Abstract
To better understand the relationship between the surface polysaccharides of pulmonary pathogens and components of the lung innate immune system, we employed selected serotypes of Klebsiella pneumoniae expressing distinct capsular polysaccharides and/or O antigen in a murine model of K. pneumoniae infection. In addition, we examined the effect of surfactant protein D (SP-D) on the cytokine response of human monocyte-derived macrophages to these serotypes in vitro. Noncapsulated mannose-containing O3 serotypes (K50/n and K55/n), which react efficiently with SP-D in vitro, triggered high levels of interleukin-1β (IL-1β) and IL-6 production. In vivo, they were more efficiently cleared from the lungs of mice but not from macrophage-depleted mice. They also were more efficiently internalized by alveolar macrophages in vivo. In contrast, galactose-containing O1 serotypes (K2/n and K21a/n), which interact poorly with SP-D, exhibited significantly lower cytokine production and less efficient pulmonary clearance and were ineffectively internalized by alveolar macrophages. These findings are consistent with in vitro results showing that production of IL-1β and IL-6 mRNA and IL-6 protein by human macrophages exposed to mannose-bearing Klebsiella O serotypes is significantly increased by SP-D. Thus, survival of inhaled bacteria in the lung depends partially on the lipopolysaccharide structure of the bacteria and their interactions with innate immunity components. We speculate that an imbalance of host SP-D and therefore cytokine levels may result in high susceptibility of the host to the pathogen.
Host defense mechanisms of the innate, nonclonal, immune system serve as the principal pathway for effective elimination of pathogens from the lung (38). Impairment of innate immunity is probably responsible for nosocomial infections caused by pathogens such as Klebsiella pneumoniae, which is one of the most frequent gram-negative bacterial pathogens isolated from hospitalized patients with severe pneumonia (4, 17).
Alveolar macrophages (AM) and polymorphonuclear leukocytes (PMNs) are potent cellular components of lung innate immunity that mediate bacterial recognition and clearance (35). The recruitment and activation of inflammatory cells at a site of infection involve the orchestrated expression of leukocyte and vascular adhesion molecules, as well as the establishment of chemotactic gradients via the generation of chemokines and cytokines (27). The attachment of the pathogen to mannose receptors (MR) on phagocytic cells can trigger the production of chemokines and cytokines (1, 43). The lung collectins surfactant protein A (SP-A) and surfactant protein D (SP-D) are also important components of pulmonary innate immunity, which, like MR, contain C-type lectin domains (6, 15). Both collectins can act as opsonins to enhance the phagocytosis of bacteria by recognizing complementary carbohydrates on bacterial surfaces and receptors on macrophages.
We have studied the interactions of K. pneumoniae with SP-D, SP-A, and MR, three C-type lectins that are believed to contribute to pulmonary innate immunity (29). Two types of K. pneumoniae glycoconjugate structures are recognized by lung C-type lectins. One of these is the capsular polysaccharide (CPS), which is recognized by both SP-A and MR. Binding of SP-A and MR to K. pneumoniae is serotype dependent. Previous studies have shown that capsulated K. pneumoniae strains that containing Manα2/3Man or Rhaα2/3Rha sequences in their CPS are directly recognized by the macrophage MR or following opsonization with SP-A, which results in attachment, ingestion, and killing of the bacteria by the phagocytic cells (2, 18, 20). Capsulated Klebsiella strains that lack such sequences in their CPS are not recognized by either MR or SP-A. Significantly, the latter strains are isolated from patients with pneumonia at a significantly higher frequency than the capsular strains recognized by these C-type lectins are isolated (28). The lipopolysaccharide (LPS) is another cell wall glycoconjugate that can serve as a target for recognition by collectins. SP-D, which selectively interacts with the noncapsulated phase variant of K. pneumoniae, preferentially binds to the conserved core region of LPS and enhances phagocytosis by opsonic mechanisms (30).
The bacterial capsule is known to inhibit phagocytic clearance. However, it has been shown that noncapsulated K. pneumoniae adheres better than the capsulated organisms to respiratory epithelial cells (26). Thus, it is likely that most of the bacteria colonizing the upper respiratory tract are in the noncapsulated phase (29). Although there is currently no evidence for an in vivo capsule switch in the lung, the phase variation phenomenon with switching from capsulated variants to noncapsulated variants occurs in vitro (28). In addition, most urine isolates from patients with Klebsiella urinary tract infections are noncapsulated, whereas the blood isolates are capsulated. This strongly suggests that Klebsiella can undergo phase variation in vivo (26).
It has been suggested that SP-D provides early protection against noncapsulated phase variants of Klebsiella because it can recognize the conserved core oligosaccharide domain of the LPS. However, it was recently found that SP-D can also interact with specific O antigens and that the binding of SP-D to serovars (serotypes) expressing LPS with a mannose-rich O antigen is significantly greater than the binding to serovars expressing a galactose-rich O antigen (37).
The present study was undertaken to determine whether differences in O-antigen structure play a role in the pulmonary response to Klebsiella. For this purpose, we employed a murine model of lung infection and evaluated various O serotypes of noncapsulated K. pneumoniae with respect to bacterial survival of the bacteria and production of inflammatory cytokines in vivo. We also examined the production of cytokines by macrophages challenged with the serotypes in vitro. We found that Klebsiella strains that efficiently bind and agglutinate SP-D via their mannose-rich O antigens are less able to infect the lungs of mice, yet they trigger higher levels of cytokine expression, than strains expressing galactose-rich O antigens.
(This work was performed by Elena Kostina in partial fulfillment of the requirements for a Ph.D. degree from Sackler Faculty of Medicine, Tel Aviv University.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Capsulated parent strains for capsular serotypes of K2, K21a, K50, and K55 were obtained as previously described (30). Spontaneously noncapsulated phase variants of capsulated parent strains K2/n, K21a/n, K50/n, and K55/n were selected from nonmucoid segments of mucoid colonies as described elsewhere (26) to obtain noncapsulated derivatives expressing O3 antigen (K50/n and K55/n) and O1 antigen (K2/n and K21a/n), as determined previously (30). Noncapsulated K. pneumoniae strains KP79 (O1), KP82 (O1), KP85 (O1), KP88 (O3), KP91 (O3), and KP94 (O3) were isolated at the Department of Medical Microbiology and Virology, University of Kiel, Kiel, Germany.
Bacteria were grown overnight on nutrient agar, harvested by scraping the confluent growth, and resuspended at the desired density in phosphate-buffered saline (PBS). CFU counting on agar plates showed that an optical density at 700 nm of 1 was equivalent to approximately 2 × 109 and 5 × 109 CFU/ml for capsulated and noncapsulated phase variants, respectively. Stock suspensions with 50-fold-higher cell densities were stored at −70°C with 20% (vol/vol) glycerol. On the day of assay, bacteria were washed three times to remove the glycerol, diluted to obtain the desired density, and incubated on ice before use for various assays. The viability of the Klebsiella strains was checked by plating serial 1:10 dilutions on MacConkey agar plates after freezing and thawing before each experiment. We found that freezing and thawing did not affect the viability of the bacteria (100% viability) (22).
