Abstract
Staphylococcus aureus capsular polysaccharides (CP) have been shown to enhance staphylococcal virulence in numerous animal models of infection. Although serotype 5 CP (CP5) and CP8 predominate among S. aureus isolates from humans, most staphylococcal isolates from bovines with mastitis in Argentina are capsule negative. This study was designed to evaluate the effects of CP5 and CP8 expression on the pathogenesis of experimental murine mastitis. Lactating mice were challenged by the intramammary route with one of three isogenic S. aureus strains producing CP5, CP8, or no capsule. Significantly greater numbers of acapsular mutant cells were recovered from the infected glands 12 days after bacterial challenge compared with the encapsulated strains. Histopathological analyses revealed greater polymorphonuclear and mononuclear leukocyte infiltration and congestion in the mammary glands of mice infected with the encapsulated strains compared with the acapsular mutant, and the serotype 5 strain elicited more inflammation than the serotype 8 strain. In vitro experiments revealed that the acapsular S. aureus strain was internalized by MAC-T bovine epithelial cells in significantly greater numbers than the CP5- or CP8-producing strain. Taken together, the results suggest that S. aureus lacking a capsule was able to persist in the murine mammary gland, whereas encapsulated strains elicited more inflammation and were eliminated faster. Loss of CP5 or CP8 expression may enhance the persistence of staphylococci in the mammary glands of chronically infected hosts.
Staphylococcus aureus is an opportunistic bacterial pathogen that can infect and replicate and persist in diverse hosts, including humans and domestic animals of economic importance (35). Bacterial components and exoproducts that promote staphylococcal virulence are numerous (28), and it has been difficult to identify the role that individual virulence determinants play in the pathogenic process. The majority of S. aureus isolates from humans produce capsular polysaccharide (CP), and serotype 5 CP (CP5) and CP8 strains are most prevalent (18). Strains that do not react with antibodies to CP5 or CP8 are currently referred to as nontypeable (NT) since serotypes 1 and 2 are extremely rare and easily identifiable because they produce mucoid colonies on solid media. Prototype strains and antibodies to detect the other putative serotypes are not available (24).
The role of the S. aureus capsule in the pathogenesis of staphylococcal infections has been evaluated in numerous animal models of infection. In general, the experimental evidence suggests that staphylococcal capsules have antiphagocytic properties and allow the organisms to persist in the blood and tissues of infected hosts (33). S. aureus mutants lacking capsule proved to be less virulent when tested in animal models of septic arthritis, abscess formation, and bacteremia (24). However, acapsular mutants were more virulent than the encapsulated serotype 5 and 8 S. aureus strains in a rat model of catheter-induced endocarditis (1). The low prevalence (<25%) of NT S. aureus among human isolates supports a significant role of CP5 and CP8 in the pathogenesis of most human infections.
In contrast, capsule production is less prevalent among S. aureus strains isolated from ruminants with mastitis (27, 30). Only ∼40% of more than 600 bovine isolates from the United States produced CP5 or CP8 (11, 34). Furthermore, only 14% of 195 epidemiologically unrelated bovine isolates of S. aureus from Argentina reacted with antibodies to CP5 or CP8 (31). We hypothesize that the lack of capsule expression may permit intracellular persistence of S. aureus and promote subclinical mammary gland infection. Since fibronectin-binding protein plays a crucial role in S. aureus adherence to and internalization in bovine epithelial cells (5, 8), masking of staphylococcal surface adhesins by the staphylococcal capsule may influence the pathogenesis of bovine mastitis. Because most cows with chronic mastitis suffer from the subclinical, persistent type of disease, we sought to determine whether capsule production influences the pathogenesis of this disease. An experimental mouse model of S. aureus-induced mastitis (9), recently validated by Brouillette and Malouin (4), was chosen to test our hypothesis. Our results indicate that S. aureus lacking a capsule persisted in the murine mammary gland longer than isogenic encapsulated strains. This finding correlated with the ability of the acapsular strain to invade bovine epithelial cells in significantly greater numbers than strains producing CP5 or CP8. Loss of capsule expression may enhance the persistence of staphylococci in the mammary glands of chronically infected hosts.
MATERIALS AND METHODS
Bacterial strains.
