitin (19). GlcNAc, in turn, putatively inhibits the effect of midgut lectins. Consequently, the removal of Sodalis by use of antibiotics decreases the number of established trypanosome infections in the gut. Additional evidence supports the role of fly lectins in regulating tsetse fly infections, since it has been shown that the legume lectins ConA, WGA, and Ricinus communis agglutinin have binding affinity for different species of Trypanosoma (75). Moreover, ConA has been shown to induce a novel form of apoptosis among EP-procyclic forms (87), suggesting the presence of a ConA-like lectin in the fly midgut. GPEET procyclics are not recognized by ConA, suggesting that a different lectin, with specificity for Gal or Glc, may recognize the glycan core of the GPI anchor and could control parasite numbers during the earlier stages of infection (71). The TsetseEP protein was recently characterized from the midgut of Glossina morsitans morsitans (14, 45). TsetseEP is a soluble protein complex that bears several Glu-Pro repeats and is strikingly similar to trypanosome EPEET procyclic. It also shares several structural features found in insect lectins and exhibits weak agglutinating activity that is competitively inhibited by d-glucosamine (14). Interestingly, TsetseEP has been found to be upregulated in response to bacterial challenge, suggesting a role in fly immunity (45). However, a comparison of midgut glycoproteins from G. m. morsitans mutant strains that are more susceptible to trypanosome infection with those of the wild type revealed that the most upregulated protein was TsetseEP (44). It is certainly possible that TsetseEP involvement in immunity is restricted to bacterial challenge and that its upregulation results in increased trypanosome survival in the gut. TsetseEP protein expression in wild-type G. m. morsitans is generally weak (14) and may correlate with the fly's relative refractoriness to infection compared to mutant trypanosome-susceptible strains. Further studies using different trypanosome species in combination with various fly species of differing susceptibilities will help elucidate this issue. Additionally, further characterization of other midgut lectins that have been identified from an expressed sequence tag (64) library may yield a functional homolog of ConA and other known lectins.
The sandfly-Leishmania parasite interaction provides the best-understood model of the importance of protein-carbohydrate recognition for parasite transmission (for a comprehensive review, see reference 99). Leishmania promastigote lipophosphoglycans (LPGs) are protein-free molecules containing the GPI core structure of Manα1-4GlcNAcα11-6myo-inositol but lacking conjugate core proteins and ethanolamine substitutions. The common backbone among LPGs is a repeating dissacharide unit composed of PO4-6Galβ1-4Manα1 with varying degrees of substitution of C-3 of Gal with Glc moieties (54). LPG has been implicated as the primary glycoconjugate involved in sandfly-parasite interactions (9, 90, 91, 98). It has been shown that allowing sandflies to first feed on mice that have been immunized with LPG and subsequently feed on nonimmunized, Leishmania-infected mice prevents normal development of the parasite in the gut (108, 109). Anti-LPG antibodies presumably affected the presentation of specific capping monosaccharides on LPG glucose side chains, thereby indirectly regulating promastigote attachment to the sandfly midgut (6).
For several years the nature of the sandfly midgut receptor for LPG remained elusive. Recently, a 35.4-kDa galectin was characterized in Phlebotomus papatasi, the primary vector for Leishmania major (54). Galectins are a widely distributed family of β-galactoside-binding lectins that contain one or two CRDs with significant sequence homology (112) and are often involved in predominantly carbohydrate mediated cell matrix lattice formation and cell-cell and cell-matrix adhesion (8). It seems that sandfly galectins are involved in defining vector susceptibility or refractoriness, since recognition by P. papatasi midgut galectins has been shown to inhibit other Leishmania spp. from successfully developing in the fly. In a similar fashion, mammalian galectins have been shown to be critical in Leishmania interactions with host cells (88, 89). Galectin-3 and especially galectin-9 not only display species-specific recognition for poly-β-Gal epitopes on Leishmania major but also facilitate L. major adhesion to host macrophages.
Although several studies have outlined the critical role of carbohydrates in the Plasmodium parasite-mosquito dynamic, interest in this line of experimentation has never fully developed, nor has it been extensively reviewed. It has been shown that a panel of legume lectins are capable of binding distinct oligosaccharides on the luminal face of the mosquito midgut (119). The importance of this carbohydrate recognition to Plasmodium development in the mosquito was then elegantly demonstrated in an ex vivo assay that showed that Plasmodium ookinete binding to the luminal face of the midgut was inhibited by periodate treatment (128). This was then further corroborated by data showing that several legume lectins (e.g., jacalin and WGA) significantly inhibited ookinete binding to putative midgut carbohydrate receptors (127). More recently, an anticarbohydrate antibody against mosquito midgut microvillus epitopes was shown to completely inhibit Plasmodium development (27). Furthermore, the ability of this antibody to recognize its cognate mosquito carbohydrate receptor was significantly inhibited by jacalin (which is specific for Gal moieties on O glycans) and WGA by a competition enzyme-linked immunosorbent assay (28), suggesting that the antibody shares similar glycan specificities with these two lectins. To date, the identity of the midgut glycoprotein target(s) is unknown.
