Abstract
Laz, a lipid-modified azurin of the human pathogens Neisseria gonorrhoeae and Neisseria meningitidis, is involved in defense against oxidative stress and copper toxicity; laz mutant strains are hypersensitive to hydrogen peroxide and copper. The N. gonorrhoeae laz mutant also has decreased survival in an ex vivo primary human ectocervical epithelial assay.
Neisseria gonorrhoeae and Neisseria meningitidis cause gonorrhoea and meningitis, respectively. During infection, pathogenic Neisseria organisms are exposed to oxidative stress (reactive oxygen species and reactive nitrogen species) generated by host defense mechanisms. As a consequence, pathogenic Neisseria spp. have evolved numerous defense mechanisms to sense and cope with oxidative stress (23, 24, 27-29, 32), some of which have been linked to virulence (32).
Azurin is a small, blue, copper-containing protein that functions in electron transport during respiration in several microorganisms (8, 14, 20, 21). However, the periplasmic azurin of Pseudomonas aeruginosa is not essential for denitrification but is involved in protection from oxidative stress (31). A P. aeruginosa azu mutant is sensitive to reactive oxygen species, including hydrogen peroxide (H2O2) and superoxide (O2.−) (31). An azurin paralogue, laz (for lipid-modified azurin), has been identified in both N. gonorrhoeae (10) and N. meningitidis (33). Laz is tethered to the outer membrane via palmityl fatty acid (26, 33) and possesses an N-terminal domain found in the H.8 protein and a C-terminal domain similar to those of other bacterial azurins (10, 15). The H.8 epitope is common to pathogenic Neisseria, leading to speculation that antigens bearing it might be involved in pathogenesis (4). Neisserial Laz is not essential for growth under aerobic and anerobic conditions in the presence of nitrite but may function in electron transport via a pathway that has not yet been identified (4). In this paper, we investigated the role of Neisseria azurin in defense against oxidative stress and survival in host epithelial cells.
Construction of N. gonorrhoeae and N. meningitidis laz mutant strains.
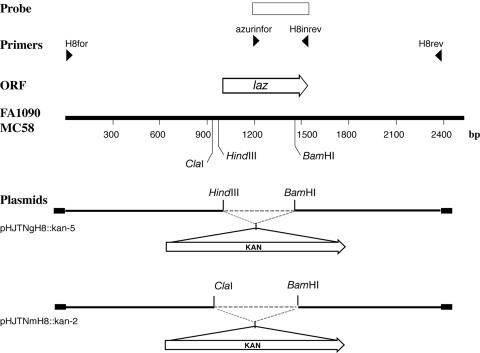
Mutation of laz from N. gonorrhoeae strain 1291 (GenBank accession P07211) and N. meningitidis strain MC58 (NMB1533) (25) involved insertion of a kanamycin resistance cassette (pUC4Kan; Amersham Biosciences) into a suitable unique restriction site in the coding region of each gene as described previously (28) (Fig. 1). Previous work has demonstrated that this cassette does not have a functional promoter in Neisseria, as it is inactive when inserted in the opposite orientation to the gene being inactivated. It also lacks an effective transcriptional terminator, as it does not reduce expression of downstream genes when inserted into an operon, and therefore it is incapable of introducing polar effects (12, 30). Briefly, the laz knockout was constructed by digesting pHJTNgH8-7 and pHJTNmH8-4 (PCR product of primers H8for [5′-AGGCGTTGTTTGAATTCG-3′] and H8rev [5′-CGGATTAATCGACCAAAG-3′] with strains N. gonorrhoeae 1291 and N. meningitidis MC58 as templates cloned into pGEM-T Easy [Promega]) with the restriction enzymes HindIII and BamHI, and ClaI and BamHI, respectively (Fig. 1). The kanamycin resistance cassette was isolated and ligated to the linearized plasmids. Transformation into N. gonorrhoeae and N. meningitidis was performed as described previously (12). Mutant strains were verified by PCR analysis using azurinfor (5′-TCCAACGACAATATGCAG-3′) and H8inrev (5′-ACCTGCCAGCCGTTACA-3′) primers and by Southern hybridization using a digoxigenin-labeled (Roche) probe that was PCR amplified using the H8infor and H8inrev primers. Digoxigenin labeling and Southern hybridization were performed according to the manufacturer's instructions.
FIG. 1.