Isogenic S. aureus strains that produce CP5, CP8, or no capsule were derived from serotype 5 strain Reynolds as recently described (36). Except for capsule production, the strains are phenotypically identical (36). The strains were negative for slime production on Congo red medium and were poor biofilm producers when cultivated in Trypticase soy broth supplemented with 1% glucose. Each strain was resistant to streptomycin (500 μg/ml), and Reynolds (CP−) was also erythromycin resistant. S. aureus strain RN6390 was generously provided by A. L. Cheung (Dartmouth Medical School, Hanover, N.H.). S. aureus was routinely cultured at 37°C for 24 h on Columbia agar supplemented with 2% NaCl to enhance CP production.
Mouse mastitis model.
CF1 outbred mice were bred and maintained in the vivarium of the Department of Microbiology, School of Medicine, University of Buenos Aires, Buenos Aires, Argentina. Animal care was done in accordance with the guidelines set forth by the National Institutes of Health (23). Groups of 22 to 30 pregnant mice were used for each experiment, and pregnancies were synchronized in order to have all births within a 24-h period. The mouse mastitis model was described previously (9). Briefly, 7 to 10 days after parturition, lactating mothers were separated from the pups and anesthetized with ketamine (60 mg/kg) (Ketalar; Parke-Davis) and xylazine (5 mg/kg) (Rompun; Bayer). The left and right fourth mammary glands (L4 and R4, respectively) were injected with 50 μl of an S. aureus suspension delivered through a 30-gauge needle and syringe and containing 105 to 106 CFU. Two hours after challenge, the mothers were returned to the pups and remained with them throughout the remainder of the experiment. Groups of lactating mice were euthanized on days 1, 4, 8, and 12, and the L4 and R4 glands were aseptically removed. Each gland was homogenized in 2 ml of tryptic soy broth (Difco, Detroit, Mich.), and dilutions of the homogenates were plated quantitatively to determine the number of CFU per gland. Mixed-inoculum experiments were also performed in which two strains were mixed at a 1:1 ratio for a total inoculum of 106 CFU per gland. Colonies of strain Reynolds (CP−) were identified by replica plating to TSA-5 μg/ml erythromycin, and in several experiments the capsular phenotypes of the strains were verified by colony immunoblotting (19).
In separate experiments, L4 and R4 mammary glands were excised for histological examination. Whole glands were fixed in 5% formalin, dehydrated with 70 to 100% ethanol, cleared with xylene, and embedded in paraffin. Tissue sections were stained with hematoxylin-eosin, periodic acid-Schiff stain, or trichromic stain and examined in a blinded fashion. To quantitatively evaluate alterations of mammary gland histology, a tissue alteration index value was determined on hematoxylin-eosin-stained sections as follows: score = 0, no infiltration, undamaged tubular epithelium; score = 1, mild polymorphonuclear (PMN) cell interstitial infiltration in isolated areas of tissue sections, undamaged tubular epithelium; score = 2, interstitial infiltration covering most fields, dispersed areas of tissue damage with loss of tissue structure, and scant images of abscess formation; score = 3, severe infiltration involving most fields, frequent areas of tissue damage with loss of tissue structure, and frequent images of abscess formation. Individual scores were applied to each mammary gland excised on day 4, 8, or 12.
Cell culture studies.
The bovine mammary epithelial cell line designated MAC-T was generously provided by Nexia Biotechnologies (Quebec, Canada). Cells were seeded (6 × 104 cells/well) in flat-bottom 24-well plates in Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, N.Y.) with 10% heat-inactivated fetal calf serum (Gibco-BRL), 1 μg/ml hydrocortisone, 5 μg/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (Sigma Chemical Co., St. Louis, Mo.) for 3 days at 37°C in 6% CO2. MAC-T cell monolayers (∼1.2 × 105 cells/well) were washed and inoculated with S. aureus to a multiplicity of infection of 15 to 20. Monolayers were washed after a 1-h incubation, and fresh medium containing 100 μg/ml gentamicin (Sigma) was added and the mixture was incubated for an additional 2 or 24 h. It should be noted that the three isogenic S. aureus strains are equally susceptible to killing by gentamicin. The epithelial cells were then washed, detached from the plates by treatment with 0.25% trypsin-0.1% EDTA, and lysed with 0.025% Triton X-100 in distilled water to release intracellular staphylococci. The CFU number was determined by quantitative plating on TSA. MAC-T cell viability was evaluated by trypan blue exclusion.
Statistical analyses.