There is evidence to suggest that malaria parasite ookinete ligands have carbohydrate recognition properties. Plasmodium, like its apicomplexan relatives, uses adhesive gliding motility for active penetration of host cells (102). Although the substrate for this motility in insects has not yet been fully defined, it is assumed to be a combination of glycoconjugates on vertebrate cells in the blood meal, the mosquito peritrophic matrix (a complex chitinous sac that envelops a blood meal), and carbohydrates on epithelial glycoproteins or glycolipids. Two parasite micronemal adhesive proteins, the membrane-bound circumsporozoite- and thrombospondin-related adhesion protein-related protein (CTRP) and the secreted vWF A-domain-like-related protein (WARP), are predominantly expressed on Plasmodium ookinetes (reviewed in reference 103). The vWF A-domain is conserved across the thrombospondin-related anonymous protein (TRAP) family of proteins and repeated in varying numbers (65, 125). For example, at the N terminus, CTRP contains six A-domains (25, 110, 124) whereas WARP contains only one (65, 125). In addition, CTRP shows evidence of seven repeated thrombospondin-1 (TSP-1)-like domains (25, 110, 124). CTRP knockout mutants are incapable of in vivo establishment in the anopheline mosquito (25, 106), and polyclonal antibodies to WARP inhibited parasite invasion of the mosquito midgut (1). These data suggest that the vWF A-domains on both WARP and CTRP may play a critical role in the midgut invasion process. TRAP, CTRP, and WARP have been shown by enzyme-linked immunosorbent assay to have binding affinity for heparin (65, 69). A more sophisticated assay using surface plasmon resonance-based technology (Biacore) showed that TRAP, as an entire molecule, has higher binding affinity for heparin than the vWF A-domains alone (2). This is most likely due to the contribution of the TSP-1 repeat domain on TRAP to heparin affinity. The vWF A-domains were also found to have two putative ligand-binding surfaces, a metal-binding site and a putative heparin binding site. In this study, however, sulfatides were shown to be incapable of completely inhibiting sporozoite infectivity for HepG2 cells, suggesting the presence of uncharacterized hepatocyte glycan or glycoconjugate receptors. In mosquitoes, TRAP, vWF A-domain, and TSP-1 knockout mutant parasites were incapable of invasion of mosquito salivary glands, but gliding motility remained unaffected (67). It remains to be seen whether surface plasmon resonance analysis of CTRP and WARP will confirm heparin specificity, especially given that GAGs or specific anionic polysaccharides have not yet been identified in mosquito midguts. It is quite likely that several critical parasitic proteins are involved in the orchestrated actions of gliding, attachment, and invasion. Recently, proteins belonging to the Limulus clotting factor C, Coch-5b2, and Lgl1 (LCCL)-lectin adhesive-like protein family were shown to be conserved across different Plasmodium species (111). The proteins are predominantly expressed in mosquito stages and are believed to have putative carbohydrate binding properties. However, the presence of multiple glycoconjugate receptors arrayed as a defined plasma membrane microdomain with putatively conserved glycan epitopes on mosquito cells has yet to be shown. Alternatively, there is some evidence for the involvement of a reciprocal arthropod recognition mechanism. Two CTL from the mosquito hemolymph, CTL4 and CTLMA2 (a mannose binding CTL), have been found to protect malaria parasites from melanization (79). Another hemolymph CTL, the mannan-binding lectin, has also been reported to have agglutinating activity (15); however, its role remains unclear at present. It is possible that mannan-binding lectin activity is opposite from that of CTLMA2, with a role more analogous to that of vertebrate mannan binding protein (70), which is involved in innate immune recognition. Other mosquito CBPs, such as the PRRs IGALE20 and gram-negative binding protein, which are upregulated in response to infection, have not yet been shown to have a direct effect on parasite development (120).
Plasmodium sporozoites that are inoculated into the vertebrate bloodstream during mosquito blood feeding make their way to the liver. Hepatocyte homing and attachment are believed to be mediated by the circumsporozoite (CS) protein and TRAP (35). CS recognizes HS proteoglycans (HSPG), which extend out through the endothelium from the basolateral surfaces of hepatocytes (12, 13, 36, 83, 104). Heparin, soluble heparan sulfate, and other sulfatides [Gal(3-SO4)β-ceramide], but not chondroitin sulfate A, have been shown to inhibit sporozoite recognition of HepG2 cells in vitro (13, 36). It was also found that antibodies against CS inhibited liver invasion, presumably preventing effective homing to the liver by blocking recognition of liver receptors, including HSPG and potentially other glycoconjugates.
Like CTRP (discussed earlier) and CS, TRAP displays binding affinity for heparin, heparan sulfate, dextran, and fucoidin but not for chondroitin sulfate A, which may correlate with parasite tropism (65, 69). The heparin binding domains for CS and TRAP consist of a conserved region II-plus adhesive motif (CSVTCG) (73) and TSP-1 repeats, respectively, both of which are clusters of basic and hydrophobic residues downstream of two cysteines. Such a cluster is also found on other cell adhesion molecules such as F-spondin, thrombospondin, and properdin. In addition, TRAP contains an A-domain that can independently bind to heparin (2, 65, 69). It is believed that in addition to assisting CS in homing to the liver, TRAP is also responsible for gliding motility and active invasion of hepatocytes (78). It is possible that to overcome the low-affinity recognition of GAG chains on HSPG, the sporozoites could either aggregate CS and/or utilize other sulfatide binding domains on TRAP in conjunction to increase its avidity for HSPG. However, the specific role of A-domains on TRAP as an adhesin was recently revised. Deletion mutants of the A-domain and TSP-1 of TRAP exhibited decreased invasive capabilities but maintained gliding motility and initial hepatocyte adhesiveness (67). The authors also found that while TSP-1 binding was HS dependent, A-domain binding was not. The data do not, however, contradict the evidence showing that A-domains by themselves have binding affinity for HS, but more importantly, they are indicative of the multivalent nature of this TRAP module for other unidentified glycan or protein receptors on hepatocytes.
