Abstract
Microsporidia of the Encephalitozoon species are frequently found as opportunistic pathogens of immunocompromised patients, but very little is known about the prevalence and significance of Encephalitozoon infection in immunocompetent individuals. It was reported previously that 8% of Dutch blood donors and 5% of pregnant French women had an immunoglobulin G (IgG) immune response against specific organelles of Encephalitozoon intestinalis. These organelles, the so-called polar tube and anchoring disk, are used to penetrate membranes of host cells during infection. The unexpectedly high percentage of immunocompetent individuals with IgG against these organelles suggested that infection of humans with microsporidia might be more common than previously recognized. In the present study, we analyzed this anti-Encephalitozoon IgG response by using indirect immunofluorescence, Western blotting, two-dimensional gel electrophoresis, and chemical deglycosylation. Our results show that the antibody response is directed against the posttranslational carbohydrate modification of the major polar tube protein (polar tube protein 1) and carbohydrate moieties of proteins in the anchoring region of the polar tube of Encephalitozoon. In addition, the antibodies were found to decrease the infectivity of E. intestinalis in vitro. The significance and possible origin of these prevalent antibodies are discussed.
Microsporidia are eukaryotic obligate intracellular parasites that share a common origin with fungi (17, 25, 26, 31). The infectious form is an environmentally resistant unicellular spore that contains the sporoplasm and an extrusion apparatus consisting of an anchoring disk and a polar tube coiled around the sporoplasm. Microsporidia have a unique mechanism for host cell invasion. During infection the host cell plasma membrane or the membranes of vacuoles surrounding internalized spores are penetrated by the rapidly extruding hollow polar tube, through which the contents of the spore are transferred (12, 14). Molecular characterization of polar tube constituents has revealed the existence of at least three distinct polar tube proteins, designated polar tube protein 1 (PTP1) (4, 5, 18), PTP2 (4), and PTP3 (23), which are unique to microsporidia and are at least partially conserved among microsporidian species (4). One of these proteins, PTP1, has recently been shown to be posttranslationally glycosylated, and the modifications may have a functional role during invasion of the host cell (33, 34).
Microsporidia have been recognized as major opportunistic pathogens in immunocompromised patients, especially those with AIDS. The clinical manifestations of infection with microsporidia of the Encephalitozoon species, E. intestinalis, E. hellem, and E. cuniculi, are mainly gastrointestinal, but rhinosinusitis, keratoconjunctivitis, hepatitis, nephritis, and encephalitis have also been reported (13, 30). Information on infection of immunocompetent individuals is scarce, and only a few cases have been described (1, 10, 20, 21, 24, 27). Diagnosis of microsporidiosis can be performed by detection of spores in patient material by microscopy or by PCR. However, in immunocompetent individuals shedding of spores is usually transient, and therefore serological techniques were developed to diagnose microsporidiosis indirectly. In these studies a high seroprevalence against the polar tube and anchoring disk of E. intestinalis was found in Dutch blood donors (8%) and pregnant French women (5%) using an enzyme-linked immunosorbent assay, counterimmunoelectrophoresis, and an immunofluorescence assay (IFA) (29). This suggested that infection of immunocompetent individuals with microsporidia might be more common than previously recognized, but the individuals could remain asymptomatic (1, 29, 31).
In this study we analyzed the immunoglobulin G (IgG) immune response of immunocompetent individuals to the polar tube and anchoring disk of E. intestinalis in order to study the antigenic constituents and the mechanism(s) underlying this commonly occurring immune response.
MATERIALS AND METHODS
Culture of microsporidia.
E. intestinalis (28), E. hellem (8), and E. cuniculi (strain GB-M1; a kind gift from E. U. Canning) were cultured in human lung mucoepidermoid cells (NCI-H292) in minimum essential medium (BioWhittaker) supplemented with 10% fetal calf serum and 2 mM glutamine at 37°C in a 5% CO2 atmosphere, essentially as described previously (27). After visual inspection of the cultures for mass spore production, the culture medium containing the spores was aspirated. Spores were pelleted by centrifugation at 1,000 × g, washed with phosphate-buffered saline (PBS), and resuspended in a small volume of PBS. The number of spores was determined by microscopy, after which the spores were stored at 4°C.
Human sera.
Human anti-polar tube sera and human control sera that were not reactive with the polar tube were obtained from a group of 300 healthy blood donors in The Netherlands and 276 healthy pregnant women in France, using consecutively collected sera. These sera were used in a previous study (29).
Indirect immunofluorescence.
The indirect immunofluorescence technique for cultured microsporidia with human sera has been described elsewhere (27). Briefly, microsporidia were grown on 18-well glass slides, fixed, and incubated with diluted human sera (1:100) or anti-polar tube protein-specific mouse antibody (1:500). Bound antibodies were detected with fluorescein isothiocyanate (FITC)-labeled anti-human or mouse IgG, examined with a Leitz fluorescence microscope, and photographed.
Confocal microscopy.
For simultaneous detection of microsporidian spore wall protein 1 (SWP1) and polar tube staining by human sera, microsporidia were incubated with diluted mouse antibody specific for anti-recombinant SWP1 (anti-recSWP1) (23) (1:100) and human anti-polar tube serum (1:100). Bound antibodies were detected with tetramethyl rhodamine isocyanate-labeled anti-mouse IgG and FITC-labeled anti-human IgG. For detection of fluorescent signals a Leica SP2 AOBS system was used. The microsporidia were imaged in a 1024 × 1024 format with a pixel size of 55 nm using a ×63 Planapo Oil objective (NA 1.32) and 2× line averaging. The excitation and detection wavelengths of FITC were 488 nm and 500 to 550 nm, respectively, and the excitation and detection wavelengths of Texas Red were 561 nm and 570 to 680 nm, respectively. In order to avoid cross talk between the two fluorophores, images were acquired in a sequential scanning mode. After this red and green images were merged to obtain a red-green-blue image.