Quantitative culture data (tissue homogenates or cell cultures) and tissue alteration index data were compared by analysis of variance (ANOVA) with posttest analysis by the Tukey-Kramer test. Tissue alteration index data were evaluated by nonparametric ANOVA with the Kruskal-Wallis test. Individual data were compared by using the Wilcoxon rank test. P values of <0.05 were considered significant.
RESULTS
Mouse mastitis model experiments.
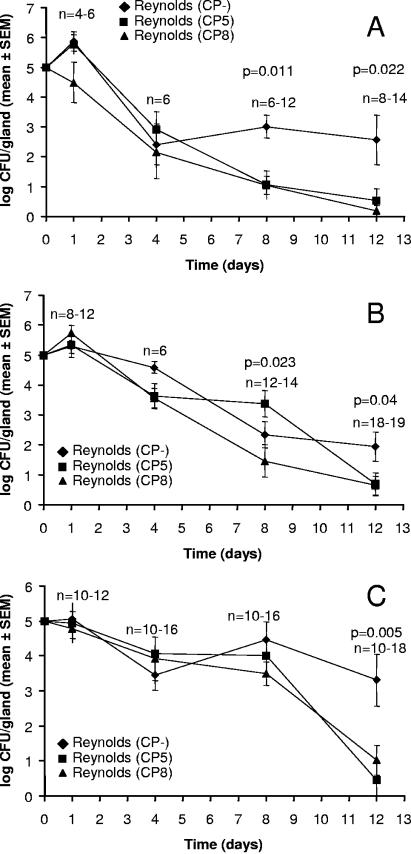
The influence of CP5 and CP8 production on persistence of S. aureus in the mammary gland was assessed by challenging groups of lactating mice with isogenic S. aureus strains that differed only in capsule expression: Reynolds (CP5), Reynolds (CP8), or Reynolds CP−. Each experiment was performed with inocula of 105, 5 × 105, or 106 CFU per gland. As shown in Fig. 1, the numbers of CFU of all three strains recovered from the infected glands slowly decreased during the course of the infection. The rate of tissue clearance for each strain was similar until days 4 to 8, at which point clearance of Reynolds (CP−) diminished compared with the encapsulated strains. The number of CFU of Reynolds (CP−) per gland was significantly higher than that of the CP-positive strains on days 8 and 12 after challenge with 105 CFU (Fig. 1A). At the higher inocula (5 × 105 [Fig. 1B] or 106 [Fig. 1C] CFU per gland), the number of CFU of Reynolds (CP−) per gland was significantly higher than that of the encapsulated strains on day 12. The staphylococcal infection remained localized to the mammary glands since the major organs from the infected animals appeared normal, and quantitative cultures of kidney, spleen, and liver homogenates were sterile.
FIG. 1.
Effect of capsule production on S. aureus virulence in the murine mastitis model. The ordinate represents the log number of CFU of Reynolds (CP−), Reynolds (CP5), and Reynolds (CP8) recovered from the mammary glands at different times after intramammary inoculation with 105 (A), 5 × 105 (B), or 106 (C) CFU/gland. The lower limit of detection by culture was 5 CFU/gland. The P values shown represent the level of significance for comparisons between Reynolds (CP−) versus Reynolds (CP5) and Reynolds (CP−) versus Reynolds (CP8). Each point represents the geometric mean from groups of 4 to 19 mice (sample sizes are shown for groups at each time point).
Several groups of mice were challenged with mixed S. aureus suspensions containing Reynolds (CP−) mixed with either Reynolds (CP5) or Reynolds (CP8). The ratio was 1:1 for the two strains, and the total inoculum was either 105 or 106 CFU/gland. The ratios of the two strains recovered from the infected glands over time did not differ significantly from the ratio of the two strains in the inoculum (data not shown). Furthermore, most of the glands were sterile by 8 to 12 days after bacterial challenge, similar to glands inoculated with the encapsulated strains.
Histopathology of infected glands.