The ability of sporozoites to adhere to cells suggests that CS may play a more dominant role in homing to the liver by acting as the primary adhesin. Based on these data, the reciprocal glycan recognition strategy proposed earlier, whereby liver-associated CBPs facilitate adherence by recognizing parasitic glycans, does not seem likely during liver invasion. However, this possibility cannot be ruled out, since several sporozoite surface proteins are GPI anchored and it is not clear whether recognition of the glycan moieties on these anchors by hepatocyte lectin-like proteins is involved in the observed adherence of A-domain or TSP-1 deletion mutant parasites. Furthermore, these protein-carbohydrate interactions represent only those that occur during the preerythrocytic stages of the parasite life cycle; there is further evidence for similar interactions occurring during the infection of red blood cells (reviewed more extensively elsewhere [38]).
SUMMARY
Extensive evidence for the role of protein-carbohydrate recognition mechanisms in vector host-pathogen interactions has been unveiled, but growth in this field has been hampered by the lack of molecular tools to study the interface between these two classes of molecules. In addition, other factors have also delayed progress in this field: for example, the general perception that prokaryotic proteins cannot be glycosylated or the assumption that all carbohydrate modifications are similar to those that have been described for mammals. Finally, underappreciation of the function of glycans beyond cell sorting, protein folding, and simple “decoration” has also contributed to the slowness of progress.
To date, the bulk of the research effort in studying protein-carbohydrate interactions between vector-borne pathogens and their host cells remains focused on the vertebrate life stages. Although the protein-carbohydrate recognition theme is, in general, conserved, there is clear evidence of modifications or variations to this theme. The literature suggests that distinct pathogen adhesion domains most likely play different roles depending on the nature of the host cell: vertebrate or invertebrate. Therefore, it may not be appropriate to extrapolate between the two groups of organisms. Despite the variations to the overall theme, one recognition event is clearly conserved across the different vector-borne pathogens. The convergent evolution of several unrelated microbial-pathogen molecules (e.g., CTRP, bacterial lipoproteins, and viral E glycoproteins) with affinity for host cell glycoconjugate polyanion carbohydrates, such as heparan sulfate, is unmistakable. This may in part result from the indistinct requirements for “consensus” heparin binding motifs of proteins. Clusters of six to eight alternating basic amino acid residues have been shown to be the requisite factors for recognition of sulfated polysaccharides (2, 20).
Pathogen recognition of glycan receptors represents the first, critical step of a very complex series of events leading to host cell invasion. The evidence suggests that molecular recognition is likely reciprocal, where low-affinity primary attachment to one set of glycoconjugates is strengthened by other coligands and coreceptors from both the pathogen and its host. Inhibiting this initial step by use of conventional methods as well as more novel approaches, such as oligosaccharide mimics or antiglycan antibodies, could result in a reduction in disease transmission. For example, antiglycan antibodies that are taken up by a mosquito along with an parasite infective blood meal can mask parasite glycan receptors that are critical for attachment (27). Clearly, one of the difficulties lies in developing a multivalent inhibitor that can effectively overcome the Velcro-like attachment. While this is no easy task, it is likely that glycobiological analyses will make significant contributions toward resolving the mechanisms of attachment of vector-borne pathogens to their invertebrate hosts.
Acknowledgments
Due to the limited number of references permitted, we were unable to include many excellent articles, and we apologize to these authors. The interested reader may want to consult references 2, 29, 31, 33, 49, 53, 58, 70, 86, 93-95, 98, 99, 102, 103, 112, 114, 115, and 120, which cover salient topics that are beyond the scope of this review. We thank Abdu Azad, Jose C. Garcia-Garcia, Stephen Higgs, Rebekah Kent, and Patricia Strickler-Dinglasan for helpful comments on the manuscript.