Restriction endonuclease, plasmid, and open reading frame (ORF) maps of the laz region. The line labeled FA1090 and MC58 represents the restriction endonuclease map of a region of the N. gonorrhoeae strain FA1090 genome sequencing project (accession number AE004969) and a region of the N. meningitidis strain MC58 genome sequencing project (accession number AE002447). The open arrow above the line indicates the orientation and the location of the azurin ORF. Below the FA1090-MC58 line, the thick black lines represent the plasmids constructed during this work. The vector, pGEM T-Easy (Promega), of each of these plasmids is represented by black boxes. The restriction endonuclease sites shown indicate where the kanamycin resistance cassette (KAN) was inserted. The rectangular box at the top represents the PCR products used as probes in this study. The laz probe was constructed from PCR products utilizing primers azurinfor and H8inrev.
The growth characteristics of the N. gonorrhoeae wild-type and laz mutant strains were indistinguishable under aerobic conditions in brain heart infusion (BHI) broth (Oxoid) at 37°C as monitored by the increase in optical density at 600 nm. Growth studies were conducted in triplicate and repeated on several occasions (data not shown).
Neisserial laz mutants are hypersensitive to H2O2 killing.
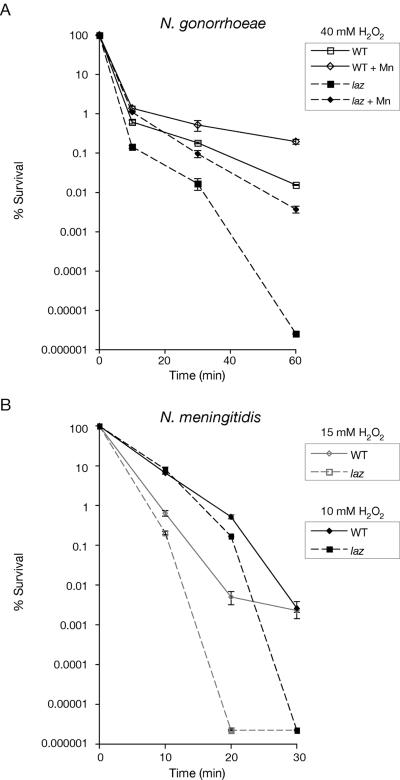
Sensitivity of the N. gonorrhoeae and N. meningitidis laz mutants to hydrogen peroxide (H2O2) was investigated using the H2O2 survival assay (13) as described by Tseng et al. (28). N. gonorrhoeae and N. meningitidis were grown overnight on BHI agar with 10% Levinthal's base (1) (37°C; 5% CO2). Medium for N. gonorrhoeae was also supplemented with IsoVitaleX (Becton Dickinson) and 100 μM MnSO4 for some experiments as indicated. Approximately 107 cells were exposed to H2O2 (10 or 15 mM for N. meningitidis; 40 mM for N. gonorrhoeae) for up to 1 h. At time intervals, samples were taken and the numbers of viable CFU were determined after overnight culture of plated serial dilutions. Each assay was done with triplicate cultures of each mutant and wild-type strain and was performed on at least three occasions. The neisserial laz mutants were more sensitive to H2O2 than their parent wild-type strains (Fig. 2a and b). These data suggest that Laz is important in H2O2 stress responses in both N. gonorrhoeae and N. meningitidis.
FIG. 2.
(A) H2O2 survival test of N. gonorrhoeae strain 1291 wild type (WT) and laz mutant. In this assay, cells were grown on BHI agar ± 100 μM MnSO4 and exposed to 40 mM H2O2. (B) H2O2 survival test of N. meningitidis strain MC58 wild type (WT) and laz mutant. In this assay, cells were grown on BHI agar and exposed to 10 mM or 15 mM H2O2. Experiments were performed in triplicate. The y axis error bars indicate ±1 standard deviation of the mean.
To determine whether azurin played a role in the Mn-dependent resistance to hydrogen peroxide killing that has been described in N. gonorrhoeae (23), H2O2 killing of N. gonorrhoeae wild type and laz was also investigated in the presence of Mn(II). Increased survival was observed for both the wild-type and the laz mutant strains that had been grown in media supplemented with Mn(II) compared to unsupplemented media (Fig. 2a), indicating that Mn resistance is azurin independent.
The sensitivity of the N. gonorrhoeae and N. meningitidis laz mutants to superoxide stress was investigated using oxidative stress killing assays with paraquat (15 mM) or xanthine (5 mM)/xanthine oxidase (350 mU/ml) as described previously (23, 28). In contrast to H2O2 killing, both the N. gonorrhoeae and N. meningitidis laz mutants behaved like their respective wild-type strains in both paraquat and xanthine/xanthine oxidase assays (data not shown).
Neisserial laz mutants are hypersensitive to copper.