Animals.
Male BALB/c mice that were 6 to 8 weeks old were used throughout this study. All mice were housed in the animal facility at Tel Aviv University. Mice were treated according to the standards of the institutional animal care and use committee of Tel Aviv University.
CP treatment of mice.
BALB/c mice were inoculated intraperitoneally with 200 mg/kg of cyclophosphamide (CP). Animal weight and white blood cell counts were monitored before the procedure and every 24 h until 4 days after CP injection, when mice were inoculated with K. pneumoniae. The weight of mice decreased from 20 ± 1.2 g on the day of CP injection to 16 ± 1.5 g 4 days after CP injection. The white blood cell counts declined from 1.25 × 107 cells/ml for nontreated mice to 1 × 104 cells/ml at 4 days after CP injection.
Pulmonary infection with K. pneumoniae.
Each mouse was inoculated by intranasal instillation of 20 μl of a Klebsiella suspension (4 × 104 CFU of a noncapsulated strain per mouse or 4 × 105 CFU of a capsulated strain per mouse). Consistent with previously published work, differences in the host response to the strains were assessed by determining the number of CFU at 72 h. This time was needed for the bacteria to reach a critical mass that induced a detectable response and for cytokines to be produced. A larger inoculum of the capsulated strain was needed to achieve detectable levels of bacteria at this time. Because the challenge was not lethal, dissemination was not assessed.
Alveolar macrophage depletion with clodronate (Cl2MDP) liposomes.
Liposome-encapsulated dichlormethylene diphosphonate (Cl2MDP) was prepared as described previously (5). Cl2MDP was a gift from Roche Diagnostics GmbH, Mannheim, Germany.
To deplete alveolar macrophages, 50 μl of either clodronate- or PBS-treated liposomes was inoculated intranasally 48 h before administration of bacteria as described above. Clodronate reduced the number of alveolar macrophages from 4.04 × 105 ± 2.31 × 105 cells to 1.7 × 104 ± 2.7 × 104 cells.
Determination of viable bacterial counts in mouse lungs.
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine hydrochloride (6.25 mg/kg) (0.25 ml per mouse) and were sacrificed by cervical dislocation 72 h after K. pneumoniae inoculation. Lungs were aseptically removed and homogenized for up to 30 s in sterile glass tubes with 1 ml of sterile saline. The lung homogenate was vigorously agitated with a Vortex mixer to disrupt bacterial aggregates before the preparation was plated for CFU counting. The colony counts before addition of SP-D and after addition of SP-D and vortexing were the same. In previous studies this procedure was found to be effective for breaking up large bacterial aggregates (30). Serial 1:10 dilutions were spread on MacConkey agar plates (100 μl/plate) and incubated for 18 to 20 h at 37°C, and the numbers of viable bacterial CFU were determined. The data were expressed as log10 CFU per lung per mouse (means ± standard deviations).
Light microscopic assay of K. pneumoniae associated with alveolar macrophages.
Mice were inoculated intranasally as described above with 4 × 106 CFU/mouse of either an O1 strain or an O3 strain. Three mice were inoculated with each strain. Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine hydrochloride (6.25 mg/kg) (0.25 ml per mouse) 16 h after the bacterial challenge. The trachea of each mouse was exposed and intubated with a polyethylene catheter (21 gauge), and a bronchoalveolar lavage (BAL) was performed with 1 ml of Dulbecco's PBS without calcium and magnesium (Beit-HaEmek, Rehovot, Israel). Approximately 0.5 to 0.7 ml of lavage fluid was retrieved from each mouse. Next, the lavage fluid was centrifuged three times at 350 × g for 10 min to remove nonphagocytized bacteria from the AM. BAL cells were resuspended in Dulbecco's PBS and then centrifuged in a cytocentrifuge at 1,500 rpm for 10 min (Eppendorf 5416 centrifuge; Eppendorf, Hamburg, Germany). Slides were stained by the Hemacolor method (17) and were examined by light microscopy in order to determine the percentage of AM associated with five or more bacteria. Three people counted at least 100 cells on each slide.
Coating of Klebsiella by SP-D.
Human SP-D dodecamers were prepared as described previously (11), and bacteria were coated with SP-D as described previously (22). The preparations contained low levels of endotoxin (<50 pg/μg of protein), as measured by a chromogenic assay. Briefly, bacterial suspensions (1 × 108 CFU/ml) were prepared in PBS supplemented with 20 mM CaCl2. Equal volumes of a bacterial suspension and PBS containing 10 μg/ml of SP-D were incubated for 60 min at 37°C. The bacteria were then washed three times by centrifugation at 12,000 × g to remove unbound SP-D. The pellet was resuspended at the original density in PBS and maintained at 4°C before it was used in assays. In previous studies we showed that the mannose-rich O3 strains employed in the present study bind SP-D efficiently, while O1 strains bind SP-D poorly in vitro (37).
Cytokine mRNA determination in mouse splenocytes.
Spleens (three spleens per group) were aseptically removed 72 h after infection with Klebsiella. The spleen cells were prepared, counted, and used for RNA extraction with Tri Reagent (TR118; Molecular Research Center Inc., Cincinnati, OH). Reverse transcription (RT) and PCR were performed using a reverse transcription system kit (A3500; Promega Corporation, Madison, WI). DNA primers for the cytokines of interest were purchased from Biotechnology General, Rehovot, Israel. For interleukin-1β (IL-1β) the upstream primer was 5′-TTGACGGACCCCAAAAGATG-3′ and the downstream primer was 5′-AGAAGGTGCTCATGTCCTCA-3′. For IL-6 the upstream primer was 5′-GTTCTCTGGGAAATCGTGGA-3′ and the downstream primer was 5′-TGTACTCCAGGTAGCTATGG-3′. For β-actin the upstream primer was 5′-ATGGATGACGATATCGCT-3′ and the downstream primer was 5′-ATGAGGTAGTCTGTCAGGT-3′.
Amplified cDNA was subjected to electrophoresis in a 1.5% agarose gel containing 100 ng/ml ethidium bromide. Gels were viewed and photographed under UV irradiation as described previously (20), and cytokine mRNA expression was measured by the TINA 2.1g program. The results were expressed as ratios of the optical density of the cytokine to the optical density of β-actin (averages ± standard errors) calculated from three separate experiments.
Preparation of lung homogenates for cytokine analysis.