Antigen preparation.
For sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) antigen preparation, purified spores (approximately 6 × 109 spores) were pelleted by centrifugation and resuspended in 4 ml of 2.5% SDS in PBS. After eight freeze-thaw cycles the suspension was sonicated on ice six times for 30 s (30-kHz microprobe; Soniprep 150; MSE, Loughborough, Great Britain) and centrifuged at 18,000 × g for 5 min. The pellet was dissolved in 2.5% SDS in PBS with 100 mM dithiothreitol (DTT) and incubated at room temperature for 48 h. The suspension was centrifuged again at 18,000 × g, and the supernatant was used as the antigen. For two-dimensional (2D) electrophoresis E. intestinalis spores (approximately 3 × 109 spores) were resuspended by vigorous vortexing in 500 μl of 2.2 M thiourea-7.7 M urea-2% Triton X-100-100 mM DTT. After incubation at room temperature for 1 h, the suspension was centrifuged at 18,000 × g for 5 min, and the supernatant was used as the antigen.
SDS-PAGE and Western blot analysis.
SDS-PAGE was performed using standard procedures. Briefly, 100 μl of the lysate was suspended in SDS-PAGE sample buffer (with 5% 2-mercaptoethanol) to obtain a final volume of 200 μl, boiled for 3 min, and size fractionated by 10% SDS-PAGE. After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes overnight. The transferred proteins were visualized using ponceau red dye staining. Incubation was performed with a multiscreen apparatus (Mini-Protean II; Bio-Rad) to create individual lanes on a single blot, unless indicated otherwise. For detection, human sera were diluted 1:500, anti-recombinant E. intestinalis PTP1 (anti-recEiPTP1) was diluted 1:2,000, anti-PTP2 (3) was diluted 1:1,000, and anti-PTP3 (23) was diluted 1:500, and the preparations were incubated with the blot for 1 h. Isotype-specific antibodies conjugated to peroxidase were obtained from DAKO (Glostrup, Denmark) and were used at a 1:2,000 dilution for 45 min. A chemiluminescent substrate (ECL) was prepared as recommended by the manufacturer (Amersham, United Kingdom). The PVDF membranes were incubated with the ECL substrate for 1 min, wrapped in plastic, and used to expose X-ray film for 3 min, 1 min, and 30 s.
2D electrophoresis and Western blot analysis.
Samples were applied to 18-cm IPG strips (pH 3 to 10; NL; Amersham Biosciences), which were allowed to rehydrate for 10 h at room temperature in the presence of the appropriate amounts of IPG buffer (protocol of Amersham Biosciences). First-dimension isoelectric focusing at 4°C was started by using 200 V for 30 min and two prefocusing steps consisting of 30 min at 400 V and 30 min at 600 V. The voltage was raised to a maximum of 3,500 V in 2.5 h, and the treatment was continued until at least 75 kV · h was reached, after which the IPG strips were stored at −80°C until they were used. For the second dimension, focused strips were equilibrated for 30 min at room temperature in 50 mM Tris-HCl (pH 8.8)-6 M urea- 30% (vol/vol) glycerol-2% SDS-1% (wt/vol) DTT, and this was followed by an identical incubation in which the DTT was replaced with 2.5% (wt/vol) iodoacetamide. Next, the strips and a broad-range marker (Bio-Rad, Hercules, CA) were placed on 12% polyacrylamide gels, and vertical electrophoresis was carried out using the Iso-Dalt system (Amersham Biosciences). After transfer of proteins to PVDF, the blot was incubated with mouse anti-recEiPTP1 (1:2,000) for 1 h, washed, and incubated with anti-mouse horseradish peroxidase conjugate (1:2,000). After detection of the PTP1 signal by ECL, antibodies were stripped from the blot by incubation in 62.5 mM Tris-HCl (pH 6.8)-2% SDS-100 mM 2-mercaptoethanol for 30 min at 50°C. The blot was rinsed twice for 5 min in PBS-Tween 20 (0.1%) and exposed to X-ray film for 10 min to ensure that all of the ECL signal was removed. This procedure was repeated twice with the same blot for two different human anti-polar tube sera diluted 1:1,000.
TFMS deglycosylation.
E. intestinalis spore proteins in PBS containing 2.5% SDS and 100 mM DTT were dialyzed overnight against PBS and centrifuged in a Speed Vac at 60°C until they were completely dry. The pellet was dissolved in 50 μl of trifluoromethanesulfonic acid (TFMS)-anisole (9:1) and incubated for 1 h at −20°C. The 50-μl mixture was then added to 500 μl of ice-cold pyridine-diethyl ether (1:9) and centrifuged for 10 min at 18,000 × g. The resulting pellet was air dried and dissolved in 100 μl of 100 mM NH4HCO3, and this was followed by overnight dialysis against 100 mM NH4HCO3 (8). As a control, recombinant human glycosylated α-galactosidase (Replagal; Transkaryotic Therapies) was treated with TFMS in the absence or presence of E. intestinalis protein extract. One half of each sample was used for PAGE followed by Coomassie blue staining, while the other half was used for Western blot analysis using anti-recEiPTP1 and two human anti-polar tube sera.
Production of anti-recEiPTP1 antibody.