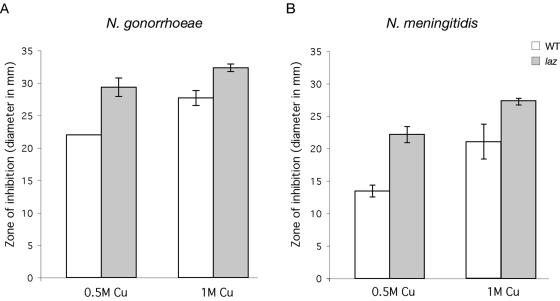
Azurins are copper-containing proteins in several different microorganisms (8, 14, 20, 21). Free ions, such as copper and iron, are dangerous to aerobic cells due to the ability of Cu+ and Fe2+ to react with H2O2 to form extremely reactive hydroxyl radicals (HO.) via the Fenton reaction (11, 16). Therefore, these ions are typically complexed with proteins within the cells to decrease the occurrence of Fenton chemistry. We were interested to determine if N. gonorrhoeae and N. meningitidis exhibited increased sensitivity to Cu2+ without a functional Laz. N. gonorrhoeae and N. meningitidis cells were grown overnight, and 106 cells were evenly spread onto BHI agar, onto which 0.5 M or 1 M CuSO4 disks were placed. The zone of growth inhibition after overnight incubation was measured as the diameter in mm. Both N. gonorrhoeae and N. meningitidis laz mutants were more sensitive to 0.5 M and 1 M CuSO4 than their respective wild-type strains (Fig. 3).
FIG. 3.
(A) Copper sensitivity test of N. gonorrhoeae strain 1291 wild type (WT) and laz mutant or (B) N. meningitidis strain MC58 wild type (WT) and laz mutant. Sensitivity was measured as the diameter (mm) of growth inhibition around a disk with 0, 0.5, or 1 M CuSO4. Experiments were performed in triplicate. The y axis error bars indicate ±1 standard deviation of the mean. There is a statistically significant difference in the mean percent survival of the laz mutant strain relative to WT in all cases (P ≤ 0.05, as determined using a Student t test).
Neisserial laz mutants have decreased survival in cervical epithelial cells.
N. gonorrhoeae infection is usually characterized by a symptomatic localized inflammatory response of the urethra in men (urethritis) and the cervix in women (cervicitis) (2, 5). N. gonorrhoeae is able to survive and replicate within epithelial cells at sites of infection in the genitourinary tract (reviewed in reference 17). Epithelial cells possess oxygen-dependent antimicrobial mechanisms (3, 6, 19, 22), but they remain to be fully characterized. The role of Laz, if any, in defense against these oxygen-dependent defenses and survival within epithelial cells was investigated using a cervical cell survival assay.
Primary human ectocervical epithelial (pex) cells were procured and maintained as described previously (7), and cell monolayers were grown to confluence in 35-mm tissue culture dishes (Falcon). To determine the ability of N. gonorrhoeae wild-type and laz mutant strains to associate with, invade, and survive within pex cells, they were challenged with either the wild-type or mutant strain and infection was allowed to progress for 1.5 h (37°C; 5% CO2). For association assays, the infection medium was removed and the cells were rinsed with phosphate-buffered saline. For invasion assays, pex cells were incubated for a further 30 min with medium containing 100 μg of gentamicin (Gibco) per ml to kill extracellular bacteria. Survival assays were performed in a similar manner, with the exception that following gentamicin treatment, the infected cell monolayers were again rinsed with phosphate-buffered saline. Fresh antibiotic-free medium was then added to each infected cell monolayer before 1 h or 2 h of incubation. Following each assay, the pex cells were lysed with 0.5% saponin to release invasive bacteria, and serial dilutions were plated to determine CFU. The percent invasion was determined as a function of the original inoculum. At all time points in the assays, the laz mutant strain had decreased survival within pex cells relative to the wild-type strain (P values were ≤0.05 as determined using a Kruskal-Wallis nonparametric analysis of variance) (Table 1).
TABLE 1.
Gonococcal association with and intracellular survival within primary human cervical epithelial cellsa
| N. gonorrhoeae strain 1291 | Association | Invasion (T = 0) | Invasion + 1 h (T = 1) | Invasion + 2 h (T = 2) |
|---|---|---|---|---|
| WT | 27.81 (2.66) | 2.80 (0.12) | 2.80 (0.25) | 6.37 (0.33) |
| laz | 18.04 (0.69) | 1.36 (0.25) | 0.94 (0.10) | 0.44 (0.08) |
Values given are the mean (variance) of the percent total association or invasion as a function of the original inoculum, determined from the number of CFU formed upon plating of the cervical cell lysates. Data were obtained from three trials performed in triplicate. At each of the four time points, there was a statistically significant difference in the mean percent survival of the laz mutant relative to N. gonorrhoeae strain 1291 wild type (WT) (P ≤ 0.05, as determined using a Kruskal-Wallis nonparametric analysis of variance). T, time.