K. pneumoniae-inoculated mice were sacrificed after 72 h by intraperitoneal injection of ketamine (100 mg/kg) and xylazine hydrochloride (6.25 mg/kg) in PBS (0.25 ml per mouse), and whole lungs were harvested for assessment of the cytokine protein level. Prior to lung removal, the pulmonary vasculature was perfused with PBS containing 5 mM EDTA via the right ventricle. After removal, whole lungs were homogenized in 3 ml of lysis buffer containing 0.5% Triton X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl2, and 1 mM MgCl2 (pH 7.40) with a tissue homogenizer (Biospec Products, Inc.). The homogenates were incubated on ice for 30 min and then centrifuged at 1,400 × g for 10 min. The supernatants were collected, passed through a 0.45-μm-pore-size filter (Gelman Sciences, Ann Arbor, MI), and then stored at −20°C for assessment of cytokine levels (40).
Murine cytokine ELISA.
The IL-6 in lung homogenates of normal and infected mice was measured by an enzyme-linked immunosorbent assay (ELISA) (MM-900 kit; Endogen, Massachusetts) performed according to the manufacturer's instructions. Triplicate samples of undiluted and diluted (1:3, 1:9) cell-free supernatants were used. Plates were read at 450 to 650 nm with an ELISA reader, and the results were expressed as the means ± standard errors of the means for two separate experiments (three mice per group).
Harvesting of human monocytes and MoDM.
Human peripheral blood monocytes were obtained from the buffy coat of normal blood bank donors. The mononuclear fraction was separated on Ficoll-Hypaque (19), and adherent monocyte monolayers (5 × 106 cells/ml in a tissue culture flask with a surface area of 75 cm2) were reconstituted with RPMI-1640 supplemented with 100 μg/ml of streptomycin, 100 U/ml of penicillin, 300 μg/ml of glutamine, and 10% newborn bovine serum. Cultured cells were further incubated for 10 to 14 days in the presence of 100 U/ml of granulocyte-macrophage colony-stimulating factor (Behringwerke, Marburg, Germany) to obtain monocyte-derived macrophages (MoDM) and to promote MR expression (21).
Induction of cytokine mRNA expression by human monocytes and MoDM.
Monocytes and MoDM monolayers (107 cells per flask) were incubated for 30 min at 37°C with either 3 ml of medium containing 1 μg/ml of LPS (Escherichia coli O111:B4; Sigma-Aldrich, St. Louis, MO) or 3 ml of medium containing 108 CFU/ml of Klebsiella that was either not coated or precoated with SP-D (10 μg/ml). The LPS was reconstituted in sterile PBS by vigorous vortexing. Total RNA extraction was performed by the Tri Reagent method according the manufacturer's protocol (Molecular Research Center Inc., Cincinnati, OH).
RT-PCR analysis was performed using a two-step RT-PCR system (Promega Corporation, Madison, WI) with 1 μg of total RNA. The sense and antisense PCR primers were used for amplification of human cytokine cDNA (BioTechnology General Ltd., Rehovot, Israel). For IL-1α the upstream primer was 5′-ATGGCAGAAGTACCTAAGCTCGC-3′ and the downstream primer was 5′-ACACAAATTGCATGGTGAAGTCAGTT-3′. For IL-6 the upstream primer was 5′-ATGAACTCCTTCTCCACAAGCGC-3′ and the downstream primer was 5′-GAAGAGCCCTCAGGCTGGACTG-3′. For β-actin the upstream primer was 5′-ATGGATGATGATATCGCCGCG-3′ and the downstream primer was 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′. PCR products were determined as described above for mouse splenocytes. The results presented below are ratios of the optical density of cytokine to the optical density of β-actin (averages ± standard errors for three separate experiments).
Induction of IL-6 protein production by human monocytes and MoDM.
Monocytes and MoDM monolayers (107 cells per flask) were incubated with 3 ml of medium containing 1 μg/ml of LPS or with 3 ml of medium containing 108 CFU/ml of noncapsulated K. pneumoniae. After 30 min of incubation the cell cultures were washed with warm Hanks balanced salt solution supplemented with RPMI-1640 with 10% newborn bovine serum and incubated overnight at 37°C in the presence of 7% CO2. After incubation the supernatants were passed through a 0.45-μm filter and stored at −20°C.
An ELISA was used to measure IL-6 in the cell supernatants as described by the manufacturer (CG50384; Endogen, Massachusetts). Triplicate samples of undiluted and diluted (1:3, 1:9) cell-free supernatants were used. Plates were read at 450 to 650 nm with an ELISA reader, and the results were expressed as the means ± standard errors of the means for three separate experiments.
Statistical analysis.
All data obtained in vitro and in vivo were analyzed by using a one-way analysis of variance (ANOVA) test and Student's two-tailed t test. Results were considered to be significantly different if the P value was <0.05.
RESULTS
Survival of noncapsulated K. pneumoniae strains in mouse lungs.
Previous studies have shown that although SP-D binds to the conserved region of all LPS serotypes (core region), the affinity of the binding is considerably greater to serotype O3 LPS, which contains dimannose sequences, than to serotype O1 LPS, which lacks such sequences. Significantly, we demonstrated that five- to sevenfold-higher concentrations of SP-D were needed to aggregate the O1 strains than to aggregate the O3 strains (37). In this study we asked whether differences in SP-D binding determine the survival of Klebsiella in mouse lungs. Mice were infected intranasally with noncapsulated O3- and O1-bearing K. pneumoniae strains, and the lung bacterial load was measured after 3 days. The number of bacteria that reached the lung by the intranasal route was very small, and there was an initial growth phase of the bacteria (48 to 72 h). During this period it was not possible to measure the rate of infection accurately. Tanabe et al. (39), who also used intranasal infection with Klebsiella, found that the bacterial counts peaked 72 h after infection. The infection in mice resolved in 6 to 7 days, and the number of CFU after 3 days reflected the process that occurred shortly after bacterial challenge. The average mean level of recovery of five different O1-bearing strains from the lung was 143 CFU, whereas the level of recovery of five O3-bearing strains was 6.3 CFU, so there was at least a 20-fold difference in survival (P < 0.013) (Table 1). Notably, we did not detect significant neutrophil infiltration 3 days after infection, which is consistent with the marked predominance of macrophages in lavage samples at this time.
TABLE 1.
Effect of LPS O-antigen type of noncapsulated K. pneumoniae strains on survival in the lungs of micea
| O antigen | Strain | Log10 CFU per mouse (mean ± SD) |
|---|---|---|
| O1 | K2/n | 2.66 ± 0.1b |
| K21a/n | 3.6 ± 0.1b | |
| KP79 | 1.36 ± 0.4 | |
| KP82 | 3.2 ± 0.1b | |
| KP85 | 3.16 ± 0.3b | |
| O3 | K50/n | 1.5 ± 0.1b |
| K55/n | 1.7 ± 0.02b | |
| KP91 | 0.8b | |
| KP94 | 0.8 ± 0.7b | |
| KP88 | 0.8b |
Normal BALB/c mice were inoculated with 4 × 104 CFU/mouse of five O1-antigen-bearing Klebsiella strains (K2/n, K21a/n, KP79, KP82, KP85) and fiveO3-bearing Klebsiella strains (K50/n, K55/n, KP88, KP91, KP94). Mice were sacrificed 72 h after bacterial inoculation, the lungs were homogenized, and the CFU were counted. The results are the results of three different experiments (four animals per group).