Editor: J. B. Kaper
REFERENCES
- 1.Abraham, E. G., S. Islam, P. Srinivasan, A. K. Ghosh, J. G. Valenzuela, J. M. Ribeiro, F. C. Kafatos, G. Dimopoulos, and M. Jacobs-Lorena. 2004. Analysis of the Plasmodium and Anopheles transcriptional repertoire during ookinete development and midgut invasion. J. Biol. Chem. 279:5573-5580. [DOI] [PubMed] [Google Scholar]
- 2.Akhouri, R. R., A. Bhattacharyya, P. Pattnaik, P. Malhotra, and A. Sharma. 2004. Structural and functional dissection of the adhesive domains of Plasmodium falciparum thrombospondin-related anonymous protein (TRAP). Biochem. J. 379:815-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backenson, P. B., J. L. Coleman, and J. L. Benach. 1995. Borrelia burgdorferi shows specificity of binding to glycosphingolipids. Infect. Immun. 63:2811-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks, D. J., J. L. Jurat-Fuentes, D. H. Dean, and M. J. Adang. 2001. Bacillus thuringiensis Cry1Ac and Cry1Fa delta-endotoxin binding to a novel 110 kDa aminopeptidase in Heliothis virescens is not N-acetylgalactosamine mediated. Insect Biochem. Mol. Biol. 31:909-918. [DOI] [PubMed] [Google Scholar]
- 5.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 6.Beverley, S. M., and S. J. Turco. 1998. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 6:35-40. [DOI] [PubMed] [Google Scholar]
- 7.Blouin, E. F., J. T. Saliki, J. de la Fuente, J. C. Garcia-Garcia, and K. M. Kocan. 2003. Antibodies to Anaplasma marginale major surface proteins 1a and 1b inhibit infectivity for cultured tick cells. Vet. Parasitol. 111:247-260. [DOI] [PubMed] [Google Scholar]
- 8.Brewer, C. F., M. C. Miceli, and L. G. Baum. 2002. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 12:616-623. [DOI] [PubMed] [Google Scholar]
- 9.Butcher, B. A., S. J. Turco, B. A. Hilty, P. F. Pimenta, M. Panunzio, and D. L. Sacks. 1996. Deficiency in β1,3-galactosyltransferase of a Leishmania major lipophosphoglycan mutant adversely influences the Leishmania-sand fly interaction. J. Biol. Chem. 271:20573-20579. [DOI] [PubMed] [Google Scholar]
- 10.Cambi, A., F. de Lange, N. M. van Maarseveen, M. Nijhuis, B. Joosten, E. M. van Dijk, B. I. de Bakker, J. A. Fransen, P. H. Bovee-Geurts, F. N. van Leeuwen, N. F. Van Hulst, and C. G. Figdor. 2004. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J. Cell Biol. 164:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlyon, J. A., M. Akkoyunlu, L. Xia, T. Yago, T. Wang, R. D. Cummings, R. P. McEver, and E. Fikrig. 2003. Murine neutrophils require α1,3-fucosylation but not PSGL-1 for productive infection with Anaplasma phagocytophilum. Blood 102:3387-3395. [DOI] [PubMed] [Google Scholar]
- 12.Cerami, C., U. Frevert, P. Sinnis, B. Takacs, P. Clavijo, M. J. Santos, and V. Nussenzweig. 1992. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70:1021-1033. [DOI] [PubMed] [Google Scholar]
- 13.Cerami, C., F. Kwakye-Berko, and V. Nussenzweig. 1992. Binding of malarial circumsporozoite protein to sulfatides [Gal(3-SO4)β1-Cer] and cholesterol-3-sulfate and its dependence on disulfide bond formation between cysteines in region II. Mol. Biochem. Parasitol. 54:1-12. [DOI] [PubMed] [Google Scholar]
- 14.Chandra, M., M. Liniger, L. Tetley, I. Roditi, and J. D. Barry. 2004. TsetseEP, a gut protein from the tsetse Glossina morsitans, is related to a major surface glycoprotein of trypanosomes transmitted by the fly and to the products of a Drosophila gene family. Insect Biochem. Mol. Biol. 34:1163-1173. [DOI] [PubMed] [Google Scholar]
- 15.Chen, C., and P. F. Billingsley. 1999. Detection and characterization of a mannan-binding lectin from the mosquito, Anopheles stephensi (Liston). Eur. J. Biochem. 263:360-366. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 17.Chu, J. J., and M. L. Ng. 2003. Characterization of a 105-kDa plasma membrane associated glycoprotein that is involved in West Nile virus binding and infection. Virology 312:458-469. [DOI] [PubMed] [Google Scholar]
- 18.Coleman, J. L., E. J. Roemer, and J. L. Benach. 1999. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect. Immun. 67:3929-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale, C., and S. C. Welburn. 2001. The endosymbionts of tsetse flies: manipulating host-parasite interactions. Int. J. Parasitol. 31:628-631. [DOI] [PubMed] [Google Scholar]
- 20.Delacoux, F., A. Fichard, S. Cogne, R. Garrone, and F. Ruggiero. 2000. Unraveling the amino acid sequence crucial for heparin binding to collagen V. J. Biol. Chem. 275:29377-29382. [DOI] [PubMed] [Google Scholar]
- 21.de la Fuente, J., J. C. Garcia-Garcia, A. F. Barbet, E. F. Blouin, and K. M. Kocan. 2004. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet. Microbiol. 98:313-322. [DOI] [PubMed] [Google Scholar]
- 22.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan. 2003. Characterization of the functional domain of major surface protein 1a involved in adhesion of the rickettsia Anaplasma marginale to host cells. Vet. Microbiol. 91:265-283. [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan. 2001. Differential adhesion of major surface proteins 1a and 1b of the ehrlichial cattle pathogen Anaplasma marginale to bovine erythrocytes and tick cells. Int. J. Parasitol. 31:145-153. [DOI] [PubMed] [Google Scholar]