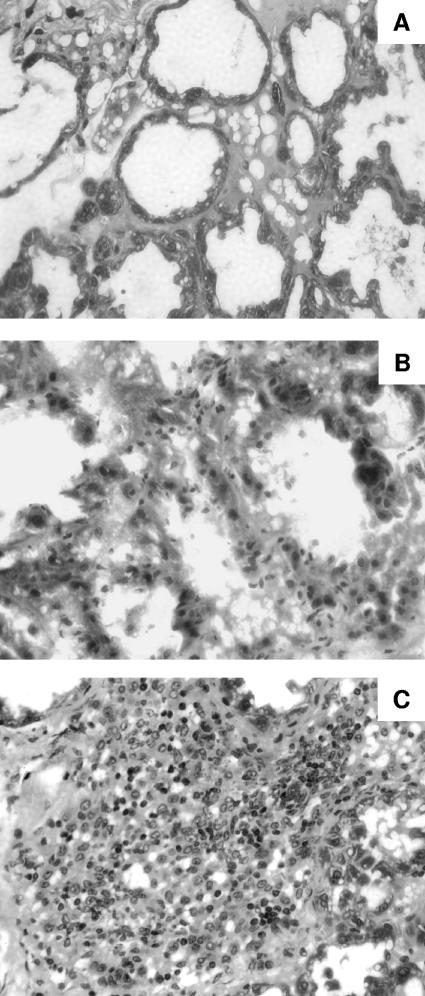
The effect of S. aureus capsule production on PMN and mononuclear cell infiltration of the infected mammary glands was assessed histologically. Groups of mice were challenged with either 105 or 106 CFU of S. aureus Reynolds (CP5), Reynolds (CP8), or Reynolds (CP−) and sacrificed 4, 8, or 12 days after challenge. Differences in histopathological findings among the three S. aureus strains were observed at a challenge dose of 105 CFU/gland (Fig. 2). PMN and mononuclear cell infiltration was more prominent on day 4 in the mammary tissues from mice challenged with 105 Reynolds (CP5) compared with Reynolds (CP8) or Reynolds (CP−) (Fig. 2). Likewise, there was a higher degree of tubular epithelium damage and more severe interstitial hemorrhaging in the tissues from mice challenged with Reynolds (CP5).
FIG. 2.
Hematoxylin-eosin-stained sections of mammary glands after challenge with 105 CFU of S. aureus. Depicted are representative sections exhibiting minor or no changes induced 4 days after challenge with Reynolds (CP−) (A), mild inflammation following challenge with Reynolds (CP8) (B), and a massive inflammatory response with marked PMN cell infiltration induced by Reynolds (CP5) (C).
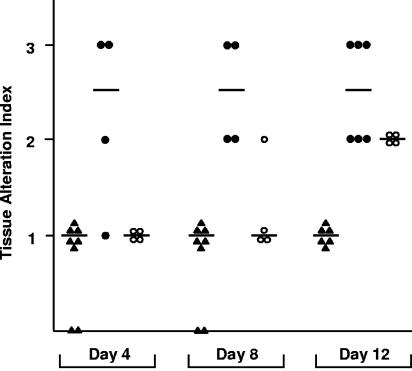
Results of histopathological findings on days 4, 8, and 12 after challenge with 105 CFU of S. aureus were quantified as the tissue alteration index and are presented in Fig. 3. Reynolds (CP5) elicited severe inflammation and extensive tissue damage in the infected mammary glands, and its score was significantly higher than that of Reynolds (CP−) on all three days (Fig. 3) and higher than that of Reynolds (CP8) on days 4 and 8. Very little tissue damage and only mild inflammation were induced in the tissues of mice inoculated with the acapsular strain. Strain Reynolds (CP8) caused an intermediate level of tissue damage resembling that of Reynolds (CP−) on days 4 and 8 but approaching that of strain Reynolds (CP5) by day 12.
FIG. 3.
Tissue alteration indexes measured in mammary gland tissue sections from mice challenged with 105 CFU/gland of Reynolds (CP−) (closed triangles), Reynolds (CP5) (closed circles), or Reynolds (CP8) (open circles). Each bar represents the median from four to eight mammary glands. Nonparametric ANOVA revealed overall significant differences among groups (P < 0.0001). Levels of significance for individual comparisons were as follows: Reynolds (CP−) versus Reynolds (CP5) on day 4, P = 0.033; Reynolds (CP−) versus Reynolds (CP5) on day 8, P = 0.004; Reynolds (CP−) versus Reynolds (CP5) on day 12, P = 0.002; Reynolds (CP−) versus Reynolds (CP8) on day 12, P = 0.010.
At a challenge dose of 106 CFU, differences in the histopathological alterations induced by the isogenic S. aureus strains were reduced. All three strains induced comparable degrees of inflammation (data not shown). This finding was not unexpected since differences in virulence can often be overcome with high inocula and S. aureus components other than CP can induce inflammation in mammalian hosts.