DNA from E. intestinalis spores was obtained by boiling purified spores at 100°C for 10 min in sterile water. The major part of the E. intestinalis ptp1 gene (coding for amino acids 23 to 371) was PCR amplified using forward primer PTP1EiD (5′-CGGGATCCACAACTGTGCTGTGTGGAGAT-3′) with a BamHI restriction site at the 5′ end and reverse primer PTP1EiR (5′-CGGAATTCGCATTGTTGTTGGCAGCAAGC-3′) with an EcoRI site at the 5′ end. PCR amplification was performed using a Perkin-Elmer DNA thermal cycler 2400 apparatus with 50-μl reaction mixture and standard conditions (Eurobio). After the DNA was denatured at 94°C for 3 min, 35 cycles consisting of 20 s of denaturation at 94°C, 30 s of annealing at 55°C, and 1 min of extension at 72°C were performed, and this was followed by a final 10-min extension step at 72°C. The PCR product was analyzed by electrophoresis on a 1% agarose gel and was purified with a QIAquick gel extraction kit (QIAGEN). After digestion with restriction enzymes BamHI and EcoRI, the PCR product was cloned into a modified prokaryotic expression vector, pGEX-4T1 (Pharmacia). This vector was modified by insertion of an in-frame eight-histidine tag between XhoI and NotI restriction sites. The resulting recombinant plasmid was introduced into the Escherichia coli BL21+ strain. E. intestinalis PTP1 was expressed as a glutathione S-transferase (GST)-PTP1-His8 fusion protein after induction with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. The recombinant protein was then purified by chromatography on an Ni-nitrilotriacetic acid column (QIAGEN), excised from Coomassie blue-stained gels, and crushed in PBS. Mouse polyclonal antibodies to recombinant E. intestinalis PTP1 were obtained by intraperitoneally inoculating Swiss mice with crushed gel samples homogenized with Freund's complete adjuvant for the first injection and with Freund's incomplete adjuvant for the subsequent injections (after 14, 21, and 28 days). Preimmune and immune sera collected 1 week after the last booster were stored at −20°C.
Infection inhibition assay.
Human lung mucoepidermoid cells (NCI-H292) were seeded in a flat-bottom 96-well plate (Costar) and grown to subconfluency. The medium was refreshed, and human anti-polar tube serum or control serum was added at various concentrations (0, 10, 20, 30, or 40%; final volume, 100 μl). Then 105 purified E. intestinalis spores were added to each well, and the infection was allowed to proceed for 24 h. The culture medium with the extracellular spores was then removed by two washes with 150 μl of medium. Fresh medium containing human anti-polar tube serum or control serum was again added to a final volume of 100 μl. Medium containing newly produced spores was collected after 48 h and used for DNA extraction and subsequent PCR analysis.
DNA extraction and PCR analysis.
To 100 μl of culture supernatant 200 μl of 5.6 M guanidine thiocyanate-18 mM EDTA-1% Triton X-100-25 mM Tris-HCl (pH 6.4) was added. From 200 μl of this suspension DNA was extracted using a High Pure PCR template preparation kit (Roche). The DNA was eluted in 200 μl of 10 mM Tris-HCl (pH 8.5). A low-cycle-number PCR (23 cycles) was performed with the isolated DNA, which amplified part of the small-subunit rRNA of E. intestinalis, as described previously (22). Amplification products were size fractionated on a 1% agarose gel and visualized by ethidium bromide staining.
RESULTS
Strong immunoreactivity of human anti-polar tube sera with E. intestinalis DTT-soluble 55-kDa antigens.
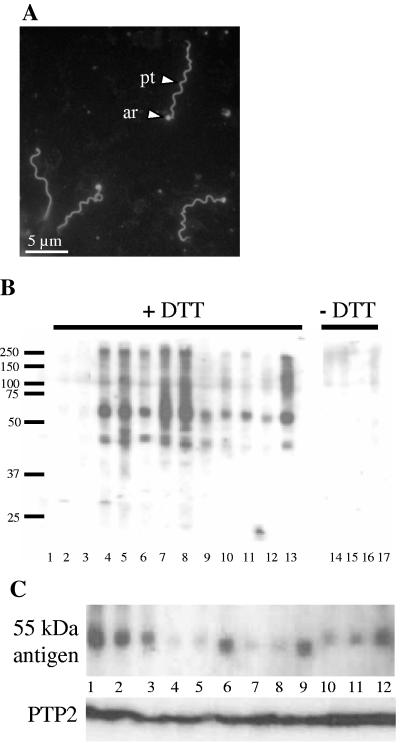
To identify the polar tube antigens targeted by human sera, a Western blot containing a fractionated total protein extract from purified E. intestinalis spores was prepared and incubated with human sera. Human sera that stained the polar tube and the anchoring region between the polar tube and the spore body of E. intestinalis in the IFA (Fig. 1A) were tested, and all of them recognized a prominent band around 55 kDa and some minor bands around 45 kDa (Fig. 1B, lanes 4 to 13). In addition, several sera produced a high-molecular-weight smear between 100 and 250 kDa. All antigens had a protein component as treatment of the extract with proteases reduced the signals to the background level (data not shown). The presence of these antigens in the protein extract depended on the presence of DTT during the extraction procedure (lanes 14 to 17), in agreement with the fact that the polar tube is known to be totally disrupted in the presence of reducing agents (19). Sera negative in the IFA of E. intestinalis spores did not recognize these antigens in a Western blot (lanes 1 to 3). Surprisingly, there appeared to be small variations in the molecular weight of the antigen recognized around 55 kDa by the anti-polar tube sera (Fig. 1C, top panel). This variation was not caused by blot artifacts since the differences were reproducible and subsequent incubation of the same blot with a mouse polyclonal antibody against PTP2 (bottom panel) at 33 kDa produced a line that was almost straight.
FIG. 1.
Reactivity of human sera with E. intestinalis in indirect IFA and on spore protein blots. (A) Human anti-polar tube serum in IFA of cultured E. intestinalis spores. The extruded polar tube (pt) and the anchoring region (ar) are strongly labeled. The spore body is not labeled. (B) Western blot analysis of E. intestinalis spore protein extract prepared in the presence of 100 mM DTT (lanes 1 to 13) or in the absence of DTT (lanes 14 to 17) and incubated with human sera. Lanes 4 to 17 were incubated with human anti-polar tube sera; in lanes 1 to 3 human control sera (negative in IFA) were used. The positions of molecular weight markers (in kDa) are indicated on the left. (C) Western blot analysis of E. intestinalis spore protein extract with 12 human anti-polar tube sera (top panel) and a mouse polyclonal antibody against E. cuniculi PTP2 (bottom panel). Note the differences in molecular weight of the 55-kDa antigen recognized by individual human sera.