- 24.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, B. R. McEwen, D. Clawson, and K. M. Kocan. 2001. Major surface protein 1a effects tick infection and transmission of Anaplasma marginale. Int. J. Parasitol. 31:1705-1714. [DOI] [PubMed] [Google Scholar]
- 25.Dessens, J. T., A. L. Beetsma, G. Dimopoulos, K. Wengelnik, A. Crisanti, F. C. Kafatos, and R. E. Sinden. 1999. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 18:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimopoulos, G., A. Richman, H. M. Muller, and F. C. Kafatos. 1997. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc. Natl. Acad. Sci. USA 94:11508-11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinglasan, R. R., I. Fields, M. Shahabuddin, A. F. Azad, and J. B. Sacci, Jr. 2003. Monoclonal antibody MG96 completely blocks Plasmodium yoelii development in Anopheles stephensi. Infect. Immun 71:6995-7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinglasan, R. R., J. G. Valenzuela, and A. F. Azad. 2005. Sugar epitopes as potential universal disease transmission blocking targets. Insect Biochem. Mol. Biol. 35:1-10. [DOI] [PubMed] [Google Scholar]
- 29.Dodd, R. B., and K. Drickamer. 2001. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology 11:71R-79R. [DOI] [PubMed] [Google Scholar]
- 30.Dorward, D. W., T. G. Schwan, and C. F. Garon. 1991. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J. Clin. Microbiol. 29:1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elgavish, S., and B. Shaanan. 1997. Lectin-carbohydrate interactions: different folds, common recognition principles. Trends Biochem. Sci. 22:462-467. [DOI] [PubMed] [Google Scholar]
- 32.Emsley, J., H. E. White, B. P. O'Hara, G. Oliva, N. Srinivasan, I. J. Tickle, T. L. Blundell, M. B. Pepys, and S. P. Wood. 1994. Structure of pentameric human serum amyloid P component. Nature 367:338-345. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 34.Fingerle, V., H. Laux, U. G. Munderloh, U. Schulte-Spechtel, and B. Wilske. 2000. Differential expression of outer surface proteins A and C by individual Borrelia burgdorferi in different genospecies. Med. Microbiol. Immunol. (Berlin) 189:59-66. [DOI] [PubMed] [Google Scholar]
- 35.Frevert, U. 1994. Malaria sporozoite-hepatocyte interactions. Exp. Parasitol. 79:206-210. [DOI] [PubMed] [Google Scholar]
- 36.Frevert, U., P. Sinnis, C. Cerami, W. Shreffler, B. Takacs, and V. Nussenzweig. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Garcia, J. C., J. de la Fuente, G. Bell-Eunice, E. F. Blouin, and K. M. Kocan. 2004. Glycosylation of Anaplasma marginale major surface protein 1a and its putative role in adhesion to tick cells. Infect. Immun. 72:3022-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaur, D., D. C. Mayer, and L. H. Miller. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413-1429. [DOI] [PubMed] [Google Scholar]
- 39.Germi, R., J. M. Crance, D. Garin, J. Guimet, H. Lortat-Jacob, R. W. Ruigrok, J. P. Zarski, and E. Drouet. 2002. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology 292:162-168. [DOI] [PubMed] [Google Scholar]
- 40.Grubhoffer, L., and F. Dusbabek. 1991. Lectin binding analysis of Argas polonicus tissue glycoproteins. Vet. Parasitol. 38:235-247. [DOI] [PubMed] [Google Scholar]
- 41.Grubhoffer, L., and L. Jindrak. 1998. Lectins and tick-pathogen interactions: a minireview. Folia Parasitol. (Prague) 45:9-13. [PubMed] [Google Scholar]
- 42.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 43.Hacker, J. K., and J. L. Hardy. 1997. Adsorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: a role for G1. Virology 235:40-47. [DOI] [PubMed] [Google Scholar]
- 44.Haddow, J. D., L. R. Haines, R. H. Gooding, R. W. Olafson, and T. W. Pearson. 2005. Identification of midgut proteins that are differentially expressed in trypanosome-susceptible and normal tsetse flies (Glossina morsitans morsitans). Insect Biochem. Mol. Biol. 35:425-433. [DOI] [PubMed] [Google Scholar]
- 45.Haines, L. R., A. M. Jackson, M. J. Lehane, J. M. Thomas, A. Y. Yamaguchi, J. D. Haddow, and T. W. Pearson. 2005. Increased expression of unusual EP repeat-containing proteins in the midgut of the tsetse fly (Glossina) after bacterial challenge. Insect Biochem. Mol. Biol. 35:413-423. [DOI] [PubMed] [Google Scholar]
- 46.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 47.Hulinska, D., P. Volf, and L. Grubhoffer. 1992. Characterization of Borrelia burgdorferi glycoconjugates and surface carbohydrates. Zentbl. Bakteriol. 276:473-480. [DOI] [PubMed] [Google Scholar]
- 48.Hung, J. J., M. T. Hsieh, M. J. Young, C. L. Kao, C. C. King, and W. Chang. 2004. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 78:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung, S. L., P. L. Lee, H. W. Chen, L. K. Chen, C. L. Kao, and C. C. King. 1999. Analysis of the steps involved in Dengue virus entry into host cells. Virology 257:156-167. [DOI] [PubMed] [Google Scholar]
- 50.Hwa, K. Y., and K. H. Khoo. 2000. Structural analysis of the asparagine-linked glycans from the procyclic Trypanosoma brucei and its glycosylation mutants resistant to concanavalin A killing. Mol. Biochem. Parasitol. 111:173-184. [DOI] [PubMed] [Google Scholar]
- 51.Ingram, G. A., J. East, and D. H. Molyneux. 1984. Naturally occurring agglutinins against trypanosomatid flagellates in the haemolymph of insects. Parasitology 89:435-451. [DOI] [PubMed] [Google Scholar]