Discussion.
The neisserial Laz proteins differ significantly from other azurins in that they contain an N-terminal domain of 39 amino acids that encodes the H.8 epitope and they are modified with lipid (33). However, like the azurin of P. aeruginosa (31), the Neisseria Laz proteins do not play a direct role in denitrification (4) but are involved in H2O2 stress responses (31).
In this study, we have characterized N. gonorrhoeae and N. meningitidis laz mutants with respect to oxidative stress induced by H2O2 and superoxide. Our data showed that the neisserial laz mutants were highly sensitive to H2O2, but unlike the azu mutant of P. aeruginosa, they showed no change in sensitivity to superoxide. The exact mechanism by which Laz confers protection from oxidative stress requires further investigation. It is already established that complexed copper ions can catalyze decomposition of peroxide molecules (18), and thus, Laz may be a defense enzyme at the cell surface involved in protection against external peroxides. The neisserial laz mutants also showed increased sensitivity to the presence of copper, suggesting that the protein may play an important role in Cu2+ ion sequestration.
Survival of the N. gonorrhoeae laz mutant strain in cervical epithelial cells was decreased relative to the wild-type strain. The role that Laz plays in the survival of N. gonorrhoeae within pex cells may be a result of a role in defense against H2O2 stress and/or copper storage (as described above). Azurin of P. aeruginosa has also been shown to interact with host cells. Azurin purified from P. aeruginosa is cytotoxic to macrophages via complex formation with the tumor suppressor protein p53. This complex formation results in reactive oxygen species generation and stabilization of p53, both of which enhance the proapoptotic activity of p53 (34-36). Copper [Cu(I)] can also modulate p53 activity by binding directly to it and inhibiting its DNA-binding activity; however, apo-azurin without copper was still cytotoxic to macrophages. The ability of P. aeruginosa to secrete azurin in the growth medium (36) and the cytotoxicity of azurin to phagocytic cells suggests that azurin is a virulence factor in P. aeruginosa (9). The neisserial Laz protein may also interact with host cells, and future studies will focus on further characterizing the function of Laz and its role in Neisseria pathogenesis.
Acknowledgments
This work was supported by Program Grant 284214 from the National Health and Medical Research Council of Australia and by U.S. Public Health Service grants AI45728, AI43924, and AI38515 from NIAID.
Editor: J. N. Weiser
REFERENCES
- 1.Alexander, H. E. 1965. The Haemophilus group, p. 724-741. In R. J. Dubos and J. G. Hirsch (ed.), Bacterial and mycotic infection in man. Pitman Medical Publishing, London, United Kingdom.
- 2.Apicella, M. A., M. Ketterer, F. K. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. [DOI] [PubMed] [Google Scholar]
- 3.Battistoni, A., F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio, and P. Valenti. 2000. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 68:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon, J. G. 1989. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin. Microbiol. Rev. 2(Suppl.):S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 6.Deitch, E. A., Y. Haskel, N. Cruz, D. Xu, and P. R. Kvietys. 1995. Caco-2 and IEC-18 intestinal epithelial cells exert bactericidal activity through an oxidant-dependent pathway. Shock 4:345-350. [DOI] [PubMed] [Google Scholar]
- 7.Edwards, J. L., J. Q. Shao, K. A. Ault, and M. A. Apicella. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farver, O., and I. Pecht. 1984. The reactivity of copper sites in the ‘blue’ copper proteins, p. 183-214. In R. Lontie (ed.), Copper proteins and copper enzymes. CRC Press Inc., Boca Raton, Fla.