Statistical differences (as determined by an ANOVA test) were found between O1 strains (K2/n, K21a/n, KP82, KP85) and each of the O3 strains (P < 0.013).
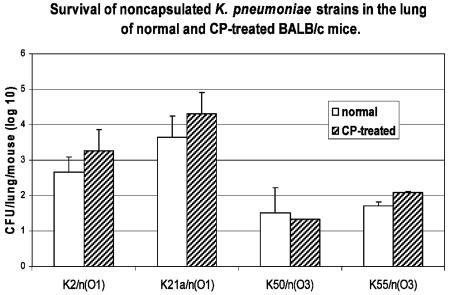
In order to examine the possible effect of PMN on the survival of the organisms in the lung in this mouse model of lung infection, we used cyclophosphamide, which causes depletion of PMN (14) but does not reduce the number of alveolar macrophages following infection (16). The viable CFU counts for K. pneumoniae O1 serotypes in the lung tissue of both CP-treated and nontreated mice were 1.5 to 2.5 orders of magnitude higher than the counts for O3 serotypes, irrespective of the capsular genetic background of the strains (P < 0.024). There was no significant difference in the pulmonary survival of K. pneumoniae between CP-treated mice and mice that were not treated (Fig. 1).
FIG. 1.
Survival of noncapsulated K. pneumoniae strains in the lungs of BALB/c mice (normal and immunosuppressed). Normal and CP-treated BALB/c mice were inoculated with 4 × 104 CFU/mouse of the K50/n, K55/n, K2/n, and K21a/n Klebsiella strains. Mice were sacrificed 72 h after bacterial inoculation, the lungs were homogenized, and the CFU were counted. The controls consisted of mock infections without bacteria. The results are expressed as means and standard deviations calculated from three different experiments (five animals per group). Statistical differences (as determined by an ANOVA test) between the O1 and O3 serotypes were found for normal (P < 0.024) and CP-treated (P < 0.02) mice.
Our previous studies indicated that macrophages recognize dimannose and dirhamnose structures in the capsular polysaccharides of Klebsiella via their MR (22). Thus, it is possible that the MR is responsible for the difference between the O1 and O3 serotypes due to removal of the capsulated variants that could arise from the noncapsulated variants during the infectious process. Therefore, we examined the fate of the capsulated parent strains of the noncapsulated variants. Mice were infected with strains that were recognized by the macrophage MR (K21a, K50) and a strain that was not recognized by the MR (K2). The results showed that the survival of the capsulated K2 strain was markedly (P < 0.001) greater than the survival of both the capsulated parent strains (K21a and K50) (63 ± 0.3 CFU for K2 and 1.2 ± 0.5 and 10 ± 2 for K21a and K50, respectively), suggesting that the survival of the capsulated parent strains was correlated with their interaction with macrophage MR and was different from the survival of their noncapsulated variants.
To establish the in vivo role of macrophages and their interactions with different K. pneumoniae serotypes in the eradication of Klebsiella from the lungs, alveolar macrophages were depleted by intranasal inoculation of clodronate (Cl2MDP)-containing liposomes. We then determined whether AM depletion altered lung bacterial clearance. Figure 2 shows that there was a 1-order-of-magnitude increase in the amount of O3-containing noncapsulated bacteria in the lungs of macrophage-depleted mice (2.57 ± 0.33 CFU/lung/mouse for clodronate-containing liposome-treated mice versus 1.5 ± 0.12 CFU/lung/mouse for PBS-containing liposome-treated mice for the K50/n Klebsiella strain [P = 0.001]; 2.38 ± 0.33 CFU/lung/mouse for clodronate-containing liposome-treated mice versus 1.73 ± 0.9 CFU/lung/mouse for PBS-containing liposome-treated mice for the K55/n Klebsiella strain [P = 0.04]). In contrast, the O1 bacterial infectivity was not affected by macrophage depletion.
FIG. 2.
Survival of noncapsulated K. pneumoniae strains in the lungs of alveolar macrophage-depleted BALB/c mice. To deplete alveolar macrophages, 50 μl of either clodronate- or PBS-containing liposomes was inoculated intranasally 48 h before bacterial administration. Clodronate-containing liposome-treated mice and PBS-containing liposome-treated BALB/c mice were inoculated with 4 × 104 CFU/mouse of the K50/n, K55/n, K2/n, and K21a/n Klebsiella strains. Mice were sacrificed 72 h after bacterial inoculation, the lungs were homogenized, and the CFU were counted. The controls consisted of mock infections without bacteria. The results are expressed as means and standards deviation calculated from two different experiments (sixanimals per group). Statistical differences (as determined by an ANOVA test) between the O1 and O3 serotypes were found for PBS-containing liposome-treated mice (P < 0.024). Also, statistical differences (as determined by the Student t test) were found between clodronate-containing liposome-treated mice and PBS-containing liposome-treated mice inoculated with the K50/n mannose-bearing O3-antigen K. pneumoniae strain (P = 0.001) and between clodronate-containing liposome-treated mice and PBS-containing liposome-treated mice inoculated with the K55/n mannose-bearing O3-antigen K. pneumoniae strain (P = 0.04). No differences were found between clodronate-containing liposome-treated mice and PBS-containing liposome-treated mice inoculated with O1-antigen K. pneumoniae strains.
Association of Klebsiella with AM in vivo.
In order to examine the role of O antigens in the interaction of bacteria with alveolar macrophages in vivo, mice were infected with either O1 antigen- or O3 antigen-bearing noncapsulated Klebsiella strains, and 16 h later BAL cells were harvested and monitored for bacterial association. No attempt was made to distinguish between attached and internalized organisms using our light microscopic assay. The results of the counting of six BAL samples for each O serotype showed that the percentage of BAL cells that had attached O3-antigen-bearing bacteria was significantly higher than the percentage of BAL cells that had attached O1-antigen-bearing bacteria (45% ± 6.5% and 23.7% ± 3.21%, respectively; P = 0.000374). Following intranasal infection in this mouse model, we found no histological evidence for acute lung inflammation (results not shown), and more than 95% of the cells in the BAL were macrophages.
Cytokine production by splenocytes and in the lungs of K. pneumoniae-infected mice.