Internalization of S. aureus within MAC-T cells.
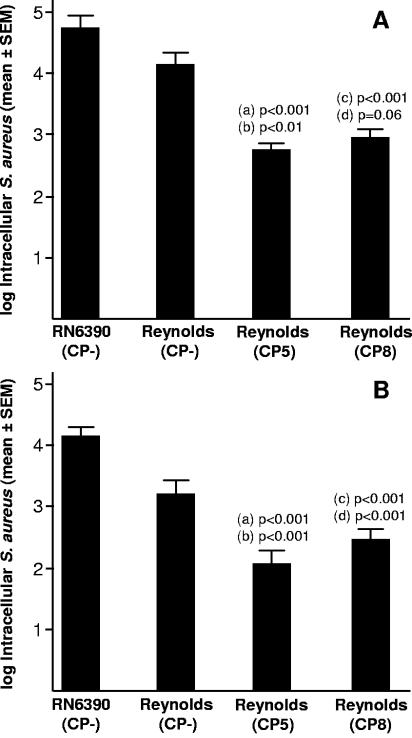
Hensen et al. (13) reported that in the early and chronic stages of mammary infection, S. aureus Newbould 305 (serotype 5) was located within the lumen of alveoli or lactiferous ducts in association with the epithelium and within phagocytic cells. We considered that Reynolds (CP−) might persist longer in the mammary glands of infected mice by invasion of interstitial tissues. Therefore, we investigated the effect of CP5 and CP8 production on S. aureus internalization into bovine epithelial cells in vitro. MAC-T cells were incubated with S. aureus at a multiplicity of infection of 15 to 20 for 1 h at 37°C, at which point the cells were washed and antibiotic-containing medium was added to kill the extracellular staphylococci. As shown in Fig. 4A, Reynolds (CP−) and unrelated NT S. aureus strain RN6390 were internalized by the epithelial cells to a greater extent than either Reynolds (CP5) or Reynolds (CP8). The overall numbers of viable staphylococci within the epithelial cells diminished <10-fold between 2 h (Fig. 4A) and 24 h (Fig. 4B). Intracellular survival of S. aureus within MAC-T cells was not affected by CP5 or CP8 production, and epithelial cell viability remained high (>95%) 24 h after antibiotic treatment. Likewise, the levels of MAC-T cell apoptosis measured 24 h after bacterial challenge were very low, and significant differences among strains were not observed. Preliminary experiments in our laboratory with S. aureus isolates (n = 8) from cows with mastitis revealed similar levels of MAC-T cell survival (>95%) and measurable numbers of intracellular staphylococci 24 h after challenge.
FIG. 4.
S. aureus internalization into MAC-T cells measured as CFU remaining after 2 h (A) or 24 h (B) of incubation after killing of extracellular bacteria. Each bar represents the number of intracellular CFU per milliliter from 11 to 24 wells (A) or 17 to 24 wells (B) run in separate experiments. Values a and c represent P values for comparisons with S. aureus RN6390, whereas values b and d are for comparisons with strain Reynolds (CP−). SEM, standard error of the mean.
DISCUSSION
The mouse model of mastitis provides a valuable tool for the study of the pathogenesis of S. aureus infection within the mammary gland (9). Our investigation into the influence of staphylococcal capsule production in this model revealed that the encapsulated S. aureus strains were cleared more readily from infected mammary glands than was an isogenic acapsular mutant. Our histologic analyses revealed that PMN cells were recruited to the mammary gland in high numbers in response to infection by the CP5- or CP8-positive S. aureus strain, and this PMN cell influx correlated with bacterial clearance. In contrast, the acapsular S. aureus mutant did not elicit much inflammation and was less efficiently cleared from the infected gland. The results of our in vitro studies suggest that the acapsular S. aureus strain may avoid immune clearance by internalization within bovine mammary epithelial cells.