To further investigate whether the antigens recognized by Western blotting indeed corresponded to the anti-polar tube and anchoring region signal obtained in IFA, preabsorption experiments were performed. Two human anti-polar tube sera were preabsorbed with narrow blot strips cut from the 55-kDa portion of blots containing total E. intestinalis spore protein extract. This showed that preincubation with blot strips from the 55-kDa region not only efficiently removed the antibodies directed to the 55-kDa antigens from the sera but also strongly reduced the recognition of the high-molecular-weight smear. When these preabsorbed sera were subsequently used for immunofluorescence analysis, the strong staining of the polar tube and anchoring region was reduced to background levels (not shown). These results indicated that the anti-55-kDa signal corresponded to the anti-polar tube staining observed in IFA.
The 55-kDa antigens recognized by human sera correspond to PTP1, the major polar tube protein in Encephalitozoon spp.
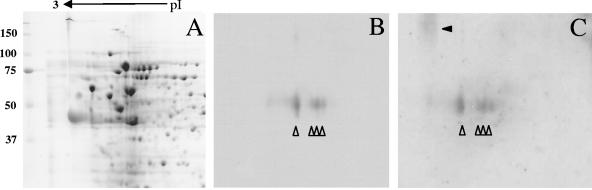
The identities of the 55-kDa antigens were investigated further by comparing their signals with those of the three known polar tube constituents of E. intestinalis (PTP1, PTP2, and the PTP3-like protein) on Western blots. Antibodies against PTP2 and PTP3 of E. cuniculi were produced in a previous study, and they are known to cross-react with homologous proteins from E. intestinalis (4, 5, 23). These antibodies could therefore be used directly for the analysis of E. intestinalis proteins. Antibodies against PTP1 of E. cuniculi did not react with E. intestinalis spore extracts due to the extensive sequence divergence of PTP1 from E. cuniculi and E. intestinalis (not shown). We therefore cloned the ptp1 sequence of E. intestinalis and produced recombinant E. intestinalis PTP1 that was N terminally fused to GST and C terminally fused to an eight-His tag. Mouse polyclonal antibodies were raised against this recEiPTP1 fusion protein. To investigate if the 55-kDa antigens recognized by the human anti-polar tube sera comigrated with one of the known E. intestinalis polar tube proteins, a Western blot with E. intestinalis spore extract was incubated with antibodies against PTP1, PTP2, and two human anti-polar tube sera. The anti-recEiPTP1 produced a relatively broad band between 50 and 60 kDa that comigrated with the 55-kDa antigens recognized by the human anti-polar tube sera. The positions of PTP2 and PTP3 did not correspond to the signal obtained with the human sera (not shown). To confirm that the human anti-polar tube sera indeed recognized PTP1 of E. intestinalis, blots of 2D gels containing total spore proteins were prepared, and part of each blot was subsequently incubated with the anti-recEiPTP1 antibody. As shown in Fig. 2, PTP1 was separated into four spots with different isoelectric points around pI 5, which is in agreement with the calculated pI (pI 4.9) (4). After stripping and incubation of the same blot with human anti-polar tube serum, the same four spots were stained. Subsequent stripping and incubation with a different human anti-polar tube serum gave identical results (not shown). This further indicated that the 55-kDa antigens of the polar tube recognized by human sera are indeed E. intestinalis PTP1.
FIG. 2.
2D Western blot analysis. A protein extract of E. intestinalis spores was fractionated by 2D electrophoresis (A) (Coomassie blue staining), transferred to a PVDF membrane and incubated with anti-recEiPTP1 (B), stripped of all binding antibody, and subsequently incubated with human anti-polar tube serum (C). The four isoelectric variants of PTP1 around pI 5 are at the same position in panels B and C (indicated by open arrowheads). The position of the high-molecular-weight smear recognized by the human anti-polar tube serum is indicated by a solid arrowhead in panel C. The positions of molecular weight markers (in kDa) are indicated on the left, and the direction of the isoelectric focusing gradient is indicated at the top.
Chemical deglycosylation experiments indicated that the PTP1 epitopes recognized by human anti-polar tube sera are not protein moieties but are carbohydrate moieties.