- 52.Isaacs, R. D. 1994. Borrelia burgdorferi bind to epithelial cell proteoglycans. J. Clin. Investig. 93:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson, A. J., F. Guirakhoo, and J. T. Roehrig. 1994. The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203:241-249. [DOI] [PubMed] [Google Scholar]
- 54.Kamhawi, S., M. Ramalho-Ortigao, V. M. Pham, S. Kumar, P. G. Lawyer, S. J. Turco, C. Barillas-Mury, D. L. Sacks, and J. G. Valenzuela. 2004. A role for insect galectins in parasite survival. Cell 119:329-341. [DOI] [PubMed] [Google Scholar]
- 55.Kamwendo, S. P., G. A. Ingram, F. L. Musisi, and D. H. Molyneux. 1993. Haemagglutinin activity in tick (Rhipicephalus appendiculatus) haemolymph and extracts of gut and salivary gland. Ann. Trop. Med. Parasitol. 87:303-305. [DOI] [PubMed] [Google Scholar]
- 56.Kamwendo, S. P., F. L. Musisi, A. J. Trees, and D. H. Molyneux. 1995. Effects of haemagglutination (lectin) inhibitory sugars on Theileria parva infection in Rhipicephalus appendiculatus. Int. J. Parasitol. 25:29-35. [DOI] [PubMed] [Google Scholar]
- 57.Karlsson, K. A. 1989. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58:309-350. [DOI] [PubMed] [Google Scholar]
- 58.Karlsson, K. A. 1995. Microbial recognition of target-cell glycoconjugates. Curr. Opin. Struct. Biol. 5:622-635. [DOI] [PubMed] [Google Scholar]
- 59.Kim, Y. S., J. H. Ryu, S. J. Han, K. H. Choi, K. B. Nam, I. H. Jang, B. Lemaitre, P. T. Brey, and W. J. Lee. 2000. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and β-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J. Biol. Chem. 275:32721-32727. [DOI] [PubMed] [Google Scholar]
- 60.Klimstra, W. B., E. M. Nangle, M. S. Smith, A. D. Yurochko, and K. D. Ryman. 2003. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 77:12022-12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kovar, V., P. Kopacek, and L. Grubhoffer. 2000. Isolation and characterization of Dorin M, a lectin from plasma of the soft tick Ornithodoros moubata. Insect Biochem. Mol. Biol. 30:195-205. [DOI] [PubMed] [Google Scholar]
- 62.Kroschewski, H., S. L. Allison, F. X. Heinz, and C. W. Mandl. 2003. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology 308:92-100. [DOI] [PubMed] [Google Scholar]
- 63.Kuhn, K. H., J. Uhlir, and L. Grubhoffer. 1996. Ultrastructural localization of a sialic acid-specific hemolymph lectin in the hemocytes and other tissues of the hard tick Ixodes ricinus (Acari; Chelicerata). Parasitol. Res. 82:215-221. [DOI] [PubMed] [Google Scholar]
- 64.Lehane, M. J., S. Aksoy, W. Gibson, A. Kerhornou, M. Berriman, J. Hamilton, M. B. Soares, M. F. Bonaldo, S. Lehane, and N. Hall. 2003. Adult midgut expressed sequence tags from the tsetse fly Glossina morsitans morsitans and expression analysis of putative immune response genes. Genome Biol. 4:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li, F., T. J. Templeton, V. Popov, J. E. Comer, T. Tsuboi, M. Torii, and J. M. Vinetz. 2004. Plasmodium ookinete-secreted proteins secreted through a common micronemal pathway are targets of blocking malaria transmission. J. Biol. Chem. 279:26635-26644. [DOI] [PubMed] [Google Scholar]
- 66.Martinez-Barragan, J. J., and R. M. del Angel. 2001. Identification of a putative coreceptor on Vero cells that participates in dengue 4 virus infection. J. Virol. 75:7818-7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matuschewski, K., A. C. Nunes, V. Nussenzweig, and R. Menard. 2002. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 21:1597-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McBride, J. W., X. J. Yu, and D. H. Walker. 2000. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene 254:245-252. [DOI] [PubMed] [Google Scholar]
- 69.McCormick, C. J., D. S. Tuckwell, A. Crisanti, M. J. Humphries, and M. R. Hollingdale. 1999. Identification of heparin as a ligand for the A-domain of Plasmodium falciparum thrombospondin-related adhesion protein. Mol. Biochem. Parasitol. 100:111-124. [DOI] [PubMed] [Google Scholar]
- 70.McGreal, E. P., L. Martinez-Pomares, and S. Gordon. 2004. Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol. Immunol. 41:1109-1121. [DOI] [PubMed] [Google Scholar]
- 71.Mehlert, A., N. Zitzmann, J. M. Richardson, A. Treumann, and M. A. Ferguson. 1998. The glycosylation of the variant surface glycoproteins and procyclic acidic repetitive proteins of Trypanosoma brucei. Mol. Biochem. Parasitol. 91:145-152. [DOI] [PubMed] [Google Scholar]
- 72.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2005. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 79:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller, H. M., I. Reckmann, M. R. Hollingdale, H. Bujard, K. J. Robson, and A. Crisanti. 1993. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 12:2881-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munoz, M. L., A. Cisneros, J. Cruz, P. Das, R. Tovar, and A. Ortega. 1998. Putative dengue virus receptors from mosquito cells. FEMS Microbiol. Lett. 168:251-258. [DOI] [PubMed] [Google Scholar]
- 75.Mutharia, L. M., and T. W. Pearson. 1987. Surface carbohydrates of procyclic forms of African trypanosomes studied using fluorescence activated cell sorter analysis and agglutination with lectins. Mol. Biochem. Parasitol. 23:165-172. [DOI] [PubMed] [Google Scholar]