In order to investigate whether the cytokine response to bacterial challenge is dependent on the O-antigen structure of the different K. pneumoniae strains, we determined the levels of IL-6 and IL-1β mRNA expressed by splenocytes and the levels of IL-6 protein in whole-lung extracts. The production of IL-1β mRNA by mouse splenocytes was significantly greater in mice infected with O3 serotypes than in mice infected with O1 serotypes (the optical density ratios were 0.25 ± 0.05 and 0.2 ± 0.025 for K2/n and K21a/n, respectively, compared with 0.37 ± 0.025 and 0.35 ± 0.05 for K50/n and K55/n, respectively; P < 0.04) (Fig. 3). The levels of IL-6 mRNA production by splenocytes following K. pneumoniae infection were dependent on both the O serotype, and the capsular genetic background of the noncapsulated derivatives. IL-6 mRNA expression was not detected in splenocytes of mice infected by the K2 strain (O1), and small amounts were found in the splenocytes of mice infected with the O1 serotype K21a strain (optical density ratio, 0.16 ± 0.05). Infection with O3 strains resulted in significantly higher mRNA expression than infection with O1 strains (optical density ratios, 0.22 ± 0.02 and 0.28 ± 0.01 for K50/n and K55/n, respectively) (P < 0.00023) (Fig. 3).
FIG. 3.
Production of IL-1β and IL-6 mRNA by splenocytes from mice infected with noncapsulated K. pneumoniae strains with different O-antigen structures: RT-PCR analysis of spleen cells (three spleens per group) from normal BALB/c mice infected with 4 × 104 CFU/mouse of noncapsulated Klebsiella strains. The results are expressed as average ratios of the optical density (OD) of cytokine to the optical density of β-actin (averages and standard errors). The data represent the results of three experiments, measured by the TINA computer program. The statistical differences for IL-1β mRNA production (as determined by an ANOVA test) between the O3-bearing strains are significantly greater than those between the O1-bearing strains (P < 0.04), and the statistical differences for IL-6 mRNA production (as determined by an ANOVA test) between the O3-bearing strains are significantly greater than those between the O1-bearing strains (P < 0.00023).
In order to evaluate the local responses to the various K. pneumoniae strains, IL-6 production was measured in whole lungs of Klebsiella-infected mice. The levels of IL-6 protein in the lungs of mice were significantly higher for O3 serotypes than for O1 serotypes (3.83 ± 0.05 and 5.34 ± 0.1 ng/ml for K50/n and K55/n, respectively, compared with 2.38 ± 0.05 and 1.85 ± 0.2 ng/ml for K2/n and K21a/n, respectively) (P < 0.0012) (Fig. 4). The cytokine response in vivo was measured 72 h after infection. As indicated in Materials and Methods, this time was needed for the bacteria to reach a critical mass which induced a detectable response and for cytokines to be produced. A similar time was reported by Tanabe and coworkers to be optimal for measuring tumor necrosis factor alpha production in the lungs of mice following intranasal infection with K. pneumoniae (39).
FIG. 4.
Production of IL-6 protein by lung tissue from mice infected with noncapsulated K. pneumoniae strains with different O-antigen structures as measured by a specific sandwich ELISA in the lungs of BALB/c mice (lungs of two mice per group). The data represent the results of three separate experiments. The controls consisted of mock infections without bacteria. The statistical differences for IL-6 protein production (as determined by an ANOVA test) between the O3-bearing strains are significantly greater than the differences between the O1-bearing strains (P < 0.0012).
Effect of SP-D and O antigens on cytokine mRNA expression and protein production by human macrophages exposed to noncapsulated K. pneumoniae strains.
In addition to the in vivo and in vitro studies with mouse macrophages, we examined cytokine production in human MoDM. SP-D-coated O3 serotype strains triggered higher levels of IL-1β mRNA than noncoated bacteria (the optical density ratios were 4.95 ± 0.03 and 3 ± 0.01 for macrophages exposed to noncoated strains K50/n and K55/n, respectively, compared with 8.8 ± 0.1 and 4.8 ± 0.08 for macrophages exposed to SP-D-coated strains K50/n and K55/n, respectively; P < 0.00004) (Fig. 5).
FIG. 5.
Expression of IL-1β mRNA by human MoDM triggered by SP-D-coated noncapsulated K. pneumoniae. Noncapsulated K. pneumoniae strains (108 CFU/ml) were incubated with human macrophages with and without precoating with human SP-D (10 μg/ml). Cytokine mRNA expression was analyzed by RT-PCR. The results are expressed as the ratios of the optical density (OD) of cytokine to the optical density of β-actin (averages and standard errors of three experiments). Statistical differences (as determined by Student's two-tailed t test) were found for IL-1β mRNA production by MoDM exposed to the K50/n (P = 0.00004) and K55/n (P = 0.000025) O3-bearing K. pneumoniae strains with SP-D and for IL-1β mRNA production by MoDM exposed to the K50/n and K55/n O3-bearing K. pneumoniae strains without SP-D.
A similar pattern was observed for IL-6 mRNA expression (the optical density ratios were 1.9 ± 0.02 and 1.8 ± 0.005 for macrophages exposed to noncoated strains K50/n and K55/n, respectively, compared with 2.6 ± 0.05 and 2.7 ± 0.01 for macrophages exposed to SP-D-coated strains K50/n and K55/n, respectively; P < 0.00038) (Fig. 6). In contrast, the expression of IL-1β mRNA by macrophages exposed to O1-bearing bacteria coated with SP-D was not significantly different from the expression by cells exposed to noncoated bacteria (Fig. 5), while the expression of IL-6 mRNA was significantly suppressed (Fig. 6). The production of IL-6 protein was also significantly suppressed by O1 bacteria treated with SP-D. In contrast, IL-6 product was significantly increased (P < 0.0134) by SP-D-treated O3 bacteria compared to the results obtained when nontreated O3 bacteria were used (Fig. 7).
FIG. 6.
Expression of IL-6 mRNA by human MoDM triggered by SP-D-coated noncapsulated K. pneumoniae. Noncapsulated K. pneumoniae strains (108 CFU/ml) were incubated with human macrophages with and without precoating with human SP-D (10 μg/ml). Cytokine mRNA expression was analyzed by RT-PCR. The results are expressed ratios of the optical density (OD) of cytokine to the optical density of β-actin (averages and standard errors of three experiments). Statistical differences (as determined by Student's two-tailed t test) were found for IL-6 mRNA production by MoDM exposed to the K50/n O3-bearing K. pneumoniae strain with and without SP-D (P = 0.035) and for IL-6 mRNA production by MoDM exposed to the K55/n O3-bearing K. pneumoniae strain with and without SP-D (P = 0.00038).
FIG. 7.
IL-6 protein production by human MoDM triggered by SP-D-coated noncapsulated K. pneumoniae. Noncapsulated K. pneumoniae strains (108 CFU/ml) were incubated with human macrophages with and without precoating with human SP-D (10 μg/ml). Cytokine protein production was measured by a specific sandwich ELISA. The results are expressed as averages and standard errors of three separate experiments. Statistical differences (as determined by Student's two-tailed t test) were found for IL-6 protein production by MoDM exposed to the K50/n O3-bearing K. pneumoniae strain (P = 0.0037) and for IL-6 protein production by MoDM exposed to the K55/n O3-bearing K. pneumoniae strain (P = 0.0134).