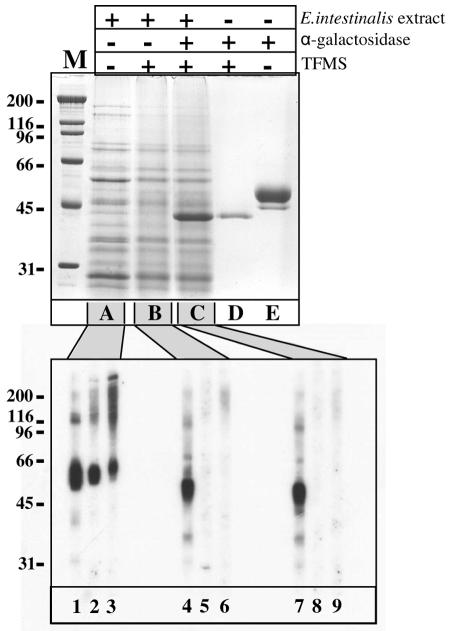
The recombinant GST-PTP1-His8 protein was of course clearly recognized by the mouse polyclonal antibody obtained after it was used for immunization, but it was not recognized by any of the human anti-polar tube sera tested, even after overexposure (not shown). This suggested that posttranslational modifications of PTP1 instead of the protein itself are crucial for recognition. PTP1 of the three Encephalitozoon species have recently been shown to be glycosylated (33, 34). To test the hypothesis that only the carbohydrate moieties of PTP1 are recognized by the human anti-polar tube sera, a total-protein extract of E. intestinalis spores was subjected to chemical deglycosylation using TFMS (Fig. 3). TFMS treatment removes all carbohydrate groups from proteins irrespective of the nature of the link between the amino acid core and the attached carbohydrate (9). TMFS had no major visible effect on the protein banding obtained by Coomassie blue staining of the extract after gel electrophoresis. This suggested that no visible protein degradation had occurred and that none of the abundant proteins isolated from the E. intestinalis spores was extensively glycosylated (Fig. 3, compare lanes A and B). Addition of a glycosylated control protein, α-galactosidase (Replagal; TKT), to the E. intestinalis spore extract showed that deglycosylation using TFMS was effective, however. The multiple forms of differentially glycosylated α-galactosidase between 45 and 50 kDa (lane E) were reduced to a single protein band at 40 kDa both in the absence (lane D) and in the presence (lane C) of the E. intestinalis spore protein extract. The protein fractions shown in lanes A, B, and C were subsequently used to produce a Western blot (Fig. 3, bottom panel) that was incubated with the anti-recEiPTP1 antibody (lanes 1, 4, and 7) and two human anti-polar tube sera (lanes 2, 5, and 8 and lanes 3, 6, and 9). TFMS treatment induced a shift in the molecular weight of PTP1 from 50 to 60 kDa to 45kDa, showing that PTP1 was deglycosylated (compare lane 1 with lanes 4 and 7), but it had no effect on the molecular weight of PTP2, PTP3, or SWP1 (not shown). Increasing the length of the TFMS treatment from 1 h to 16 h did not lead to a further decrease in the molecular weight of PTP1 (not shown). The overall mouse polyclonal antibody reactivity, however, was not greatly reduced upon TFMS treatment. In sharp contrast, the recognition of two human anti-polar tube sera was reduced to background levels following deglycosylation; the signals around 55 kDa and the high-molecular-weight smear were no longer visible after TFMS treatment (compare lanes 2 to 3 with lanes 5 to 6 and 8 to 9).
FIG. 3.
SDS-PAGE and Western blot analysis of E. intestinalis spore protein extract before and after TFMS treatment. A Coomassie blue-stained gel of E. intestinalis spore protein extract (top panel, lane A) was deglycosylated with TFMS in the absence (lane B) or in the presence (lane C) of the control protein α-galactosidase. The patterns for the control glycoprotein alone before TFMS treatment (lane E) and after TFMS treatment (lane D) are also shown. Protein fractions A, B, and C were size fractionated by SDS-PAGE, transferred to a single PVDF membrane (bottom panel), and incubated with anti-recEiPTP1 (lanes 1, 4 and 7) and two human anti-polar tube sera (lanes 2 and 3). The molecular weight of PTP1 decreased after TFMS treatment (lanes 4 and 7), while the reactivity of the human sera was lost after TFMS treatment (lanes 5, 6, 8, and 9). The positions of molecular weight markers (in kDa) are indicated on the left (lane M).
High-molecular-weight glycosylated antigens that are also specifically labeled by human anti-polar tube sera are probably anchoring region components.
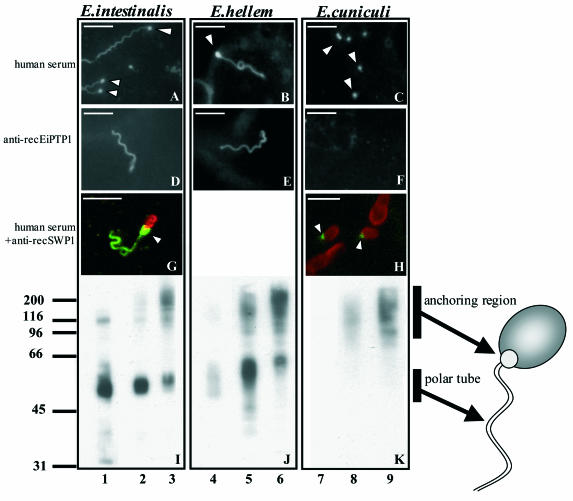
To test whether the human anti-polar tube antibodies also reacted with polar tube constituents of other microsporidia and to further study what they recognize, in vitro cultured spores from E. hellem and E. cuniculi were used for IFA and Western blot experiments. As shown in Fig. 4, extruded polar tubes from E. intestinalis spores were stained with both human anti-polar tube serum and mouse polyclonal anti-recEiPTP1 antibody. Interestingly, most human anti-polar tube sera not only stained the polar tube but also intensely stained what appeared to be the anchoring region, located between the beginning of the filament and the spore wall (Fig. 4A). This anchoring region was available to antibodies only in spores in which the polar tube was extruded. The anti-recEiPTP1 antibody efficiently recognized the polar tube but did not recognize the anchoring region (Fig. 4D). Similar results were obtained for in vitro cultured spores from E. hellem (Fig. 4B and E). In contrast, PTP1 of E. cuniculi was not recognized by the mouse polyclonal anti-recEiPTP1 antibody (Fig. 4F). Human anti-polar tube sera were also not able to stain the extruded filaments from E. cuniculi but gave bright punctate staining (Fig. 4C). The location of the strongly stained structure at the proximal end of the polar tube was investigated further by double immunostaining using a mouse antibody against spore wall protein 1 (anti-recSWP1) (23) and a human anti-polar tube serum, followed by confocal microscopy (Fig. 4G and H). From the double staining of E. intestinalis spores it became apparent that this structure partially overlapped with the SWP1 staining and connects the extruded polar tube with the spore body (Fig. 4G). In E. cuniculi spores, the punctate staining with human anti-polar tube sera (Fig. 4C) was located at the anterior pole of the spore (Fig. 4H).
FIG. 4.