Intramammary challenge with Reynolds (CP5) caused a more intense inflammatory reaction in the mammary gland than did Reynolds (CP8). This finding is intriguing, considering the recent report (36) that Reynolds (CP5) was more virulent in a murine bacteremia model than was Reynolds (CP8). Reynolds (CP5) and Reynolds (CP8) showed similar levels of virulence in the mouse model of experimental mastitis. The pathogenesis of the infection in the mammary gland is clearly distinct from that in the bacteremia model, since in the latter model (36) the serotype 5 and 8 strains persisted longer in the bloodstreams of infected mice than did the acapsular mutant. Moreover, the capsule type 5 and 8 strains were more resistant than the acapsular mutant to opsonophagocytic killing by human or mouse leukocytes (36). There is no evidence to date that purified CP5 or CP8 displays intrinsic chemotactic activity for PMN cells in vitro (J. Lee, unpublished observations) or activates the complement system (36). However, purified CP8 elicited a localized inflammatory response characterized by neutrophil infiltration in a mouse model of surgical wound infection (J. C. Lee and A. O. Tzianabos, 104th Gen. Meet. Am. Soc. Microbiol., abstr. D-145, 2004). How production of CP5 or CP8 influences the levels of the various chemoattractants and inhibitors (6) that regulate PMN cell migration into the mouse mammary gland is unclear and deserves further investigation.
It is plausible that the number of PMN cells present at the site of infection may dictate whether a particular interaction between S. aureus and the mammary gland leads to acute or chronic disease. CP5 and CP8 appear to up-regulate the recruitment of PMN cells in the mouse model of mammary infection, and this may influence the outcome of the host-bacterium interaction (7, 10). Reynolds (CP5) and Reynolds (CP8) both caused an acute infection in lactating mice, and clearance of the bacterial infection occurred at the expense of the integrity of the mammary tissue. Likewise, persistent infection was not observed when a mixed inoculum containing encapsulated and acapsular S. aureus was delivered to the mice. We postulate that the robust inflammatory response induced by the encapsulated strain effectively cleared both S. aureus strains from the infected gland.
Optimal clearance of S. aureus from the mammary gland occurred at a challenge dose of 105 CFU. At a challenge dose of 106 CFU of S. aureus, it appeared that the exacerbated host response and an abundance of toxic bacterial products contributed to the observed damage of the mammary tissue. The differential influence of CP5 versus CP8 capsule production on the pathogenesis of bovine mastitis has not yet been evaluated. Host factors and staphylococcal virulence factors in addition to capsule probably affect the host-bacterium interaction, resulting in clinical or subclinical infection (22, 32).
S. aureus internalization into bovine epithelial cells is well documented (12), and this intracellular environment may provide a niche in which S. aureus can persist and avoid killing by PMN cells and certain antibiotics (3). Our results indicate that capsule production diminished S. aureus internalization by MAC-T cells in vitro, but neither CP5 nor CP8 affected bacterial survival once S. aureus was internalized. The interaction between adhesins expressed on the bacterial surface (25) and host cell receptors is critical for S. aureus internalization into bovine mammary epithelial cells (17, 29). Indeed, an isogenic mutant lacking expression of fibronectin-binding protein exhibited a 30% reduction in the ability to adhere to cultured MAC-T bovine cells and a 95% reduction in invasion compared with the parental strain (5, 8). Capsule expression has been shown to reduce S. aureus adherence to endothelial cells and platelets in vitro (26; A. Risley, C. Cywes, T. Foster, and J. Lee, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. D-254, 2004). Moreover, the infectious dose of encapsulated S. aureus strains exceeds that of acapsular mutants in the endocarditis model of infection (1), presumably by masking adhesins critical for staphylococcal binding to the damaged heart valve. The different factors involved in bacterial adhesion and invasion that influence the pathogenesis of bovine S. aureus mastitis have been reviewed by Kerro Dego et al. (16).
Lack of S. aureus capsule expression during infection may be due to mutations within the capsule gene locus, mutations within regulatory loci like agr, or regulation by environmental conditions encountered by S. aureus in the early phases of infection (24). S. aureus fails to produce capsule during the exponential phase of growth (20), in the presence CO2 concentrations of ≥1%, or under alkaline conditions (14, 15). CP expression has been shown to be down-regulated during chronic staphylococcal lung infection of cystic fibrosis patients (14). Acapsular S. aureus cells that are internalized avoid uptake and killing by neutrophils. The entry of S. aureus into host cells through certain ligands and their receptors may trigger apoptosis (2), leading to the release of viable staphylococci into the extracellular milieu. Cycles of internalization by epithelial cells and subsequent release of acapsular S. aureus into the extracellular space may lead to chronic subclinical disease. The feasibility that genotypic and phenotypic changes may occur during infection, leading to the emergence of acapsular S. aureus, is currently under investigation in our laboratories.