DISCUSSION
K. pneumoniae can undergo phase variation between capsulated and noncapsulated phenotypes (26). It has been hypothesized that noncapsulated organisms preferentially adhere to epithelial cells and colonize the upper respiratory tract (36). Thus, noncapsulated variants are likely to be the first organisms to enter the lung. If the noncapsulated bacteria escape efficient recognition, they can multiply in the lung and shift to the capsulated phase.
Previous studies have shown that noncapsulated K. pneumoniae strains expressing LPS structures whose O antigen is rich in mannose residues, such as O3, bind better to SP-D and are more efficiently aggregated by SP-D than strains expressing O antigens lacking these structures, such as the galactose-rich O1 antigen (37). Furthermore, SP-D promotes the killing of K. pneumoniae by macrophages in vitro (30). In previous studies we found that the mannose receptor of macrophages interacts poorly with noncapsulated O1 or O3 strains compared to the interaction with capsulated strains bearing dimannose structures in their capsular polysaccharides, suggesting that the mannose structures on O3 LPS are different from those on the capsular polysaccharides (22, 29).
In view of these observations, we examined the correlation between SP-D binding by various noncapsulated variants of Klebsiella and their abilities to cause lung infection in a mouse model. Significantly higher numbers of noncapsulated O1-bearing strains K2/n and K21a/n, which were not recognized by the SP-D, than of the noncapsulated K50/n and K55/n O3-mannose-bearing strains were recovered from the lungs of infected mice. These findings are also consistent with epidemiological studies showing that serotypes lacking mannose sequences in their CPS and O1-bearing Klebsiella serotypes are the most common serotypes found among clinical isolates from pneumonia patients (10). A possible explanation for the serotype-dependent differences is the interaction of the bacteria with SP-D, with the resulting effects on bacterial agglutination and phagocytosis by alveolar macrophages (29). Recently, SP-D was also found to inhibit growth of some gram-negative bacteria by altering the permeability of the outer membrane (42).
The recognition and phagocytosis of bacteria by macrophages at the initial stage of infection and the rate of bacterial proliferation are key elements in the overall survival of the bacteria. Differential interaction and opsonization by SP-D of O1 and O3 serotypes may affect their clearance by AM and thus be partially responsible for the differences in infectivity. This was tested by infection of AM-depleted mice and association of bacteria with AM in BAL fluid of Klebsiella-infected mice.
Eradication of O3 mannose-containing serotypes was attenuated in macrophage-depleted mice, while clearance of serotypes lacking O1 mannose was not changed. Our proposed explanation for this is that O1 serotypes are not efficiently opsonized by SP-D and thus are ineffectively cleared by macrophages. Accordingly, removal of macrophages did not affect the infectivity of the O1 bacteria. By contrast, O3 serotypes are efficiently opsonized by SP-D, which permits efficient internalization by macrophages. Removal of macrophages thus impaired O3 clearance in the mice. It was also observed that the association of O1-bearing K. pneumoniae with lung AM was significantly less than the association of O3 bearing bacteria. We therefore suggest that impaired clearance of the O1 serotypes results in part from low opsonization by SP-D and removal by alveolar macrophages.
In order to examine the effect of PMN on the survival of the organisms in the lung in this mouse model of lung infection, we used cyclophosphamide, which causes depletion of PMN (14). It was found that PMN play a minor role in the eradication of Klebsiella from the lungs. Cyclophosphamide treatment that reduced PMN blood levels by 1,000-fold did not have a significant effect on the survival of either O1 or O3 bacteria in the lungs (Fig. 1). In addition, only minute amounts of PMN were found by histological examination and in BAL fluid of infected mice.
Although other C-type lectins, such as SP-A and macrophage MR, could play a role in the eradication of noncapsulated bacteria, their involvement seems less likely. First, SP-A and macrophage MR recognize the same capsular serotypes, and noncapsulated variants interact poorly with these C-type lectins (22, 29). Second, when the K2 and K21a strains, which have the same O1 antigen, are in the capsulated form, they express different capsular polysaccharide structures, which influence their clearance from the lung. In particular, the K21a strain capsule is recognized by macrophage MR and is opsonized by SP-A (29). In contrast, there is no significant difference in the lung clearance of the noncapsulated variants of these strains, which have the same O antigen. Nevertheless, contributions by MR and SP-A can be excluded only by using appropriate knockout models.
Previous studies have shown that SP-D can efficiently interact with mannose-containing glycoconjugates (23, 33). These molecules include the mannose oligosaccharides of influenza A virus hemagglutinin (12), commercial preparations of yeast mannan (24), and the GpA glycoprotein of Pneumocystis carinii (25). Significantly, all the repeating units of the O1 antigen lack mannose but contain galactose (32), a sugar that reacts very weakly with SP-D (37).
Regulation of cytokine production by inflammatory cells (e.g., alveolar macrophages) is now considered a critical determinant of the innate immune response to microorganisms (3, 47). The kinetics and synthesis of pro- and anti-inflammatory cytokine production have been evaluated previously with a K. pneumoniae murine pneumonia model (44, 45). IL-1β and IL-6 are typical examples of multifunctional cytokines involved in the regulation of hematopoiesis and inflammation. IL-1β plays an important role in inflammatory processes and induces a wide range of hematological and metabolic responses (8). IL-1 can augment the capacity of mononuclear phagocytes to produce other inflammatory mediators, such as prostaglandins, and IL-6. A clinical study previously showed that there was localized production of IL-1β, IL-6, and tumor necrosis factor alpha in infected lungs of patients with community-acquired pneumonia (7). In this regard, we observed that noncapsulated K. pneumoniae strains with O3 mannose-bearing antigen triggered high levels of IL-1β and IL-6 mRNA expression in splenocytes and resulted in high levels of IL-6 protein in the lungs of infected mice, while serotypes with the O1 antigen lacking mannose sequences did not.
In addition to the in vivo studies with mice we also examined the ability of O1 and O3 bacteria to stimulate human mononuclear phagocytes and the role of SP-D in this stimulation. In vitro experiments indicated that coating O3 mannose-containing serotypes (K50/n and K55/n) with SP-D significantly augmented the mRNA and protein IL-6 responses, as well as the mRNA IL-1β responses in human MoDM. Production of IL-6, but not production of IL-1β, was significantly suppressed by O1 SP-D-treated bacteria compared to the production in nontreated bacteria. In the absence of SP-D there were small differences between the strains with regard to IL-1β and IL-6 induction, suggesting that the linear mannose residues of the repeating units of the O3 antigen do not influence interactions with MR-bearing macrophages, as was also found in our previous study (22). The findings are consistent with the in vivo results showing that IL-6 expression by alveolar macrophages of mice decreased following infection with O1 bacteria but increased following infection with O3 bacteria. At present, it is difficult to explain the downregulation of IL-6 production in human MoDM exposed to SP-D-coated O1-bearing bacteria (K2/n and K21a/n). It is possible that small amounts of SP-D presented to macrophages by O1 serotypes downregulate their capacity to produce cytokines, which is consistent with the suggestion that lung SP-D can block proinflammatory mediator production (9).