IFA and Western blot analysis of Encephalitozoon spp. Cultured E. intestinalis, E. hellem, and E. cuniculi were incubated with human anti-polar tube serum (A to C) and anti-recEiPTP1 (D to F). E. intestinalis and E. cuniculi spores were also double immunostained (G and H) with human anti-polar tube serum and detected with an FITC-conjugated secondary antibody (green fluorescence), and the anti-spore wall antibody, anti-recSWP1, was detected with a tetramethyl rhodamine isocyanate-conjugated secondary antibody (red fluorescence). The arrowheads indicate the strongly fluorescent anchoring region. Bars = 5 μm. Spore protein extracts of E. intestinalis (I), E. hellem (J), and E. cuniculi (K) were used for SDS-PAGE, transferred to PVDF membranes, and incubated with anti-recEiPTP1 (lanes 1, 4, and 7) and the two human anti-polar tube sera (lanes 2, 3, 5, 6, 8, and 9). The locations of glycosylated PTP1 in the polar tube and of the glycosylated proteins in the high-molecular-weight smear of the anchoring region are indicated schematically on the right. The positions of molecular weight markers (in kDa) are indicated on the left.
Western blot analysis of extracts from E. intestinalis (Fig. 4I), E. hellem (Fig. 4J), and E. cuniculi (Fig. 4K) indicated that, as in E. intestinalis, E. hellem PTP1 and the high-molecular-weight smear were recognized by human anti-polar tube sera. Human anti-polar tube sera did not stain PTP1 of E. cuniculi but did recognize a dot at the anterior pole of the spore (Fig. 4C and H) and the high-molecular-weight smear (Fig. 4K), suggesting that these antigens were the same and were derived from the anchoring region.
Human anti-polar tube sera partially inhibit infection by E. intestinalis in vitro.
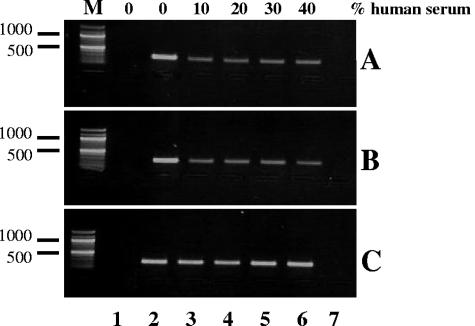
To evaluate the effect of human anti-polar tube antibody on the infection efficiency of cultured lung mucoepidermoid host cells by E. intestinalis, an in vitro assay was used. E. intestinalis spores were added to the host cells in the presence of various concentrations of human anti-polar tube sera or a control serum (Fig. 5). The production of spores was analyzed by semiquantitative PCR, which amplified part of the small-subunit rRNA gene of E. intestinalis. Both anti-polar tube sera were able to partially inhibit spore production at the lowest serum concentration (10%), based on the decrease in the PCR signal after addition of human anti-polar tube serum to the culture medium (Fig. 5A and B, lanes 3). Higher concentrations of anti-polar tube sera in the culture medium did not lead to a further increase in infection inhibition (lanes 4 to 6). The control serum had no effect on spore production (Fig. 5C).
FIG. 5.
Human polar tube sera partially inhibit E. intestinalis infection in vitro. Human lung mucoepidermoid cells were infected with E. intestinalis spores in the absence (lanes 2) or in the presence (lanes 3 to 6) of various concentrations (10 to 40%) of two human anti-polar tube sera (A and B) or normal human serum (C). The production of spores was analyzed by semiquantitative PCR amplification of part of the small-subunit rRNA gene of E. intestinalis. The PCR signal specifically decreased in the cultures with human anti-polar tube serum. Lanes 1 contained noninfected host cells, and for lanes 7 no template was added to the PCR mixture. The size marker (lanes M) was a 100-bp ladder.
DISCUSSION
The polar tube of microsporidia is a highly specialized structure and is essential for invasion of the host cell. Molecular analysis of the components of the polar tube has revealed the presence of several protein components, so-called polar tube proteins, which have no apparent homology to other proteins and represent novel structural protein families (4, 23, 31). Recently, PTP1 was found to be posttranslationally modified by O mannosylation. This modification appeared to have functional importance for the invasion process (33, 34). During infection of humans by microsporidia both the polar tube and the spore body induce a vigorous humoral IgG immune response that can be detected by both Western blotting and immunofluorescence analysis. Interestingly, from a detailed serological study of an immunocompetent patient infected with E. cuniculi, it appeared that the antibody titers against the spore wall and the polar tube did not develop simultaneously (27). In a previous study members of our group detected high antibody titers against the polar tube and a structure that was likely to be the residual anchoring disk of E. intestinalis in 8% of 300 Dutch blood donors and in 5% of 276 pregnant French women (29). These observations suggest that microsporidiosis might be a common infection that remains largely asymptomatic in immunocompetent individuals. To test this hypothesis, we analyzed the frequently observed humoral immune response against the polar tube of E. intestinalis. From our results (especially the changes in immune recognition upon deglycosylation [Fig. 3]) it became clear that the carbohydrate modifications of PTP1 and carbohydrate structures in the anchoring region are essential for recognition by human anti-polar tube sera. Interestingly, it appeared that the IgG epitopes on PTP1 and the anchoring region in E. intestinalis largely overlap as preabsorption of anti-PTP1 antibodies also strongly reduced recognition of the anchoring region. However, anti-recombinant PTP antibodies do not stain the anchoring region, indicating that the glycoprotein(s) in this region is not PTP1 (Fig. 4) (3, 18, 20). The identity of the antigens from the anchoring region could be of interest. However, the combined IFA and Western blot results suggested that these antigens migrate as a smear between 100 and 250 kDa, making identification difficult. A recent study of the microsporidian polar tube showed that only PTP1 is glycosylated. No evidence of glycosylated high-molecular-weight complexes from the anchoring region was presented. Probably, glycosylation of this region was not observed due to the high electron density of the spore body as determined by electron microscopy, which obscured labeling of this region. However, the portion of the tube near the spore was labeled most intensely (34). Glycosylation of PTP1 was surmised to be functional in the parasite-host interaction by creating a “sticky” polar tube capable of adhering to the host cell membrane receptors. The glycosylation of the anchoring region might have a function similar to that of PTP1 glycosylation in facilitating the attachment of the spore body to the host cell membrane during invasion. Various parasites have been shown to use carbohydrate receptors for adhesion and invasion of host cells (2, 15, 35).