Our results do not contradict the premise that capsule may play some role in the pathogenesis of S. aureus ruminant mastitis (13). CP5 and CP8 may participate in concert with other extracellular factors in the pathogenesis of acute clinical mastitis (21). However, a significant number of S. aureus isolates from mastitis do not produce capsule (11, 31, 34). Acapsular S. aureus cells may have an advantage over encapsulated strains in subclinical disease because they can persist in the mammary gland. Determinant features of the host cell-S. aureus interaction that lead to bacterial persistence may be a decreased inflammatory response in the mammary gland, resulting in diminished phagocytic killing of S. aureus in the early stages of disease. This scenario may allow S. aureus cells lacking a capsule to interact more effectively with host cell receptors, leading to internalization within epithelial cells, intracellular persistence, and chronic disease.
The results of our study demonstrate that an acapsular S. aureus mutant was able to persist longer than isogenic serotype 5 and 8 strains in the infected mammary glands of lactating mice. The encapsulated strains induced more inflammation than the acapsular mutant, and this may contribute to their enhanced clearance by the host immune response. Our in vitro studies revealed that the acapsular S. aureus mutant was more readily internalized by bovine epithelial cells than the isogenic encapsulated strains. We speculate that S. aureus may persist in the intracellular milieu of epithelial cells within the infected gland, where they are protected from competent phagocytes. The mechanisms by which increased migration of PMN cells occurred in response to infection by encapsulated S. aureus strains remain unknown and warrant further research.
Acknowledgments
This study was partially supported by grants from the NIH Fogarty Foundation (FIRCA 1-R03-TW006264) and the NIAID (RO1 AI29040) to J.C.L., the Agencia Nacional de Promoción de la Ciencia y la Tecnología, Argentina (ANPCYT PICT 05-10648), and the Secretaría de Ciencia y Técnica, Universidad de Buenos Aires, Buenos Aires, Argentina (UBACyT M-009).
We thank Lorena Medina for dedicated technical assistance and Sabrina Soldavini for help in animal care and performance of animal experiments.
Editor: V. J. DiRita
REFERENCES
- 1.Baddour, L. M., C. Lowrance, A. Albus, J. H. Lowrance, S. K. Anderson, and J. C. Lee. 1992. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J. Infect. Dis. 165:749-753. [DOI] [PubMed] [Google Scholar]
- 2.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley, A. 2002. Bovine mastitis: an evolving disease. Vet. J. 164:116-128. [DOI] [PubMed] [Google Scholar]
- 4.Brouillette, E., and F. Malouin. 2005. The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect. 7:560-568. [DOI] [PubMed] [Google Scholar]
- 5.Brouillette, E., B. Talbot, and F. Malouin. 2003. The fibronectin-binding proteins of Staphylococcus aureus may promote mammary gland colonization in a lactating mouse model of mastitis. Infect. Immun. 71:2292-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Haas, C. J. C., K. E. Veldkamp, A. Peschel, F. Weerkamp, W. J. B. Van Wamel, E. C. J. M. Heezius, Myriam, J. J. G. Poppelier, K. P. M. Van Kessel, and J. A. G. van Strijp. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199:687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detilleux, J. C. 2004. Neutrophils in the war against Staphylococcus aureus: predator-prey models to the rescue. J. Dairy Sci. 87:3716-3724. [DOI] [PubMed] [Google Scholar]
- 8.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin-binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez, M. I., V. E. García, M. M. Gherardi, M. C. Cerquetti, and D. O. Sordelli. 1998. Intramammary immunization with live-attenuated Staphylococcus aureus protects mice from experimental mastitis. FEMS Immunol. Med. Microbiol. 20:21-27. [DOI] [PubMed] [Google Scholar]
- 10.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 11.Guidry, A., A. Fattom, A. Patel, and C. O'Brien. 1997. Prevalence of capsular serotypes among Staphylococcus aureus isolates from cows with mastitis in the United States. Vet. Microbiol. 59:53-58. [DOI] [PubMed] [Google Scholar]
- 12.Hebert, A., K. Sayasith, S. Senechal, P. Dubreuil, and J. Lagace. 2000. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol. Lett. 193:57-62. [DOI] [PubMed] [Google Scholar]