The correlation between the survival of Klebsiella in mouse lungs and cytokine production by SP-D-treated bacteria is interesting. SP-D-coated O3-bearing bacteria, which trigger enhanced IL-1β and IL-6 production, were also eradicated faster from the lungs of infected mice. In contrast, noncapsulated O1 serotypes (K2/n and K21a/n), which are significantly more virulent in the murine pneumonia model, triggered the same or lower IL-1β and IL-6 production in vitro when they were coated with SP-D. We suggest that this phenomenon depends on opsonization of noncapsulated O3 serotypes by SP-D, which results in faster elimination of the bacteria by macrophages and enhanced proinflammatory cytokine production during the rapid clearance of the inoculated bacteria from the lung. This speculation was confirmed by the in vivo results showing that the differences between the survival of O3-bearing serotypes and the survival of O1-bearing serotypes disappeared following alveolar macrophage depletion in the murine pneumonia model. Indeed, it was previously shown that defective immunological host responses, including decreased IL-1β, IL-6, and IFN-γ production, underlie the progression of K. pneumoniae pulmonary infection to systemic septicemia (46). Moreover, administration of IL-1 has been shown to enhance nonspecific resistance in animals to several gram-positive and gram-negative bacteria and fungi (41).
Because SP-D has been shown to bind or acts as an opsonin for many microorganisms, including E. coli, Salmonella enterica serovar Paratyphi, K. pneumoniae, P. carinii, and influenza A virus, it was of interest to identify its role in vivo (13, 23, 31, 34). In this study we found that lung clearance is enhanced for bacteria that express O antigen recognized by SP-D in vitro. The process of bacterial phagocytosis may trigger induction of a cytokine response, which is designed to set the stage for the induction of clonal immunity and to enhance the bacterial clearance. Although specific receptors involved in SP-D-mediated bacterial uptake have not been defined, macrophages are known to possess receptors that can engage collectin-ligand complexes, with resulting effects on the expression of cytokines (9).
The results of this study support the idea that the virulence of K. pneumoniae strains is strongly correlated with their CPS and O-antigen structure and involves innate immune components, such as macrophages, SP-D, and cytokines. The presence in the lung of both capsulated and noncapsulated K. pneumoniae requires mechanisms that can successfully defend against both bacterial forms. Thus, we suggest that alveolar macrophages and their MR function primarily against capsulated Klebsiella, while SP-D and macrophages are responsible for the elimination of noncapsulated bacteria. We speculate that an imbalance of such host defense mechanisms increases the susceptibility of the host to this pathogen.
Acknowledgments
This study was supported by grant 2001055 from the United States-Israel Binational Science Foundation and by the Elsa and Leo Abramson Fund.
We thank Naam Kariv, Tel Aviv University, for his assistance in performing the in vivo experiments.
Editor: J. N. Weiser
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanism of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-628. [DOI] [PubMed] [Google Scholar]
- 2.Athamna, A., I. Ofek, Y. Keisari, S. Markowitz, G. S. Dutton, and N. Sharon. 1991. Lectinophagocytosis of encapsulated Klebsiella pneumoniae mediated by surface lectin of guinea pig alveolar macrophages and human monocyte-derived macrophages. Infect. Immun. 59:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banyer, J. L., N. H. R. Hamilton, I. A. Ramshaw, and A. J. Ramsey. 2000. Cytokines and innate and adaptive immunity. Rev. Immunodiagn. 2:359-373. [PubMed] [Google Scholar]
- 4.Bartlett, J. G., O'Keefe, P., F. P. Tally, T. J. Louie, and S. L. Gorbach. 1986. Bacteriology of hospital-acquired pneumonia. Arch. Intern. Med. 146:868-871. [PubMed] [Google Scholar]
- 5.Broug-Holub, E., G. B. Toews, J. F. van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine III, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumoniae: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crouch, E., K. Hartshorn, and I. Ofek. 2000. Collectins and pulmonary innate immunity. Immunol. Rev. 173:52-65. [DOI] [PubMed] [Google Scholar]
- 7.Dehoux, M. S., A. Boutten, and J. Ostinelli. 1994. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 150:710-716. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello, C. A. 1991. Interleukin-1 and interleukin-1 antagonism. Blood 77:1627-1652. [PubMed] [Google Scholar]
- 9.Gardai, S. J., Y.-Q. Xiao, M. Dickinson, J. A. Nick, D. R. Voelker, K. Greene, and P. M. Henson. 2003. By binding SIRPα or Calreticulin/CD19, lung collectins act as a dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13-23. [DOI] [PubMed] [Google Scholar]
- 10.Hansen, D. S., F. Mestre, S. Alberti, S. Hernandez-Alles, D. Alvarez, A. Domenech-Sanchez, J. Gil, S. Merino, J. M. Tomas, and V. J. Benedi. 1999. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J. Clin. Microbiol. 37:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartshorn, K., D. Chang, K. Rust, and E. Crouch. 1996. Interaction of recombinant human pulmonary surfactant protein D and SP-D multimers with influenza A. Am. J. Physiol. 271:1753-1762. [DOI] [PubMed] [Google Scholar]
- 12.Hartshorn, K., M. R. White, D. Voelker, J. P. Coburn, K. S. Zaner, and E. Crouch. 2000. Mechanism of binding of surfactant protein D to influenza A virus: importance of binding to hemagglutinin to antiviral activity. Biochem. J. 351:449-457. [PMC free article] [PubMed] [Google Scholar]
- 13.Hartshorn, K. L., E. Crouch, M. R. White, P. Eggleton, A. I. Tauber, D. Chang, and K. N. Sastry. 1994. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Investig. 94:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawgood, S., and F. R. Poulain. 2001. The pulmonary collectins and surfactant metabolism. Annu. Rev. Physiol. 63:495-519. [DOI] [PubMed] [Google Scholar]
- 16.Hickman-Davis, J. M., J. R. Lindsey, and S. Matalon. 2001. Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis. Infect. Immun. 69:6401-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis, V. R., V. P. Munn, A. K. Highsmith, D. H. Culver, and J. M. Hughes. 1985. The epidemiology of nosocomial infection caused by Klebsiella pneumoniae. Infect. Control 6:68-74. [DOI] [PubMed] [Google Scholar]