Although all human anti-polar tube sera recognized PTP1, there appeared to be slight differences in the molecular weight of the PTP1 recognized by individual sera (Fig. 1B). This suggests that there is a spectrum of PTP1 molecules with various degrees of O mannosylation that result in slightly different antibody recognition on a blot, which is not uncommon for glycosylated antigens (6). Interestingly, glycosylation of PTP1 and the anchoring region appeared to be very similar in E. intestinalis and E. hellem but different in E. cuniculi. Human anti-polar tube sera efficiently recognized the anchoring region of all three species and PTP1 of E. intestinalis and E. hellem, but not PTP1 of E. cuniculi. This suggests that the glycosylation patterns of E. cuniculi PTP1 are different from those of the other two species. Possibly, the difference reflects host specificity, with glycosylation of PTP1 being optimized for adherence to the receptor(s) of the most important host.
The exact mechanism by which the prevalent and abundant anti-polar tube IgG antibodies are induced remains obscure. Studies of the humoral response during systemic infection with microsporidia have shown that in addition to polar tube constituents many other microsporidial proteins are recognized (7, 16, 27, 32). Humans probably have frequent contact with microsporidia, by the oral or respiratory route, without progress toward systemic disease (30). Whether these contacts could induce only anti-polar tube antibodies is still unknown. Presentation of immunogenic carbohydrate moieties to the epithelial surface of the gut in general does not elicit systemic IgG antibody responses. In AIDS patients worldwide E. intestinalis is the predominant opportunistic infecting Encephalitozoon species, and E. cuniculi infection has been reported only infrequently (13, 30). In agreement with these data is our observation in humans of the presence of anti-polar tube antibodies to E. intestinalis but the absence of such antibodies (directed only to the polar tube) to E. cuniculi (26; data not shown). O mannosylation, especially of cell wall components, is widespread in bacteria and several eukaryotes. This suggests that the antibody response could also be induced by a more common pathogen with O mannosylation patterns very similar to those of Encephalitozoon, which could be a more distantly related species of microsporidia or fungi. However, we could not find any cross-reactivity of the anti-polar tube antibodies against the two most common fungi that cause systemic disease, Aspergillus spp. and Candida spp. (27; data not shown).
Human anti-polar tube sera were found to partially inhibit infection of host cells by E. intestinalis in vitro. This indicates that these antibodies may contribute to antibody-mediated protection against microsporidial cell invasion by binding to the carbohydrate part of PTP1, thereby preventing efficient adherence of the polar tube to the host cell. An antibody against the microsporidial exospore was also shown to decrease infection of host cells in vitro. Similar to our results, the decrease was not dependent on the antibody concentration (11). In vivo, the anti-polar tube antibodies could further influence the host-parasite interaction by affecting antibody-mediated mechanisms, such as opsonin-dependent phagocytosis, again stressing the importance of these broad-specificity antibodies.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Bergquist, N. R., G, Stintzing, L. Smedman, T. Waller, and T. Andersson. 1984. Diagnosis of encephalitozoonosis in man by serological tests. Br. Med. J. 288:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, X. M., and N. F. LaRusso. 2000. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118:368-379. [DOI] [PubMed] [Google Scholar]
- 3.Delbac, F., F. Duffieux, D. David, G. Metenier, and C. P. Vivares. 1998. Immunocytochemical identification of spore proteins in two microsporidia, with emphasis on extrusion apparatus. J. Eukaryot. Microbiol. 45:224-231. [DOI] [PubMed] [Google Scholar]
- 4.Delbac, F., I. Peuvel, G. Metenier, E. Peyretaillade, and C. P. Vivares. 2001. Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect. Immun. 69:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delbac, F., P. Peyret, G. Metenier, D. David, A. Danchin, and C. P. Vivares. 1998. On proteins of the microsporidian invasive apparatus: complete sequence of a polar tube protein of Encephalitozoon cuniculi. Mol. Microbiol. 29:825-834. [DOI] [PubMed] [Google Scholar]
- 6.Desrosiers, R. R., Y. Bertrand, Q. T. Nguyen, M. Demeule, R. Gabathuler, M. L. Kennard, S. Gauthier, and R. Beliveau. 2003. Expression of melanotransferrin isoforms in human serum: relevance to Alzheimer's disease. Biochem. J. 374:463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didier, E. S., J. A. Shadduck, P. J. Didier, N. Millichamp, and C. R. Vossbrinck. 1991. Studies on ocular microsporidia. J. Protozool. 38:635-638. [PubMed] [Google Scholar]
- 8.Didier, E. S., P. J. Didier, D. N. Friedberg, S. M. Stenson, J. M. Orenstein, R. W. Yee, F. O. Tio, R. M. Davis, C. Vossbrinck, N. Millichamp, and J. A. Shadduck. 1991. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J. Infect. Dis. 163:617-621. [DOI] [PubMed] [Google Scholar]
- 9.Edge, A. S. 2003. Deglycosylation of glycoproteins with trifluoromethanesulphonic acid: elucidation of molecular structure and function. Biochem. J. 376:339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enriquez, F. J., D. Taren, A. Cruz-Lopez, M. Muramoto, J. D. Palting, and P. Cruz. 1998. Prevalence of intestinal encephalitozoonosis in Mexico. Clin. Infect. Dis. 26:1227-1229. [DOI] [PubMed] [Google Scholar]