- 9.Goto, M., T. Yamada, K. Kimbara, J. Horner, M. Newcomb, T. K. Gupta, and A. M. Chakrabarty. 2003. Induction of apoptosis in macrophages by Pseudomonas aeruginosa azurin: tumour-suppressor protein p53 and reactive oxygen species, but not redox activity, as critical elements in cytotoxicity. Mol. Microbiol. 47:549-559. [DOI] [PubMed] [Google Scholar]
- 10.Gotschlich, E. C., and M. E. Seiff. 1987. Identification and gene structure of an azurin-like protein with a lipoprotein signal peptide in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 43:253-255. [Google Scholar]
- 11.Imlay, J. A., S. M. Chin, and S. Linn. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640-642. [DOI] [PubMed] [Google Scholar]
- 12.Jennings, M. P., D. W. Hood, I. R. Peak, M. Virji, and E. R. Moxon. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18:729-740. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, S. R., B. M. Steiner, D. D. Cruce, G. H. Perkins, and R. J. Arko. 1993. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect. Immun. 61:1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakutani, T., H. Watanabe, K. Arima, and T. Beppu. 1981. A blue protein as an inactivating factor for nitrite reductase from Alcaligenes faecalis strain S-6. J Biochem. 89:463-472. [DOI] [PubMed] [Google Scholar]
- 15.Kawula, T. H., S. M. Spinola, D. G. Klapper, and J. G. Cannon. 1987. Localization of a conserved epitope and an azurin-like domain in the H.8 protein of pathogenic Neisseria. Mol. Microbiol. 1:179-185. [DOI] [PubMed] [Google Scholar]
- 16.Koppenol, W. H. 1993. The centennial of the Fenton reaction. Free Radic. Biol. Med. 15:645-651. [DOI] [PubMed] [Google Scholar]
- 17.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 18.Pires dos Santos, M. L., A. Faljoni-Alario, A. S. Mangrich, and A. M. da Costa Ferreira. 1998. Antioxidant and pro-oxidant properties of some di-Schiff base copper(II) complexes. J. Inorg. Biochem. 71:71-78. [Google Scholar]
- 19.Rochelle, L. G., B. M. Fischer, and K. B. Adler. 1998. Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radic. Biol. Med. 24:863-868. [DOI] [PubMed] [Google Scholar]
- 20.Ryden, L. 1984. Structure and evolution of the small blue proteins, p. 157-182. In R. Lontie (ed.), Copper proteins and copper enzymes. CRC Press Inc., Boca Raton, Fla.
- 21.Ryden, L., and J. Lundgren. 1976. Homology relationships among the small blue proteins. Nature 261:344-346. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt, H. H., and U. Walter. 1994. NO at work. Cell 78:919-925. [DOI] [PubMed] [Google Scholar]
- 23.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 24.Skaar, E. P., D. M. Tobiason, J. Quick, R. C. Judd, H. Weissbach, F. Etienne, N. Brot, and H. S. Seifert. 2002. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc. Natl. Acad. Sci. USA 99:10108-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 26.Trees, D. L., and S. M. Spinola. 1990. Localization of and immune response to the lipid-modified azurin of the pathogenic Neisseria. J. Infect. Dis. 161:336-339. [DOI] [PubMed] [Google Scholar]
- 27.Tseng, H. J., A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2003. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect. Immun. 71:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 29.Turner, S. M., E. G. Reid, H. Smith, and J. A. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae, a lipoprotein from a Gram-negative bacterium. Biochem. J. 373:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Ley, P., M. Kramer, A. Martin, J. C. Richards, and J. T. Poolman. 1997. Analysis of the icsBA locus required for biosynthesis of the inner core region from Neisseria meningitidis lipopolysaccharide. FEMS Microbiol. Lett. 146:247-253. [DOI] [PubMed] [Google Scholar]
- 31.Vijgenboom, E., J. E. Busch, and G. W. Canters. 1997. In vivo studies disprove an obligatory role of azurin in denitrification in Pseudomonas aeruginosa and show that azu expression is under control of rpoS and ANR. Microbiology 143:2853-2863. [DOI] [PubMed] [Google Scholar]
- 32.Wilks, K. E., K. L. Dunn, J. L. Farrant, K. M. Reddin, A. R. Gorringe, P. R. Langford, and J. S. Kroll. 1998. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect. Immun. 66:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods, J. P., J. F. Dempsey, T. H. Kawula, D. S. Barritt, and J. G. Cannon. 1989. Characterization of the neisserial lipid-modified azurin bearing the H.8 epitope. Mol. Microbiol. 3:583-591. [DOI] [PubMed] [Google Scholar]
- 34.Yamada, T., M. Goto, V. Punj, O. Zaborina, M. L. Chen, K. Kimbara, D. Majumdar, E. Cunningham, T. K. Das Gupta, and A. M. Chakrabarty. 2002. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc. Natl. Acad. Sci. USA 99:14098-14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada, T., M. Goto, V. Punj, O. Zaborina, K. Kimbara, T. K. Das Gupta, and A. M. Chakrabarty. 2002. The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect. Immun. 70:7054-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaborina, O., N. Dhiman, M. Ling Chen, J. Kostal, I. A. Holder, and A. M. Chakrabarty. 2000. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology 146:2521-2530. [DOI] [PubMed] [Google Scholar]