- 18.Kabha, K., L. Nissimov, A. Athamna, Y. Keisari, H. Parolis, L. A. S. Parolis, R. M. Grue, J. Schlepper-Schafer, A. R. B. Ezekowitz, D. E. Ohman, and I. Ofek. 1995. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect. Immun. 63:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keisari, Y. 1996. Human mononuclear phagocytes in tissue culture, p. 153-160. In G. E. Jones (ed.), Methods molecular biology, vol. 20. Human cell culture protocols. Human Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 20.Keisari, Y., K. Kabha, L. Nissimov, J. Schlepper-Schaefer, and I. Ofek. 1997. Phagocyte-bacteria interactions. Adv. Dent. Res. 11:43-49. [DOI] [PubMed] [Google Scholar]
- 21.Keisari, Y., G. Robin, L. Nissimov, H. Wang, A. Mesika, R. Dimri, and I. Ofek. 2000. Role of cytokines in the maturation and function of macrophages: effect of GM-CSF and IL-4, p. 73-89. In Y. Keisari and I. Ofek (ed.), The biology and pathology of innate immunity mechanisms. Kluwer Academic/Plenum Publishing New York, N.Y. [DOI] [PubMed]
- 22.Keisari, Y., H. Wang, A. Mesika, R. Matatov, L. Nissimov, E. Crouch, and I. Ofek. 2001. Surfactant protein D-coated Klebsiella pneumoniae stimulates cytokine production in mononuclear phagocytes. J. Leukoc. Biol. 70:135-141. [PubMed] [Google Scholar]
- 23.Kuan, S. F., K. Rust, and E. Crouch. 1992. Interaction of surfactant protein D with bacterial lipopolysaccharides: surfactant protein D is an Escherichia coli binding protein in bronchoalveolar lavage. J. Clin. Investig. 90:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim, B. L., J. Y. Wang, U. Holmskov, H. J. Hoppe, and K. B. Reid. 1994. Expression of carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of Gram-negative bacteria. Biochem. Biophys. Res. Commun. 202:1674-1682. [DOI] [PubMed] [Google Scholar]
- 25.Limper, A. H., E. Crouch, O'Riordan, D. M., D. Chang, Z. Vuk-Pavlovic, J. E. Standing, K. Y. Kwon, and A. Adlakha. 1995. Surfactant protein D modulates interaction of Pneumocystis carinii with alveolar macrophages. J. Lab. Clin. Med. 126:416-422. [PubMed] [Google Scholar]
- 26.Matatov, R., J. Goldhar, E. Skutelsky, I. Sechter, R. Perry, R. Podschun, H. Sahly, K. Thankavel, S. N. Abraham, and I. Ofek. 1999. Inability of encapsulated Klebsiella pneumoniae to assemble functional type I fimbriae on their surface. FEMS Microbiol. Lett. 179:123-130. [DOI] [PubMed] [Google Scholar]
- 27.Nelson, S. 2001. Novel nonantibiotic therapies for pneumonia. Cytokines and host defense. Chest 119:419S-425S. [DOI] [PubMed] [Google Scholar]
- 28.Ofek, I., Y. Goldhar, Y. Keisari, and N. Sharon. 1995. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 49:239-276. [DOI] [PubMed] [Google Scholar]
- 29.Ofek, I., E. Crouch, and Y. Keisari. 2000. The role of C-type lectins in the innate immunity against pulmonary pathogens, p. 27-36. In Y. Keisari and I. Ofek (ed.), The biology and pathology of innate immunity mechanisms. Kluwer Academic/Plenum Publishing New York, N.Y. [DOI] [PubMed]
- 30.Ofek, I., A. Mesika, M. Kalina, Y. Keisari, R. Podschun, H. Sahly, D. Chang, D. McGregor, and E. Crouch. 2001. Surfactant protein D enhances the phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect. Immun. 69:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Riordan, D. M., J. E. Standing, K. Y. Kwon, D. Chang, E. Crouch, and A. H. Limper. 1995. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J. Clin. Investig. 95:2699-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ørskov, I., and F. Ørskov. 1984. Serotyping of Klebsiella, p. 143-164. In T. Bergan (ed.), Methods in microbiology. Academic Press, London, United Kingdom.
- 33.Person, A., D. Chang, and E. Crouch. 1990. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J. Biol. Chem. 265:5755-5763. [PubMed] [Google Scholar]
- 34.Pikaar, J. C., W. F. Voorhout, L. M. van Golde, J. Verhoev, J. A. Van Strijp, and J. F. van Iwaarden. 1995. Opsonic activities of surfactant proteins A and D in phagocytosis of gram-negative bacteria by alveolar macrophages. J. Infect. Dis. 172:481-489. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds, H. Y. 1988. Normal and defective respiratory host defenses, 2nd ed., p. 1-33. Raven Press, New York, N.Y.
- 36.Sahly, H., R. Podschun, T. A. Oelschlaeger, M. Greiwe, H. Parolis, D. Hasty, J. Kekow, U. Ullmann, I. Ofek, and S. Sela. 2000. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 68:6744-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahly, H., I. Ofek, R. Podschun, H. Brade, Y. He, U. Ullmann, and E. Crouch. 2002. Surfactant protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich O-antigen. J. Immunol. 169:3267-3274. [DOI] [PubMed] [Google Scholar]
- 38.Standiford, T. J., S. L. Kunkel, and R. M. Strieter. 1997. Role of chemokines in antibacterial host defense. Methods Enzymol. 288:220-241. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe, M., T. Matsumoto, K. Shibuya, K. Tateda, S. Miyazaki, A. Nakane, Y. Iwakura, and K. Yamaguchi. 2005. Compensatory response of IL-1 gene knockout mice after pulmonary infection with Klebsiella pneumoniae. J. Med. Microbiol. 54:7-13. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, W. C., R. M. Strieter, D. A. Zisman, J. M. Wilkowski, K. A. Bucknell, G. Chen, and T. J. Standiford. 1997. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect. Immun. 65:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogels, M. T. E., and J. W. M. van der Meer. 1992. Use of immune modulators in nonspecific therapy of bacterial infection. Antimicrob. Agents Chemother. 36:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, H., A. Kuzmenko, S. Wan, L. Schaffer, A. Weiss, J. H. Fisher, K. S. Kim, and F. X. McCormack. 2003. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Investig. 111:1589-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yammamoto, Y., T. W. Klein, and H. Friedman. 1997. IL-6 involvement of mannose receptor in cytokine interleukin-1β, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida, K., T. Matsumoto, K. Tateda, K. Uchida, S. Tsujimoto, and K. Yamaguchi. 2000. Role of bacterial capsule in local and systemic inflammatory responses of mice during pulmonary infection with K. pneumoniae. J. Med. Microbiol. 49:1003-1010. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida, K., T. Matsumoto, K. Tateda, K. Uchida, S. Tsujimoto, and K. Yamaguchi. 2001. Induction of interleukin-10 and down-regulation of cytokine production by Klebsiella pneumoniae capsule in mice with pulmonary infection. J. Med. Microbiol. 50:456-461. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida, K., T. Matsumoto, K. Tated, K. Uchida, S. Tsujimoto, Y. Iwakurai, and K. Yamaguchi. 2001. Protection against pulmonary infection with Klebsiella pneumoniae in mice by interferon-gamma through activation of phagocytic cells and stimulation of production of other cytokines. J. Med. Microbiol. 50:959-964. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, P., R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]