- 11.Enriquez, F. J., G. Wagner, M. Fragoso, and O. Ditrich. 1998. Effects of an anti-exospore monoclonal antibody on microsporidial development in vitro. Parasitology 117:515-520. [DOI] [PubMed] [Google Scholar]
- 12.Franzen, C. 2004. Microsporidia: how can they invade other cells? Trends Parasitol. 20:275-276. [DOI] [PubMed] [Google Scholar]
- 13.Franzen, C., and A. Muller. 2001. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 3:389-400. [DOI] [PubMed] [Google Scholar]
- 14.Franzen, C., A. Muller, P. Hartmann, and B. Salzberger. 2005. Cell invasion and intracellular fate of Encephalitozoon cuniculi (Microsporidia). Parasitology 130:285-292. [DOI] [PubMed] [Google Scholar]
- 15.Hespanhol, R. C., M. de Nazare C. Soeiro, M. B. Meuser, S. L. de Nazareth, M. Meirelles, and S. Corte-Real. 2005. The expression of mannose receptors in skin fibroblast and their involvement in Leishmania (L.) amazonensis invasion. J. Histochem. Cytochem. 53:35-44. [DOI] [PubMed] [Google Scholar]
- 16.Hollister, W. S., E. U. Canning, and A. Willcox. 1991. Evidence for widespread occurrence of antibodies to Encephalitozoon cuniculi (Microspora) in man provided by ELISA and other serological tests. Parasitology 102:33-43. [DOI] [PubMed] [Google Scholar]
- 17.Keeling, P. J. 2003. Congruent evidence from alpha-tubulin and beta-tubulin gene phylogenies for a zygomycete origin of microsporidia. Fungal Genet. Biol. 38:298-309. [DOI] [PubMed] [Google Scholar]
- 18.Keohane, E. M., G. A. Orr, H. S. Zhang, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1998. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol. Biochem. Parasitol. 94:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keohane, E. M., G. A. Orr, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1996. Purification and characterization of a microsporidian polar tube protein. Mol. Biochem. Parasitol. 79:255-259. [DOI] [PubMed] [Google Scholar]
- 20.Matsubayashi, H., T. Koike, I. Mikata, H. Takei, and S. Hagiwara. 1959. A case of Encephalitozoon-like body infection in man. AMA Arch. Pathol. 67:181-187. [PubMed] [Google Scholar]
- 21.Muller, A., R. Bialek, A. Kamper, G. Fatkenheuer, B. Salzberger, and C. Franzen. 2001. Detection of microsporidia in travelers with diarrhea. J. Clin. Microbiol. 39:1630-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notermans, D. W., R. Peek, M. D. de Jong, E. M. Wentink-Bonnema, R. Boom, and T. van Gool. 2005. Detection and identification of Enterocytozoon bieneusi and Encephalitozoon species in stool and urine specimens by PCR and differential hybridization. J. Clin. Microbiol. 43:610-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peuvel, I., P. Peyret, G. Metenier, C. P. Vivares, and F. Delbac. 2002. The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol. Biochem. Parasitol. 122:69-80. [DOI] [PubMed] [Google Scholar]
- 24.Raynaud, L., F. Delbac, V. Broussolle, M. Rabodonirina, V. Girault, M. Wallon, G. Cozon, C. P. Vivares, and F. Peyron. 1998. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J. Clin. Microbiol. 36:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomarat, F., C. P. Vivares, and M. Gouy. 2004. Phylogenetic analysis of the complete genome sequence of Encephalitozoon cuniculi supports the fungal origin of microsporidia and reveals a high frequency of fast-evolving genes. J. Mol. Evol. 59:780-791. [DOI] [PubMed] [Google Scholar]
- 26.Van de Peer, Y., A. Ben Ali, and A. Meyer. 2000. Microsporidia: accumulating molecular evidence that a group of amitochondriate and suspectedly primitive eukaryotes are just curious fungi. Gene 246:1-8. [DOI] [PubMed] [Google Scholar]
- 27.Van Gool, T., C. Biderre, F. Delbac, E. Wentink-Bonnema, R. Peek, and C. P. Vivares. 2004. Serodiagnostic studies in an immunocompetent individual infected with Encephalitozoon cuniculi. J. Infect. Dis. 189:2243-2249. [DOI] [PubMed] [Google Scholar]
- 28.Van Gool, T., E. U. Canning, H. Gilis, M. A. van den Bergh Weerman, J. K. Eeftinck Schattenkerk, and J. Dankert. 1994. Septata intestinalis frequently isolated from stool of AIDS patients with a new cultivation method. Parasitology 109:281-289. [DOI] [PubMed] [Google Scholar]
- 29.Van Gool, T., J. C. Vetter, B. Weinmayr, A. Van Dam, F. Derouin, and J. Dankert. 1997. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J. Infect. Dis. 175:1020-1024. [DOI] [PubMed] [Google Scholar]
- 30.Weber, T., D. A. Schwartz, and R. T. Bryan. 2000. Microsporidia, p. 2920-2933. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, Pa.
- 31.Weiss, L. M. 2001. Microsporidia: emerging pathogenic protists. Acta Trop. 78:89-102. [DOI] [PubMed] [Google Scholar]
- 32.Weiss, L. M., A. Cali, E. Levee, D. LaPlace, H. Tanowitz, D. Simon, and M. Wittner. 1992. Diagnosis of Encephalitozoon cuniculi infection by Western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am. J. Trop. Med. Hyg. 47:456-462. [DOI] [PubMed] [Google Scholar]
- 33.Xu, Y., P. Takvorian, A. Cali, and L. M. Weiss. 2003. Lectin binding of the major polar tube protein (PTP1) and its role in invasion. J. Eukaryot. Microbiol. 50:600-601. [DOI] [PubMed] [Google Scholar]
- 34.Xu, Y., P. M. Takvorian, A. Cali, G. Orr, and L. M. Weiss. 2004. Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans. Infect. Immun. 72:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Z., M. Duchene, and S. L. Stanley, Jr. 2002. A monoclonal antibody to the amebic lipophosphoglycan-proteophosphoglycan antigens can prevent disease in human intestinal xenografts infected with Entamoeba histolytica. Infect. Immun. 70:5873-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]