- 13.Hensen, S. M., M. J. Pavicic, J. A. Lohuis, J. A. De Hoog, and B. Poutrel. 2000. Location of Staphylococcus aureus within the experimentally infected bovine udder and the expression of capsular polysaccharide type 5 in situ. J. Dairy Sci. 83:1966-1975. [DOI] [PubMed] [Google Scholar]
- 14.Herbert, S., D. Worlitzsch, B. Dassy, A. Boutonnier, J. M. Fournier, G. Bellon, A. Dalhoff, and G. Doring. 1997. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J. Infect. Dis. 176:431-438. [DOI] [PubMed] [Google Scholar]
- 15.Herbert, S., S. W. Newell, C. Lee, K. P. Wieland, B. Dassy, J. M. Fournier, C. Wolz, and G. Doring. 2001. Regulation of Staphylococcus aureus type 5 and type 8 capsular polysaccharides by CO2. J. Bacteriol. 183:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerro Dego, O., J. E. van Dijk, and H. Nederbragt. 2002. Factors involved in the early pathogenesis of bovine Staphylococcus aureus mastitis with emphasis on bacterial adhesion and invasion. A review. Vet. Q. 24:181-198. [DOI] [PubMed] [Google Scholar]
- 17.Lammers, A., P. J. Nuijten, and H. E. Smith. 1999. The fibronectin-binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol. Lett. 180:103-109. [DOI] [PubMed] [Google Scholar]
- 18.Lee, C. Y., and J. C. Lee. 2000. Staphylococcal capsule, p. 361-366. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 19.Lee, J. C., M. J. Liu, J. Parsonnet, and R. D. Arbeit. 1990. Expression of type 8 capsular polysaccharide and production of toxic shock syndrome toxin 1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 28:2612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luong, T., S. Sau, M. Gomez, J. C. Lee, and C. Y. Lee. 2002. Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and. sarA. Infect. Immun. 70:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsunaga, T., S. Kamata, N. Kakiichi, and K. Uchida. 1993. Characteristics of Staphylococcus aureus isolated from peracute, acute and chronic bovine mastitis. J. Vet. Med. Sci. 55:297-300. [DOI] [PubMed] [Google Scholar]
- 22.Mullarky, I. K., C. Su, N. Frieze, Y. H. Park, and L. M. Sordillo. 2001. Staphylococcus aureus agr genotypes with enterotoxin production capabilities can resist neutrophil bactericidal activity. Infect. Immun. 69:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council. 1996. Guide for the care and use of laboratory animals (NIH guide, revised). National Research Council, Washington, D.C.
- 24.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus capsular polysaccharide. Clin. Microbiol. Rev. 17:218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 26.Pohlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Doring, J. C. Lee, J. M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of the capsular polysaccharide, the global regulator agr, and the bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poutrel, B., A. Boutonnier, L. Sutra, and J. M. Fournier. 1988. Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J. Clin. Microbiol. 26:38-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, N.Y.
- 29.Sinha, B., P. Francois, Y. A. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. H. Kraus, G. Peter, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sompolinsky, D., Z. Samra, W. W. Karakawa, W. F. Vann, R. Schneerson, and Z. Malik. 1985. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J. Clin. Microbiol. 22:828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sordelli, D. O., F. R. Buzzola, M. I. Gómez, L. Steele-Moore, D. Berg, E. Gentilini, M. Catalano, A. J. Reitz, T. Tollersrud, G. Denamiel, P. Jeric, and J. C. Lee. 2000. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: genetic and epidemiologic analyses. J. Clin. Microbiol. 38:846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutra, L., and B. Poutrel. 1993. Virulence factors involved in the pathogenesis of bovine intramammary infections caused by S. aureus. J. Med. Microbiol. 40:79-89. [DOI] [PubMed] [Google Scholar]
- 33.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tollersrud, T., K. Kenny, A. J. Reitz, and J. C. Lee. 2000. 2000. Genetic and serologic evaluation of capsule production by bovine mammary isolates of Staphylococcus aureus and other Staphylococcus spp. from Europe and the United States. J. Clin. Microbiol. 38:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldvogel, F. A. 2000. Staphylococcus aureus, p. 2069-2092. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Churchill Livingstone, Philadelphia, Pa.
- 36.Watts, J. A., D. Ke, Q. Wang, A. Pillay, A. Nicholson-Weller, and J. C. Lee. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 73:3502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]