- 76.Myles, K. M., D. J. Pierro, and K. E. Olson. 2003. Deletions in the putative cell receptor-binding domain of Sindbis virus strain MRE16 E2 glycoprotein reduce midgut infectivity in Aedes aegypti. J. Virol. 77:8872-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nussenzweig, I., and I. Menard. 2000. Analysis of a malaria sporozoite protein family required for gliding motility and cell invasion. Trends Microbiol. 8:94-97. [DOI] [PubMed] [Google Scholar]
- 79.Osta, M. A., G. K. Christophides, and F. C. Kafatos. 2004. Effects of mosquito genes on Plasmodium development. Science 303:2030-2032. [DOI] [PubMed] [Google Scholar]
- 80.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pal, U., X. Li, T. Wang, R. R. Montgomery, N. Ramamoorthi, A. M. Desilva, F. Bao, X. Yang, M. Pypaert, D. Pradhan, F. S. Kantor, S. Telford, J. F. Anderson, and E. Fikrig. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457-468. [DOI] [PubMed] [Google Scholar]
- 82.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pancake, S. J., G. D. Holt, S. Mellouk, and S. L. Hoffman. 1993. Malaria sporozoites and circumsporozoite protein bind sulfated glycans: carbohydrate binding properties predicted from sequence homologies with other lectins. Parasitologia 35(Suppl.):77-80. [PubMed] [Google Scholar]
- 84.Parveen, N., M. Caimano, J. D. Radolf, and J. M. Leong. 2003. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol. Microbiol. 47:1433-1444. [DOI] [PubMed] [Google Scholar]
- 85.Parveen, N., and J. M. Leong. 2000. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:1220-1234. [DOI] [PubMed] [Google Scholar]
- 86.Pearson, A. M. 1996. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 8:20-28. [DOI] [PubMed] [Google Scholar]
- 87.Pearson, T. W., R. P. Beecroft, S. C. Welburn, S. Ruepp, I. Roditi, K. Y. Hwa, P. T. Englund, C. W. Wells, and N. B. Murphy. 2000. The major cell surface glycoprotein procyclin is a receptor for induction of a novel form of cell death in African trypanosomes in vitro. Mol. Biochem. Parasitol. 111:333-349. [DOI] [PubMed] [Google Scholar]
- 88.Pelletier, I., T. Hashidate, T. Urashima, N. Nishi, T. Nakamura, M. Futai, Y. Arata, K. Kasai, M. Hirashima, J. Hirabayashi, and S. Sato. 2003. Specific recognition of Leishmania major poly-β-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 278:22223-22230. [DOI] [PubMed] [Google Scholar]
- 89.Pelletier, I., and S. Sato. 2002. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 277:17663-17670. [DOI] [PubMed] [Google Scholar]
- 90.Pimenta, P. F., E. M. Saraiva, E. Rowton, G. B. Modi, L. A. Garraway, S. M. Beverley, S. J. Turco, and D. L. Sacks. 1994. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc. Natl. Acad. Sci. USA 91:9155-9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pimenta, P. F., S. J. Turco, M. J. McConville, P. G. Lawyer, P. V. Perkins, and D. L. Sacks. 1992. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science 256:1812-1815. [DOI] [PubMed] [Google Scholar]
- 92.Rey, F. A. 2003. Dengue virus envelope glycoprotein structure: new insight into its interactions during viral entry. Proc. Natl. Acad. Sci. USA 100:6899-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rini, J. M. 1995. Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 24:551-577. [DOI] [PubMed] [Google Scholar]
- 94.Roseman, S. 2001. Reflections on glycobiology. J. Biol. Chem. 276:41527-41542. [DOI] [PubMed] [Google Scholar]
- 95.Rostand, K. S., and J. D. Esko. 1997. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rudin, W., and H. Hecker. 1979. Functional morphology of the midgut of Aedes aegypti L. (Insecta, Diptera) during blood digestion. Cell Tissue Res. 200:193-203. [DOI] [PubMed] [Google Scholar]
- 97.Rudin, W., and H. Hecker. 1989. Lectin-binding sites in the midgut of the mosquitoes Anopheles stephensi Liston and Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 75:268-279. [DOI] [PubMed] [Google Scholar]
- 98.Sacks, D., and S. Kamhawi. 2001. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu. Rev. Microbiol. 55:453-483. [DOI] [PubMed] [Google Scholar]
- 99.Sacks, D. L. 2001. Leishmania-sand fly interactions controlling species-specific vector competence. Cell. Microbiol. 3:189-196. [DOI] [PubMed] [Google Scholar]
- 100.Salas-Benito, J. S., and R. M. del Angel. 1997. Identification of two surface proteins from C6/36 cells that bind dengue type 4 virus. J. Virol. 71:7246-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304:248-253. [DOI] [PubMed] [Google Scholar]
- 103.Sinden, R. E. 2002. Molecular interactions between Plasmodium and its insect vectors. Cell. Microbiol. 4:713-724. [DOI] [PubMed] [Google Scholar]
- 104.Sinnis, P., P. Clavijo, D. Fenyo, B. T. Chait, C. Cerami, and V. Nussenzweig. 1994. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J. Exp. Med. 180:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Templeton, T. J. 2004. Borrelia outer membrane surface proteins and transmission through the tick. J. Exp. Med. 199:603-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Templeton, T. J., D. C. Kaslow, and D. A. Fidock. 2000. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol. Microbiol. 36:1-9. [DOI] [PubMed] [Google Scholar]
- 107.Terra, W. R., C. Ferreria, B. P. Jordao, and R. J. Dillon. 1996. Compartmentalization of digestion, p. 153-194. In M. J. Lehane and P. F. Billingsley (ed.), The biology of the insect midgut. Chapman and Hall, London, United Kingdom.
- 108.Tonui, W. K., P. A. Mbati, C. O. Anjili, A. S. Orago, S. J. Turco, J. I. Githure, and D. K. Koech. 2001. Transmission blocking vaccine studies in leishmaniasis. II. Effect of immunisation using Leishmania major derived 63 kilodalton glycoprotein, lipophosphoglycan and whole parasite antigens on the course of L. major infection in BALB/c mice. East Afr. Med. J. 78:90-92. [PubMed] [Google Scholar]
- 109.Tonui, W. K., J. S. Mejia, L. Hochberg, M. L. Mbow, J. R. Ryan, A. S. Chan, S. K. Martin, and R. G. Titus. 2004. Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with L. major. Infect. Immun. 72:5654-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trottein, F., T. Triglia, and A. F. Cowman. 1995. Molecular cloning of a gene from Plasmodium falciparum that codes for a protein sharing motifs found in adhesive molecules from mammals and plasmodia. Mol. Biochem. Parasitol. 74:129-141. [DOI] [PubMed] [Google Scholar]
- 111.Trueman, H. E., J. D. Raine, L. Florens, J. T. Dessens, J. Mendoza, J. Johnson, C. C. Waller, I. Delrieu, A. A. Holders, J. Langhorne, D. J. Carucci, J. R. Yates III, and R. E. Sinden. 2004. Functional characterization of an LCCL-lectin domain containing protein family in Plasmodium berghei. J. Parasitol. 90:1062-1071. [DOI] [PubMed] [Google Scholar]
- 112.Vasta, G. R., H. Ahmed, and E. W. Odom. 2004. Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr. Opin. Struct. Biol. 14:617-630. [DOI] [PubMed] [Google Scholar]
- 113.Vickerman, K. 1973. The mode of attachment of Trypanosoma vivax in the proboscis of the tsetse fly Glossina fuscipes: an ultrastructural study of the epimastigote stage of the trypanosome. J. Protozool. 20:394-404. [DOI] [PubMed] [Google Scholar]
- 114.Vyas, N. K., M. N. Vyas, M. C. Chervenak, D. R. Bundle, B. M. Pinto, and F. A. Quiocho. 2003. Structural basis of peptide-carbohydrate mimicry in an antibody-combining site. Proc. Natl. Acad. Sci. USA 100:15023-15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wadstrom, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223-233. [DOI] [PubMed] [Google Scholar]
- 116.Wang, E., A. C. Brault, A. M. Powers, W. Kang, and S. C. Weaver. 2003. Glycosaminoglycan binding properties of natural Venezuelan equine encephalitis virus isolates. J. Virol. 77:1204-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang, S., R. He, and R. Anderson. 1999. PrM- and cell-binding domains of the dengue virus E protein. J. Virol. 73:2547-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Welburn, S. C., I. Maudlin, and D. H. Molyneux. 1994. Midgut lectin activity and sugar specificity in teneral and fed tsetse. Med. Vet. Entomol. 8:81-87. [DOI] [PubMed] [Google Scholar]
- 119.Wilkins, S., and P. F. Billingsley. 2001. Partial characterization of oligosaccharides expressed on midgut microvillar glycoproteins of the mosquito, Anopheles stephensi Liston. Insect Biochem. Mol. Biol. 31:937-948. [DOI] [PubMed] [Google Scholar]
- 120.Xu, X., Y. Dong, E. G. Abraham, A. Kocan, P. Srinivasan, A. K. Ghosh, R. E. Sinden, J. M. C. Ribeiro, M. Jacobs-Lorena, F. C. Kafatos, and G. Dimopoulos. 2005. Transcriptome analysis of Anopheles stephensi-Plasmodium berghei interactions. Mol. Biochem. Parasitol. 142:76-87. [DOI] [PubMed] [Google Scholar]
- 121.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yazi Mendoza, M., J. S. Salas-Benito, H. Lanz-Mendoza, S. Hernandez-Martinez, and R. M. del Angel. 2002. A putative receptor for dengue virus in mosquito tissues: localization of a 45-kDa glycoprotein. Am. J. Trop. Med. Hyg. 67:76-84. [DOI] [PubMed] [Google Scholar]
- 123.Yu, X. Q., and M. R. Kanost. 2004. Immulectin-2, a pattern recognition receptor that stimulates hemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev. Comp. Immunol. 28:891-900. [DOI] [PubMed] [Google Scholar]
- 124.Yuda, M., H. Sakaida, and Y. Chinzei. 1999. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J. Exp. Med. 190:1711-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuda, M., K. Yano, T. Tsuboi, M. Torii, and Y. Chinzei. 2001. von Willebrand factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol. Biochem. Parasitol. 116:65-72. [DOI] [PubMed] [Google Scholar]
- 126.Zambrano, M. C., A. A. Beklemisheva, A. V. Bryksin, S. A. Newman, and F. C. Cabello. 2004. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect. Immun. 72:3138-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zieler, H., C. F. Garon, E. R. Fischer, and M. Shahabuddin. 2000. A tubular network associated with the brush-border surface of the Aedes aegypti midgut: implications for pathogen transmission by mosquitoes. J. Exp. Biol. 203:1599-1611. [DOI] [PubMed] [Google Scholar]
- 128.Zieler, H., J. P. Nawrocki, and M. Shahabuddin. 1999. Plasmodium gallinaceum ookinetes adhere specifically to the midgut epithelium of Aedes aegypti by interaction with a carbohydrate ligand. J. Exp. Biol. 202:485-495. [DOI] [PubMed] [Google Scholar]